Association between Statin Use and Sepsis Risk in Patients with Dementia: A Retrospective Cohort Study
Abstract
:1. Introduction
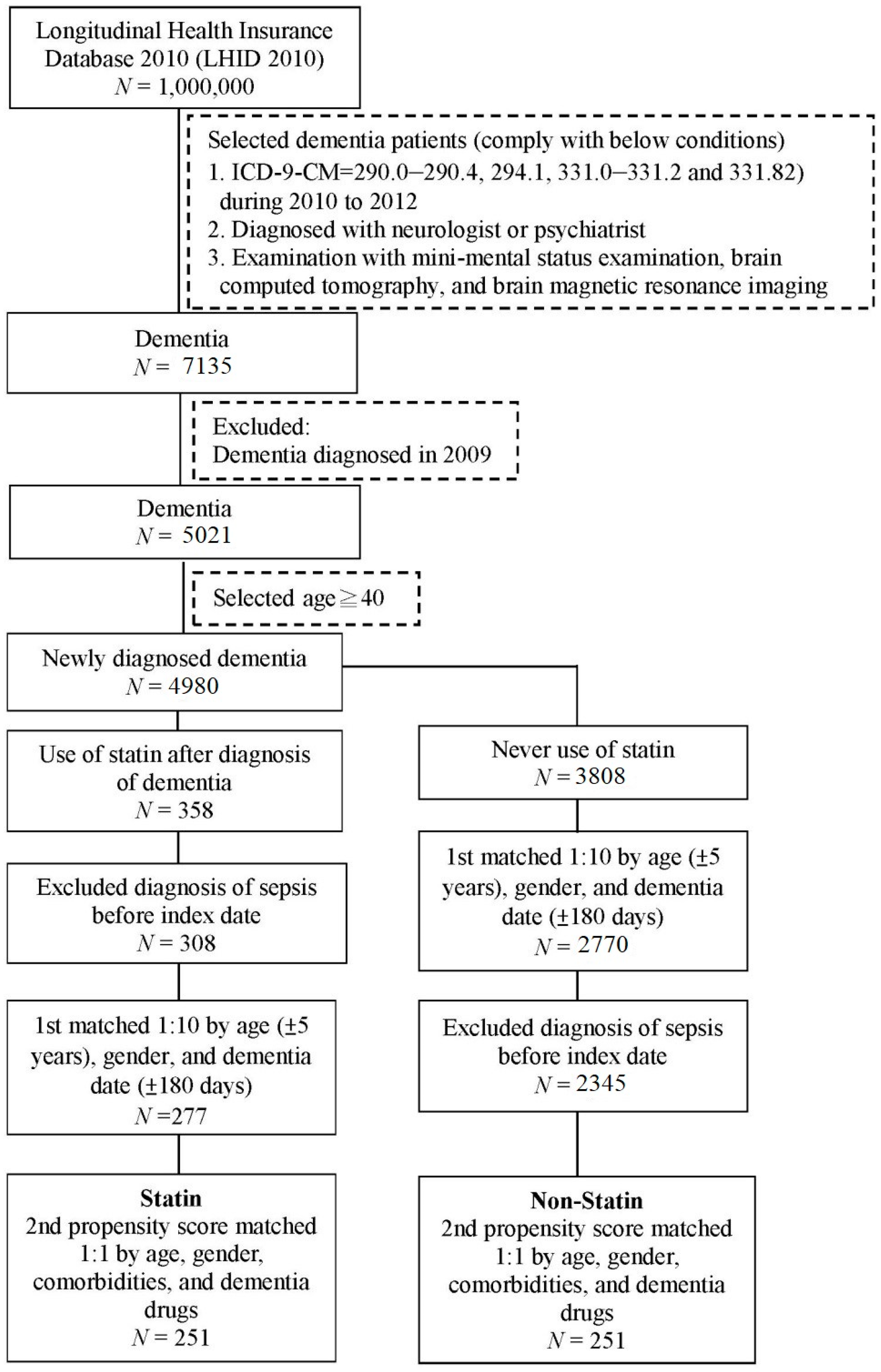
2. Materials and Methods
2.1. Data Source
2.2. Study Groups
2.3. Main Outcome Measurement
2.4. Covariates and Matching
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sirtori, C.R. The pharmacology of statins. Pharmacol. Res. 2014, 88, 3–11. [Google Scholar] [CrossRef]
- Carneiro, A.V.; Costa, J.; Borges, M. Statins for primary and secondary prevention of coronary heart disease. A scientific review. Revista portuguesa de cardiologia 2004, 23, 95–122. [Google Scholar] [PubMed]
- Majumdar, S.R.; McAlister, F.A.; Eurich, D.T.; Padwal, R.S.; Marrie, T.J. Statins and outcomes in patients admitted to hospital with community acquired pneumonia: Population based prospective cohort study. BMJ 2006, 333, 999. [Google Scholar] [CrossRef]
- Grudzinska, F.S.; Dosanjh, D.P.; Parekh, D.; Dancer, R.C.; Patel, J.; Nightingale, P.; Walton, G.M.; Sapey, E.; Thickett, D.R. Statin therapy in patients with community-acquired pneumonia. Clin. Med. 2017, 17, 403–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammad, S.; Nguyen, H.; Nguyen, M.; Abdel-Rasoul, M.; Nguyen, V.; Nguyen, C.D.; Nguyen, K.T.; Li, L.; Kitzmiller, J.P. Pleotropic effects of statins: Untapped potential for statin pharmacotherapy. Curr. Vasc. Pharmacol. 2018. [Google Scholar]
- Parker, B.A.; Capizzi, J.A.; Grimaldi, A.S.; Clarkson, P.M.; Cole, S.M.; Keadle, J.; Chipkin, S.; Pescatello, L.S.; Simpson, K.; White, C.M.; et al. Effect of statins on skeletal muscle function. Circulation 2013, 127, 96–103. [Google Scholar] [PubMed]
- Simic, I.; Reiner, Z. Adverse effects of statins-myths and reality. Curr. Pharm. Des. 2015, 21, 1220–1226. [Google Scholar] [CrossRef] [PubMed]
- Agouridis, A.P.; Kostapanos, M.S.; Elisaf, M.S. Statins and their increased risk of inducing diabetes. Exp. Opin. Drug Saf. 2015, 14, 1835–1844. [Google Scholar] [CrossRef]
- Maki, K.C.; Dicklin, M.R.; Baum, S.J. Statins and diabetes. Cardiol. Clin. 2015, 33, 233–243. [Google Scholar]
- Karahalil, B.; Hare, E.; Koc, G.; Uslu, I.; Senturk, K.; Ozkan, Y. Hepatotoxicity associated with statins. Arch. Ind. Hyg. Toxicol. 2017, 68, 254–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellosta, S.; Corsini, A. Statin drug interactions and related adverse reactions: An update. Exp. Opin. Drug Saf. 2018, 17, 25–37. [Google Scholar] [CrossRef]
