Abnormalities on Chest Computed Tomography and Lung Function Following an Intense Dust Exposure: A 17-Year Longitudinal Study
Abstract
1. Introduction
2. Methods
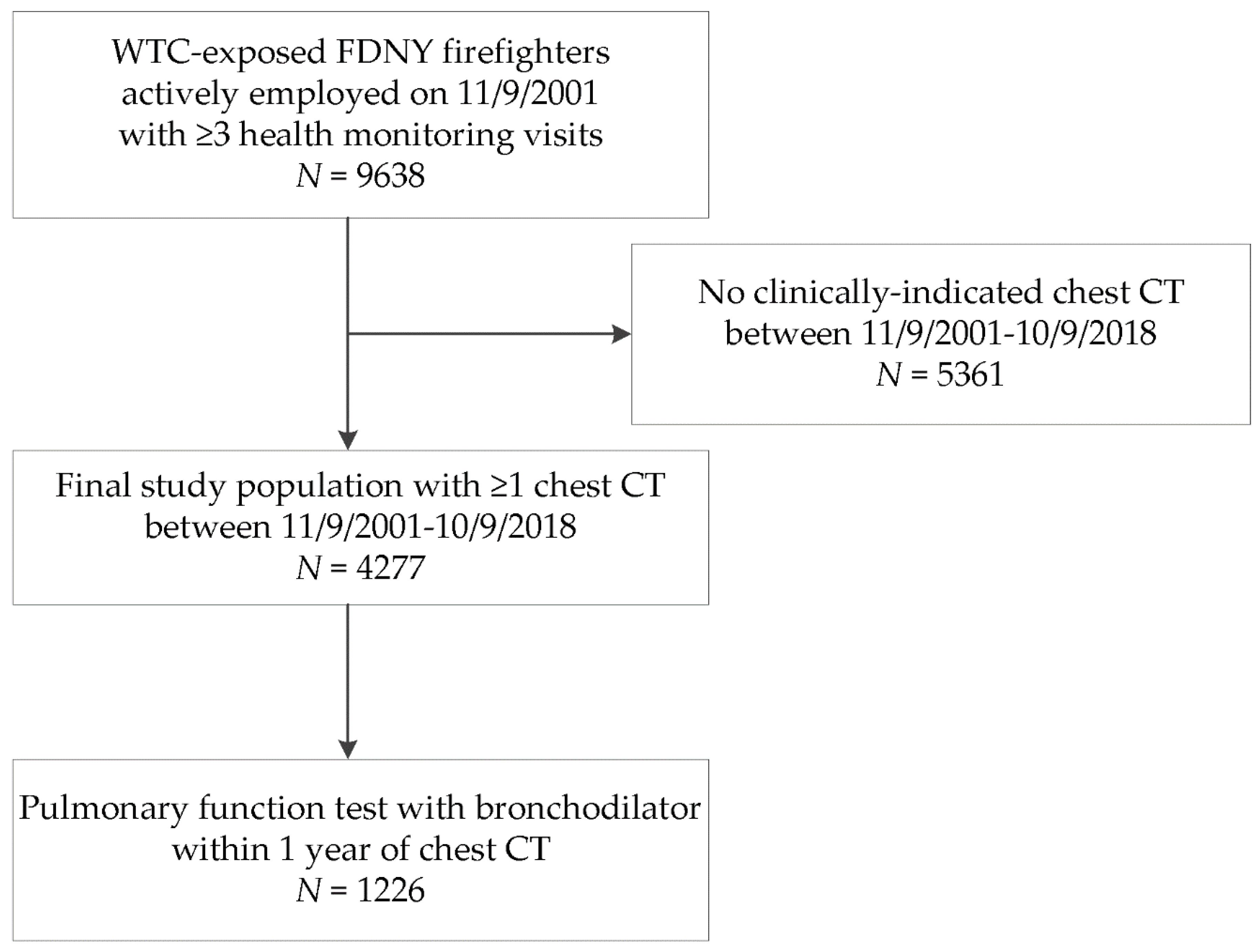
2.1. Study Population
2.2. CT Scans
2.3. Participant Characteristics
2.4. World Trade Center Dust Exposure
2.5. Longitudinal Respiratory Symptoms
2.6. Longitudinal Screening Spirometry
2.7. Complete Pulmonary Function Testing
2.8. Statistical Analyses
3. Results
3.1. Population Characteristics
3.2. CT Abnormalities and WTC Exposure
3.3. CT Abnormalities and Respiratory Symptoms
3.4. CT Abnormalities and Longitudinal Spirometry
3.5. CT Abnormalities and Lung Volumes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lioy, P.J.; Weisel, C.P.; Millette, J.R.; Eisenreich, S.; Vallero, D.; Offenberg, J.; Buckley, B.; Turpin, B.; Zhong, M.; Cohen, M.D.; et al. Characterization of the dust/smoke aerosol that settled east of the world trade center (wtc) in lower manhattan after the collapse of the wtc 11 september 2001. Environ. Health Perspect 2002, 110, 703–714. [Google Scholar] [CrossRef]
- Aldrich, T.K.; Gustave, J.; Hall, C.B.; Cohen, H.W.; Webber, M.P.; Zeig-Owens, R.; Cosenza, K.; Christodoulou, V.; Glass, L.; Al-Othman, F.; et al. Lung function in rescue workers at the world trade center after 7 years. N. Engl. J. Med. 2010, 362, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Weiden, M.D.; Ferrier, N.; Nolan, A.; Rom, W.N.; Comfort, A.; Gustave, J.; Zeig-Owens, R.; Zheng, S.; Goldring, R.M.; Berger, K.I.; et al. Obstructive airways disease with air trapping among firefighters exposed to world trade center dust. Chest 2010, 137, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, T.K.; Vossbrinck, M.; Zeig-Owens, R.; Hall, C.B.; Schwartz, T.M.; Moir, W.; Webber, M.P.; Cohen, H.W.; Nolan, A.; Weiden, M.D.; et al. Lung function trajectories in world trade center-exposed new york city firefighters over 13 years: The roles of smoking and smoking cessation. Chest 2016, 149, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Zeig-Owens, R.; Singh, A.; Aldrich, T.K.; Hall, C.B.; Schwartz, T.; Webber, M.P.; Cohen, H.W.; Kelly, K.J.; Nolan, A.; Prezant, D.J.; et al. Blood leukocyte concentrations, fev1 decline, and airflow limitation. A 15-year longitudinal study of world trade center-exposed firefighters. Ann. Am. Thorac. Soc. 2018, 15, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Liu, C.; Putman, B.; Zeig-Owens, R.; Hall, C.B.; Schwartz, T.; Webber, M.P.; Cohen, H.W.; Berger, K.I.; Nolan, A.; et al. Predictors of asthma/copd overlap in fdny firefighters with world trade center dust exposure: A longitudinal study. Chest 2018, 154, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Regan, E.A.; Lynch, D.A.; Curran-Everett, D.; Curtis, J.L.; Austin, J.H.; Grenier, P.A.; Kauczor, H.U.; Bailey, W.C.; DeMeo, D.L.; Casaburi, R.H.; et al. Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern. Med. 2015, 175, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Paulin, L.M.; Smith, B.M.; Koch, A.; Han, M.; Hoffman, E.A.; Martinez, C.; Ejike, C.; Blanc, P.D.; Rous, J.; Barr, R.G.; et al. Occupational exposures and computed tomographic imaging characteristics in the spiromics cohort. Ann. Am. Thorac. Soc. 2018, 15, 1411–1419. [Google Scholar] [CrossRef]
