Biomarker Effects in Carassius auratus Exposure to Ofloxacin, Sulfamethoxazole and Ibuprofen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Test Organisms
2.3. Exposure Test
2.4. Chemical Analysis
2.5. Enzyme Assay
2.6. Statistical Analysis
2.7. Calculation of Hazard Quotient
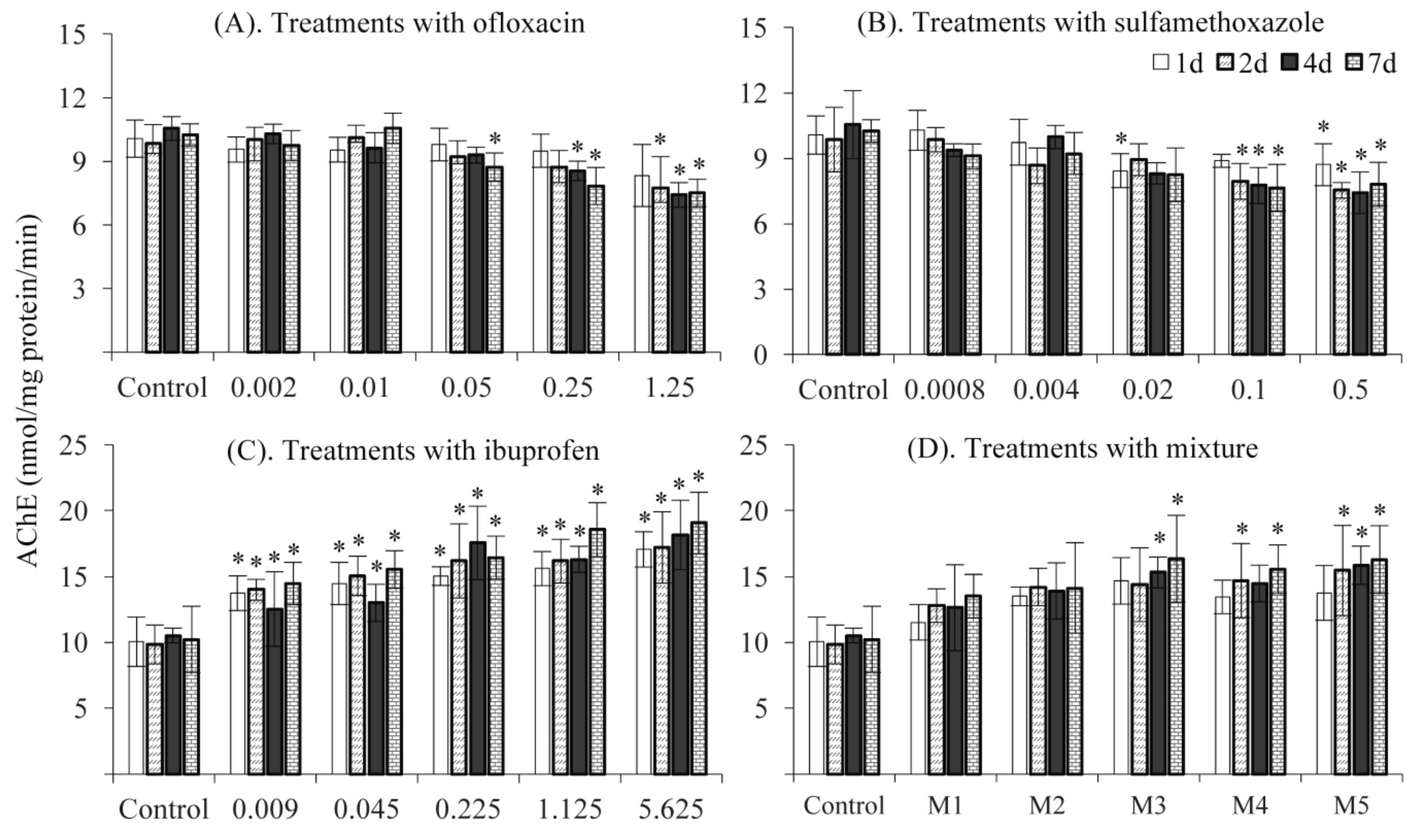
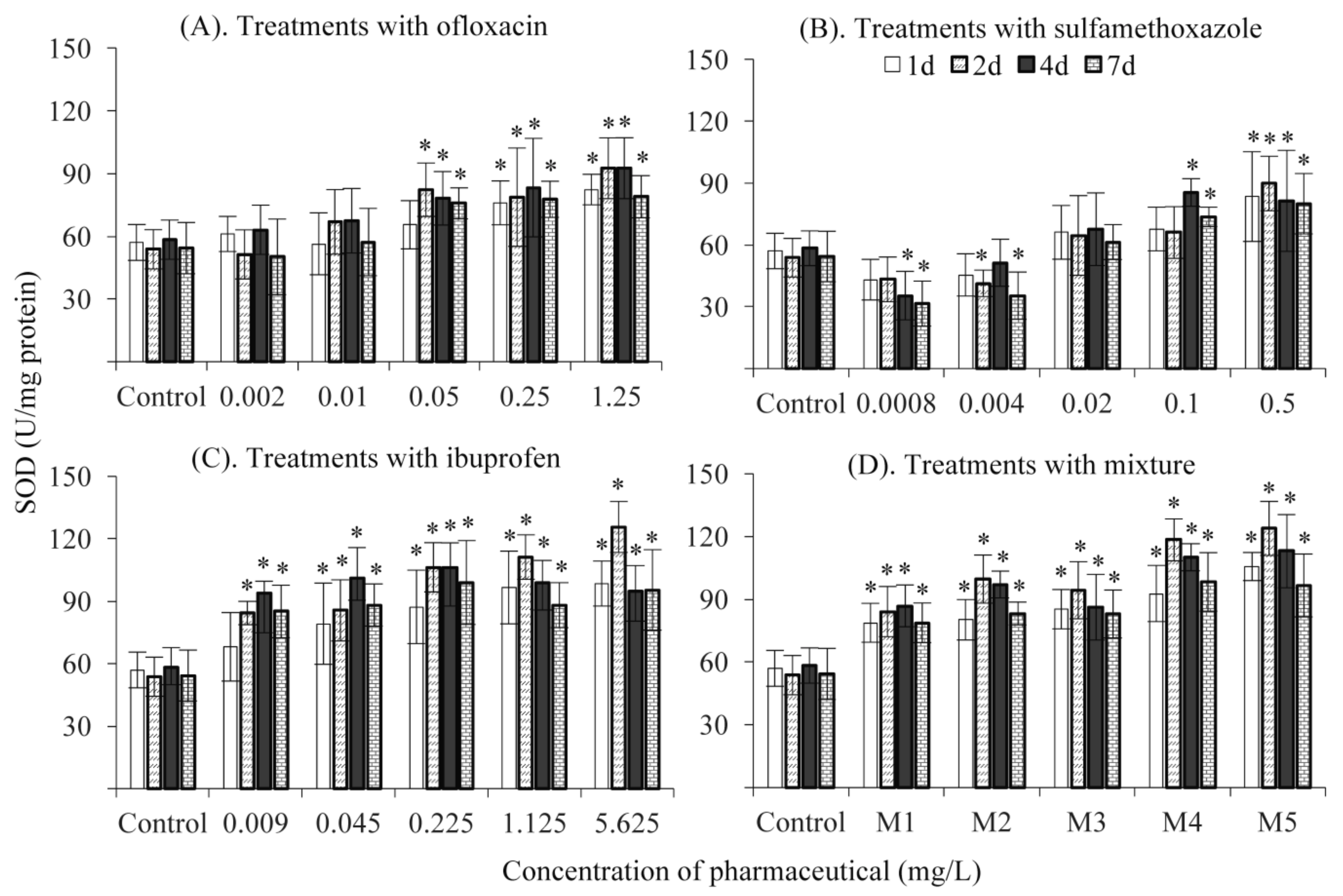
3. Results and Discussion
3.1. Verification of Exposure Concentration
3.2. AChE Activity
3.3. EROD Activity
3.4. SOD Activity
3.5. Environmental Implications
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ternes, T.A.; Joss, A.; Siegrist, H. Peer Reviewed: Scrutinizing Pharmaceuticals and Personal Care Products in Wastewater Treatment. Environ. Sci. Technol. 2004, 38, 392–399. [Google Scholar] [CrossRef]
- Cappello, T.; Denise, F.; Maria, M.; Andrea, C.; Marta, B.; Maria, J.B.; Angela, M.; Salvatore, F.; Cinta, P. Sex steroids and metabolic responses in mussels Mytilus galloprovincialis exposed to drospirenone. Ecotoxicol. Environ. Saf. 2017, 143, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999−2000: A national reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Ashton, D.; Hilton, M.; Thomas, K.V. Investigating the environmental transport of human pharmaceuticals to streams in the United Kingdom. Sci. Total Environ. 2004, 333, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Ginebreda, A.; Muñoz, I.; Alda, M.L.; Brix, R.; López-Doval, J.; Barceló, D. Environmental risk assessment of pharmaceuticals in rivers: Relationships between hazard indexes and aquatic macroinvertebrate diversity indexes in the Llobregat River (NE Spain). Environ. Int. 2010, 36, 153–162. [Google Scholar] [CrossRef]
- Ye, J.P.; Zou, S.C.; Zhang, G.; Xu, W.H. Characteristics of selected antibiotics in the aquatic environment of the Pearl River Delta, south China. Ecol. Environ. 2007, 16, 384–388. [Google Scholar]
- Trovó, A.G.; Nogueira, R.F.P.; Agüera, A.; Fernandez-Alba, A.R.; Sirtori, C.; Malato, S. Degradation of sulfamethoxazole in water by solar photo-Fenton. Chemical and toxicological evaluation. Water Res. 2009, 43, 3922–3931. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, Y.; Tang, X. In vitro and in vivo evaluation of ofloxacin sustained release pellets. Int. J. Pharm. 