- Malfitano, A.M.; Marasco, G.; Proto, M.C.; Laezza, C.; Gazzerro, P.; Bifulco, M. Statins in neurological disorders: An overview and update. Pharmacol. Res. 2014, 88, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Hendrie, H.C.; Hake, A.; Lane, K.; Purnell, C.; Unverzagt, F.; Smith-Gamble, V.; Murrell, J.; Ogunniyi, A.; Baiyewu, O.; Callahan, C.; et al. Statin use, incident dementia and alzheimer disease in elderly african americans. Ethn. Dis. 2015, 25, 345–354. [Google Scholar] [CrossRef]
- Ling, Q.; Tejada-Simon, M.V. Statins and the brain: New perspective for old drugs. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 66, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Golomb, B.A.; Evans, M.A. Statin adverse effects: A review of the literature and evidence for a mitochondrial mechanism. Am. J. Cardiovasc. Drugs 2008, 8, 373–418. [Google Scholar] [CrossRef]
- Reinhart, K.M.; Woods, J.A. Strategies to preserve the use of statins in patients with previous muscular adverse effects. Am. J. Health-Syst. Pharm. 2012, 69, 291–300. [Google Scholar] [CrossRef]
- Liu, A.; Wu, Q.; Guo, J.; Ares, I.; Rodriguez, J.L.; Martinez-Larranaga, M.R.; Yuan, Z.; Anadon, A.; Wang, X.; Martinez, M.A. Statins: Adverse reactions, oxidative stress and metabolic interactions. Pharmacol. Ther. 2019, 195, 54–84. [Google Scholar] [CrossRef]
- Kim, H.I.; Park, S. Sepsis: Early recognition and optimized treatment. Tuberc. Respir. Dis. 2019, 82, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E. New definitions for sepsis and septic shock: Continuing evolution but with much still to be done. JAMA 2016, 315, 757–759. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Wu, Y.T.; Ali, G.C.; Guerchet, M.; Prina, A.M.; Chan, K.Y.; Prince, M.; Brayne, C. Prevalence of dementia in mainland china, Hong Kong and Taiwan: An updated systematic review and meta-analysis. Int. J. Epidemiol. 2018, 47, 709–719. [Google Scholar] [CrossRef]
- Janda, S.; Young, A.; Fitzgerald, J.M.; Etminan, M.; Swiston, J. The effect of statins on mortality from severe infections and sepsis: A systematic review and meta-analysis. J. Crit. Care 2010, 25, 656.e7–656.e22. [Google Scholar] [CrossRef]
- Falagas, M.E.; Makris, G.C.; Matthaiou, D.K.; Rafailidis, P.I. Statins for infection and sepsis: A systematic review of the clinical evidence. J. Antimicrob. Chemother. 2008, 61, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Almog, Y.; Shefer, A.; Novack, V.; Maimon, N.; Barski, L.; Eizinger, M.; Friger, M.; Zeller, L.; Danon, A. Prior statin therapy is associated with a decreased rate of severe sepsis. Circulation 2004, 110, 880–885. [Google Scholar] [CrossRef]
- Merx, M.W.; Liehn, E.A.; Graf, J.; van de Sandt, A.; Schaltenbrand, M.; Schrader, J.; Hanrath, P.; Weber, C. Statin treatment after onset of sepsis in a murine model improves survival. Circulation 2005, 112, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Covelli, D.; Vannucchi, G.; Campi, I.; Curro, N.; D’Ambrosio, R.; Maggioni, M.; Gianelli, U.; Beck-Peccoz, P.; Salvi, M. Statins may increase the risk of liver dysfunction in patients treated with steroids for active graves’ orbitopathy. J. Clin. Endocrinol. Metab. 2015, 100, 1731–1737. [Google Scholar] [CrossRef]
- Golomb, B.A. Do statins reduce the risk of infection? BMJ 2011, 343, d7134. [Google Scholar] [CrossRef]
- Van den Hoek, H.L.; Bos, W.J.; de Boer, A.; van de Garde, E.M. Statins and prevention of infections: Systematic review and meta-analysis of data from large randomised placebo controlled trials. BMJ 2011, 343, d7281. [Google Scholar] [CrossRef]
- Hackam, D.G.; Mamdani, M.; Li, P.; Redelmeier, D.A. Statins and sepsis in patients with cardiovascular disease: A population-based cohort analysis. Lancet 2006, 367, 413–418. [Google Scholar] [CrossRef]
- Lee, M.G.; Lee, C.C.; Lai, C.C.; Hsu, T.C.; Porta, L.; Lee, M.; Chang, S.S.; Chien, K.L.; Chen, Y.M. Preadmission statin use improves the outcome of less severe sepsis patients—A population-based propensity score matched cohort study. Br. J. Anaesth. 2017, 119, 645–654. [Google Scholar] [CrossRef]
- Yasuda, H.; Yuen, P.S.; Hu, X.; Zhou, H.; Star, R.A. Simvastatin improves sepsis-induced mortality and acute kidney injury via renal vascular effects. Kidney Int. 2006, 69, 1535–1542. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, L.; Zhao, X.; Yang, W.; Zhang, R. An experimental study of the protective effect of simvastatin on sepsis-induced myocardial depression in rats. Biomed. Pharmacother. 2017, 94, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Kruger, P.; Bailey, M.; Bellomo, R.; Cooper, D.J.; Harward, M.; Higgins, A.; Howe, B.; Jones, D.; Joyce, C.; Kostner, K.; et al. A multicenter randomized trial of atorvastatin therapy in intensive care patients with severe sepsis. Am. J. Respir. Crit. Care Med. 2013, 187, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Ji, M.; Si, X. The effects of statin therapy on mortality in patients with sepsis: A meta-analysis of randomized trials. Medicine 2018, 97, e11578. [Google Scholar] [CrossRef]