2008, 360, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Zivanovic, L.; Zigic, G.; Zecevic, M. Investigation of chromatographic conditions for the separation of ofloxacin and its degradation products. J. Chromatogr. A. 2006, 1119, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Isidori, M.; Lavorgna, M.; Nardelli, A.; Pascarella, L.; Parrella, A. Toxic and genotoxic evaluation of six antibiotics on non-target organisms. Sci. Total Environ. 2005, 346, 87–98. [Google Scholar] [CrossRef]
- Parolini, M.; Binelli, A.; Provini, A. Chronic effects induced by ibuprofen on the freshwater bivalve Dreissena polymorpha. Ecotoxicol. Environ. Saf. 2011, 74, 1586–1594. [Google Scholar] [CrossRef]
- Carballa, M.; Omil, F.; Lema, J.M.; Llompart, M.; García-Jares, C.; Rodríguez, I.; Gómez, M.; Ternes, T. Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant. Water Res. 2004, 38, 2918–2926. [Google Scholar] [CrossRef]
- Zorita, S.; Mårtensson, L.; Mathiasson, L. Occurrence and removal of pharmaceuticals in a municipal sewage treatment system in the south of Sweden. Sci. Total Environ. 2009, 407, 2760–2770. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, L.; Rysz, M.; Wang, Y.; Zhang, H.; Alvarez, P.J.J. Occurrence and transport of tetracycline, sulfonamide, quinolone, and macrolide antibiotics in the Haihe River Basin, China. Environ. Sci. Technol. 2011, 45, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.H.; Thomas, K.V. The occurrence of selected pharmaceuticals in wastewater effluent and surface waters of the lower Tyne catchment. Sci. Total Environ. 2006, 356, 143–153. [Google Scholar] [CrossRef]
- Yang, X.F.; Lu, G.H.; Liu, J.C.; Yan, Z.H. Multigenerational chronic effects of pharmaceuticals on Daphnia magna at environmentally relevant concentrations. China Environ. Sci. 2013, 33, 538–545. [Google Scholar]
- Maisano, M.; Trapani, M.R.; Parrino, V.; Parisi, M.G.; Cappello, T.; D’Agata, A.; Benenati, G.; Natalotto, A.; Mauceri, A.; Cammarata, M. Haemolytic activity and characterization of nematocyst venom from Pelagia noctiluca (Cnidaria: Scyphozoa). It. J. Zool. 2013, 80, 168–176. [Google Scholar] [CrossRef]