- Muldoon, M.F.; Barger, S.D.; Ryan, C.M.; Flory, J.D.; Lehoczky, J.P.; Matthews, K.A.; Manuck, S.B. Effects of lovastatin on cognitive function and psychological well-being. Am. J. Med. 2000, 108, 538–546. [Google Scholar] [CrossRef]
- Muldoon, M.F.; Ryan, C.M.; Sereika, S.M.; Flory, J.D.; Manuck, S.B. Randomized trial of the effects of simvastatin on cognitive functioning in hypercholesterolemic adults. Am. J. Med. 2004, 117, 823–829. [Google Scholar] [CrossRef]
- Foreman, M.G.; Mannino, D.M.; Moss, M. Cirrhosis as a risk factor for sepsis and death: Analysis of the national hospital discharge survey. Chest 2003, 124, 1016–1020. [Google Scholar] [CrossRef]
- Doi, K.; Leelahavanichkul, A.; Hu, X.; Sidransky, K.L.; Zhou, H.; Qin, Y.; Eisner, C.; Schnermann, J.; Yuen, P.S.; Star, R.A. Pre-existing renal disease promotes sepsis-induced acute kidney injury and worsens outcome. Kidney Int. 2008, 74, 1017–1025. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, J.; Wang, G.; Liu, Q.; Guo, K.; Li, J. Association between diabetes mellitus and outcomes of patients with sepsis: A meta-analysis. Med. Sci. Monit. 2017, 23, 3546–3555. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Martin, J.F.; Martin-Fernandez, M.; Lopez-Mestanza, C.; Duque, P.; Almansa, R. Shared features of endothelial dysfunction between sepsis and its preceding risk factors (aging and chronic disease). J. Clin. Med. 2018, 7, 400. [Google Scholar] [CrossRef] [PubMed]

| Variable | Statin (N = 251) | Non-Statin (N = 251) | |||
|---|---|---|---|---|---|
| n | % | n | % | p-Value | |
| Age, mean ± SD | 77.8 ± 8.8 | 77.5 ± 7.8 | 0.715 | ||
| Sex | 0.720 | ||||
| Female | 136 | 54.2 | 140 | 55.8 | |
| Male | 115 | 45.8 | 111 | 44.2 | |
| Hypertension | 208 | 82.9 | 216 | 86.1 | 0.324 |
| Hyperlipidemia | 131 | 52.2 | 119 | 47.4 | 0.284 |
| Diabetes | 130 | 51.8 | 146 | 58.2 | 0.151 |
| Cerebrovascular disease | 155 | 61.8 | 151 | 60.2 | 0.714 |
| Renal disease | 38 | 15.1 | 45 | 17.9 | 0.400 |
| Liver disease | 9 | 3.6 | 10 | 4.0 | 0.815 |
| Asthma | 17 | 6.8 | 15 | 6.0 | 0.715 |
| Malignancy | 21 | 8.4 | 21 | 8.4 | 1.000 |
| Parkinsonism | 30 | 12.0 | 32 | 12.7 | 0.786 |
| Dementia drugs | 18 | 7.2 | 21 | 8.4 | 0.617 |
| Variable | N | No. of Sepsis Event | Crude Hazard Ratio (HR) | 95% CI | Adjusted Hazard Ratio (HR) † | 95% CI |
|---|---|---|---|---|---|---|
| Statin | ||||||
| No | 251 | 24 | 1 | 1 | ||
| Yes | 251 | 27 | 1.36 | 0.78–2.36 | 1.42 | 0.81–2.50 |
| Age | 502 | 51 | 1.03 | 0.99–1.07 | 1.03 | 0.99–1.07 |
| Sex | ||||||
| Female | 276 | 26 | 1 | 1 | ||
| Male | 226 | 25 | 1.25 | 0.72–2.17 | 1.16 | 0.66–2.05 |
| Hypertension | 424 | 43 | 0.93 | 0.44–1.99 | 0.92 | 0.43–2.00 |
| Hyperlipidemia | 250 | 21 | 0.68 | 0.39–1.19 | 0.76 | 0.43–1.36 |
| Diabetes | 276 | 29 | 1.15 | 0.66–1.99 | 1.35 | 0.75–2.45 |
| Cerebrovascular disease | 306 | 37 | 1.65 | 0.89–3.05 | 1.66 | 0.87–3.17 |
| Renal disease | 83 | 10 | 1.40 | 0.7–2.8 | 1.43 | 0.70–2.92 |
| Liver disease | 19 | 4 | 2.11 | 0.76–5.86 | 2.09 | 0.71–6.14 |
| Asthma | 32 | 7 | 2.03 | 0.91–4.53 | 2.02 | 0.90–4.55 |
| Malignancy | 42 | 3 | 0.73 | 0.23–2.33 | 0.78 | 0.24–2.56 |
| Parkinsonism | 62 | 12 | 2.12 | 1.11–4.05 | 1.93 | 0.99–3.79 |
| Dementia drugs | 39 | 3 | 0.66 | 0.21–2.12 | 0.84 | 0.25–2.80 |
| Variable | Statin | Non-Statin | ||||
|---|---|---|---|---|---|---|
| N | No. of Sepsis Event | N | No. of Sepsis Event | HR | 95% CI | |
| Age | ||||||
| <70 | 49 | 4 | 33 | 1 | 2.78 | 0.31–24.84 |
| ≥70 | 202 | 23 | 218 | 23 | 1.42 | 0.79–2.53 |
| p for interaction = 0.544 | ||||||
| Gender | ||||||
| Female | 136 | 11 | 140 | 15 | 0.86 | 0.40–1.88 |
| Male | 115 | 16 | 111 | 9 | 2.29 * | 1.01–5.21 |
| p for interaction = 0.124 | ||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, L.-T.; Tang, C.-Y.; Yang, S.-F.; Yeh, H.-W.; Yeh, Y.-T.; Wang, Y.-H.; Chou, M.-C.; Yeh, C.-B.; Chan, C.-H. Association between Statin Use and Sepsis Risk in Patients with Dementia: A Retrospective Cohort Study. Int. J. Environ. Res. Public Health 2019, 16, 1626. https://doi.org/10.3390/ijerph16091626
Yeh L-T, Tang C-Y, Yang S-F, Yeh H-W, Yeh Y-T, Wang Y-H, Chou M-C, Yeh C-B, Chan C-H. Association between Statin Use and Sepsis Risk in Patients with Dementia: A Retrospective Cohort Study. International Journal of Environmental Research and Public Health. 2019; 16(9):1626. https://doi.org/10.3390/ijerph16091626
Chicago/Turabian StyleYeh, Liang-Tsai, Chuan-Yi Tang, Shun-Fa Yang, Han-Wei Yeh, Ying-Tung Yeh, Yu-Hsun Wang, Ming-Chih Chou, Chao-Bin Yeh, and Chi-Ho Chan. 2019. "Association between Statin Use and Sepsis Risk in Patients with Dementia: A Retrospective Cohort Study" International Journal of Environmental Research and Public Health 16, no. 9: 1626. https://doi.org/10.3390/ijerph16091626
APA StyleYeh, L. -T., Tang, C. -Y., Yang, S. -F., Yeh, H. -W., Yeh, Y. -T., Wang, Y. -H., Chou, M. -C., Yeh, C. -B., & Chan, C. -H. (2019). Association between Statin Use and Sepsis Risk in Patients with Dementia: A Retrospective Cohort Study. International Journal of Environmental Research and Public Health, 16(9), 1626. https://doi.org/10.3390/ijerph16091626