- Matozzo, V.; Formenti, A.; Donadello, G.; Marin, M.G. A multi-biomarker approach to assess effects of Triclosan in the clam Ruditapes philippinarum. Mar. Environ. Res. 2012, 74, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, G.; Yang, X.; Wang, C. Single and combined effects of selected pharmaceuticals at sublethal concentrations on multiple biomarkers in Carassius auratus. Ecotoxicology 2012, 21, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lu, G.; Cui, J.; Wang, P. Sublethal effects of pesticide mixtures on selected biomarkers of Carassius auratus. Environ. Toxicol. Pharmacol. 2009, 28, 414–419. [Google Scholar] [CrossRef]
- Guilhermino, L.; Lopes, M.C.; Carvalho, A.P.; Soared, A.M.V.M. Inhibition of acetylcholinesterase activity as effect criterion in acute tests with juvenile Daphnia magna. Chemosphere 1996, 32, 727–738. [Google Scholar] [CrossRef]
- Lu, G.H.; Wang, C.; Zhu, Z. The Dose–Response Relationships for EROD and GST Induced by Polyaromatic Hydrocarbons in Carassius auratus. Bull. Environ. Contam. Toxicol. 2009, 82, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Marklunds, M.G. Involvement of superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1975, 47, 469–474. [Google Scholar] [CrossRef]
- Ludwicki, J.K.; Góralczyk, K.; Struciński, P.; Wojtyniak, B.; Rabczenko, D.; Toft, G.; Lindh, C.H.; Jönsson, B.A.G.; Lenters, V.; Heederik, D.; et al. Hazard quotient profiles used as a risk assessment tool for PFOS and PFOA serum levels in three distinctive European populations. Environ. Int. 2015, 74, 112–118. [Google Scholar] [CrossRef]
- Wang, Z.; Du, Y.; Yang, C.; Liu, X.; Zhang, J.; Li, E.; Zhang, Q.; Wang, X. Occurrence and ecological hazard assessment of selected antibiotics in the surface waters in and around Lake Honghu, China. Sci. Total Environ. 2017, 609, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Roex, E.W.M.; Keijzers, R.; van Gestel, C.A.M. Acetylcholinesterase inhibition and increased food consumption rate in the zebrafish, Danio rerio, after chronic exposure to parathion. Aquat Toxicol. 2003, 64, 451–460. [Google Scholar] [CrossRef]
- Dos Santos Miron, D.; Crestani, M.; Rosa Shettinger, M.; Maria Morsch, V.; Baldisserotto, B.; Angel Tierno, M.; Moraes, G.; Vieira, V.L.P. Effects of the herbicides clomazone, quinclorac, and metsulfuron methyl on acetylcholinesterase activity in the silver catfish (Rhamdia quelen) (Heptapteridae). Ecotoxicol. Environ. Saf. 2005, 61, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Dutta, H.M.; Arends, D.A. Effects of endosulfan on brain acetylcholinesterase activity in juvenile Bluegill sunfish. Environ. Res. 2003, 91, 157–162. [Google Scholar] [CrossRef]
- Sturm, A.; Radau, T.S.; Hahn, T.; Schulz, R. Inhibition of rainbow trout acetylcholinesterase by aqueous and suspended particle-associated organophosphorous insecticides. Chemosphere 2007, 68, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Van Dyk, J.S.; Pletschke, B. Review on the use of enzymes for the detection of organochlorine, organophosphate; carbamate pesticides in the environment. Chemosphere 2011, 82, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Nunes, B.; Carvalho, F.; Guilhermino, L. Acute and chronic effects of clofibrate and clofibric acid on the enzymes acetylcholinesterase, lactate dehydrogenase and catalase of the mosquitofish, Gambusia holbrooki. Chemosphere 2004, 57, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, E.M.; Abrahamson, A.; Brunström, B.; Brandt, I. Cytochrome p4501a induction in rainbow trout gills; liver following exposure to waterborne indigo, benzo(a)pyrene; 3,3′,4,4′,5-pentachlorobiphenyl. Aquat. Toxicol. 2006, 79, 226–232. [Google Scholar] [CrossRef]
- Whyte, J.J.; Jung, R.E.; Schmitt, C.J.; Tillitt, D.E. Ethoxyresorufin-O-deethylase (EROD) activity in fish as a biomarker of chemical exposure. Crit. Rev. Toxicol. 2000, 30, 347–570. [Google Scholar] [CrossRef]
- Martín-Díaz, M.L.; Gagné, F.; Blaise, C. The use of biochemical responses to assess ecotoxicological effects of Pharmaceutical and Personal Care Products (PPCPs) after injection in the mussel Elliptio complanata. Environ. Toxicol. Pharmacol. 2009, 28, 237–242. [Google Scholar] [CrossRef]
- Bartram, A.E.; Winter, M.J.; Huggett, D.B.; McCormack, P.; Constantine, L.A.; Hetheridge, M.J.; Hutchinson, T.H.; Kinter, L.B.; Ericson, J.F.; Sumpter, J.P.; et al. In vivo and in vitro liver and gill EROD activity in rainbow trout (Oncorhynchus mykiss) exposed to the beta-blocker propranolol. Environ. Toxicol. 2012, 27, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Laville, N.; Ait-Aissa, S.; Gomez, E.; Casellas, C.; Porcher, J.M. Effects of human pharmaceuticals on cytotoxicity, EROD activity and ROS production in fish hepatocytes. Toxicology 2004, 196, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Jackson, M.J. Exercise-Induced Oxidative Stress: Cellular Mechanisms and Impact on Muscle Force Production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yu, H.; Zhang, J.; Yin, Y.; Shi, H.; Wang, X. Bioaccumulation, depuration and oxidative stress in fish Carassius auratus under phenanthrene exposure. Chemosphere 2006, 63, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Li, P.; Randak, T. Ecotoxocological effects of short-term exposure to a human pharmaceutical Verapamil in juvenile rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physol C Toxicol. Pharmacol. 2010, 152, 385–391. [Google Scholar] [CrossRef]
- Li, Z.H.; Velisek, J.; Zlabek, V.; Grabic, R.; Machova, J.; Kolarova, J.; Randak, T. Hepatic antioxidant status and hematological parameters in rainbow trout, Oncorhynchus mykiss, after chronic exposure to carbamazepine. Chem. Biol. Interact. 2010, 183, 98–104. [Google Scholar] [CrossRef]
- Binelli, A.; Parolini, M.; Cogni, D.; Pedriali, A.; Provini, A. A multi-biomarker assessment of the impact of the antibacterial trimethoprim on the non-target organism Zebra mussel (Dreissena polymorpha). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009, 150, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Huddleston, J.; Zak, J.; Jeter, R. Antimicrobial susceptibilities of Aeromonas spp. isolated from environmental sources. Appl. Environ. Microbiol. 2006, 72, 7036–7042. [Google Scholar] [CrossRef]
- Cristale, J.; Katsoyiannis, A.; Sweetman, A.J.; Jones, K.C.; Lacorte, S. Occurrence and risk assessment of organophosphorus and brominated flame retardants in the River Aire (UK). Environ. Pollut. 2013, 179, 194–200. [Google Scholar] [CrossRef]
- Sánchez-Avila, J.; Tauler, R.; Lacorte, S. Organic micropollutants in coastal waters from NW Mediterranean Sea: Sources distribution; potential risk. Environ. Int. 2012, 46, 50–62. [Google Scholar] [CrossRef] [PubMed]

| Pharmaceutical Information | Environmentally Measured Concentration (μg/L) |
|---|---|
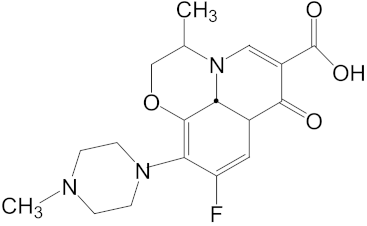 Ofloxacin (Molecular Weight= 361.38, logKow = −0.39) | 0.11, Shenzhen River [6] 8.77, Llobregat River [5] 0.18, Haihe River [14] |
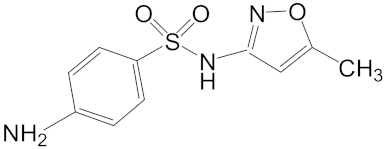 Sulfamethoxazole (Molecular Weight = 253.3 logKow = 0.89) | 0.88, Shenzhen River [6] 11.92, Llobregat River [5] 0.5, England Stream [4] |
 Ibuprofen (Molecular Weight = 206.29, logKow = 3.97) | 9.89, Llobregat River [5] 1.11, England Stream [4] 2.37, Tyner River [15] |
| Pharmaceutical | Nominal Concentration (NC) & Measured Concentration (MC) | Recovery Rate (%) | RSD (%) | DL (ng/L) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Ofloxacin | NC | 0.002 | 0.01 | 0.05 | 0.25 | 1.25 | 100.7 | 6.2 | 2 |
| MC | 0.002 | 0.012 | 0.046 | 0.21 | 1.25 | ||||
| Sulfamethoxazole | NC | 0.0008 | 0.004 | 0.02 | 0.1 | 0.5 | 85.2 | 4.7 | 1 |
| MC | 0.0007 | 0.0047 | 0.0213 | 0.1022 | 0.5121 | ||||
| Ibuprofen | NC | 0.009 | 0.045 | 0.225 | 1.125 | 5.625 | 83.6 | 4 | 2 |
| MC | 0.084 | 0.041 | 0.212 | 0.953 | 5.421 | ||||
| MIX | NC | 0.002 + 0.0008 + 0.009 | 0.01 + 0.004 + 0.045 | 0.05 + 0.02 + 1.125 | 0.25 + 0.1 + 1.125 | 1.25 + 0.5 + 5.625 | |||
| MC | 0.002 + 0.0008 + 0.009 | 0.013 + 0.0036 + 0.045 | 0.056 + 0.022 + 0.243 | 0.264 + 0.0.119 + 0.017 | 1.119 + 0.486 + 5.501 | ||||
| Pharmaceutical | NOEC (µg/L) | AF | PNEC (µg/L) | MEC (µg/L) | HQ Value |
|---|---|---|---|---|---|
| Ofloxacin | 39.68 | 10 | 3.97 | 3.02 | 0.76 |
| Sulfamethoxazole | 16.02 | 10 | 1.60 | 4.43 | 2.77 |
| Ibuprofen | 1.80 | 10 | 0.18 | 4.46 | 24.78 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Xu, X.; Wei, X.; Wan, J.; Zhang, Y. Biomarker Effects in Carassius auratus Exposure to Ofloxacin, Sulfamethoxazole and Ibuprofen. Int. J. Environ. Res. Public Health 2019, 16, 1628. https://doi.org/10.3390/ijerph16091628
Yang X, Xu X, Wei X, Wan J, Zhang Y. Biomarker Effects in Carassius auratus Exposure to Ofloxacin, Sulfamethoxazole and Ibuprofen. International Journal of Environmental Research and Public Health. 2019; 16(9):1628. https://doi.org/10.3390/ijerph16091628
Chicago/Turabian StyleYang, Xiaofan, Xiaoping Xu, Xueyu Wei, Jie Wan, and Yu Zhang. 2019. "Biomarker Effects in Carassius auratus Exposure to Ofloxacin, Sulfamethoxazole and Ibuprofen" International Journal of Environmental Research and Public Health 16, no. 9: 1628. https://doi.org/10.3390/ijerph16091628
APA StyleYang, X., Xu, X., Wei, X., Wan, J., & Zhang, Y. (2019). Biomarker Effects in Carassius auratus Exposure to Ofloxacin, Sulfamethoxazole and Ibuprofen. International Journal of Environmental Research and Public Health, 16(9), 1628. https://doi.org/10.3390/ijerph16091628