- Marchetti, N.; Garshick, E.; Kinney, G.L.; McKenzie, A.; Stinson, D.; Lutz, S.M.; Lynch, D.A.; Criner, G.J.; Silverman, E.K.; Crapo, J.D.; et al. Association between occupational exposure and lung function, respiratory symptoms, and high-resolution computed tomography imaging in copdgene. Am. J. Respir. Crit. Care Med. 2014, 190, 756–762. [Google Scholar] [CrossRef]
- Mendelson, D.S.; Roggeveen, M.; Levin, S.M.; Herbert, R.; de la Hoz, R.E. Air trapping detected on end-expiratory high-resolution computed tomography in symptomatic world trade center rescue and recovery workers. J. Occup. Env. Med. 2007, 49, 840–845. [Google Scholar] [CrossRef]
- de la Hoz, R.E.; Liu, X.; Doucette, J.T.; Reeves, A.P.; Bienenfeld, L.A.; Wisnivesky, J.P.; Celedon, J.C.; Lynch, D.A.; San Jose Estepar, R. Increased airway wall thickness is associated with adverse longitudinal first-second forced expiratory volume trajectories of former world trade center workers. Lung 2018, 196, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Galban, C.J.; Han, M.K.; Boes, J.L.; Chughtai, K.A.; Meyer, C.R.; Johnson, T.D.; Galban, S.; Rehemtulla, A.; Kazerooni, E.A.; Martinez, F.J.; et al. Computed tomography-based biomarker provides unique signature for diagnosis of copd phenotypes and disease progression. Nat. Med. 2012, 18, 1711–1715. [Google Scholar] [CrossRef]
- Martinez, C.H.; Diaz, A.A.; Meldrum, C.; Curtis, J.L.; Cooper, C.B.; Pirozzi, C.; Kanner, R.E.; Paine, R., 3rd; Woodruff, P.G.; Bleecker, E.R.; et al. Age and small airway imaging abnormalities in subjects with and without airflow obstruction in spiromics. Am. J. Respir. Crit. Care Med. 2017, 195, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Berger, K.I.; Reibman, J.; Oppenheimer, B.W.; Vlahos, I.; Harrison, D.; Goldring, R.M. Lessons from the world trade center disaster: Airway disease presenting as restrictive dysfunction. Chest 2013, 144, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Weakley, J.; Webber, M.P.; Gustave, J.; Kelly, K.; Cohen, H.W.; Hall, C.B.; Prezant, D.J. Trends in respiratory diagnoses and symptoms of firefighters exposed to the world trade center disaster: 2005–2010. Prev. Med. 2011, 53, 364–369. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. Gold executive summary. Am. J. Respir. Crit. Care Med. 2017, 195, 557–582. [Google Scholar] [CrossRef]
- Tan, W.C.; Hague, C.J.; Leipsic, J.; Bourbeau, J.; Zheng, L.; Li, P.Z.; Sin, D.D.; Coxson, H.O.; Kirby, M.; Hogg, J.C.; et al. Findings on thoracic computed tomography scans and respiratory outcomes in persons with and without chronic obstructive pulmonary disease: A population-based cohort study. PLoS ONE 2016, 11, e0166745. [Google Scholar] [CrossRef]
- Woodruff, P.G.; Barr, R.G.; Bleecker, E.; Christenson, S.A.; Couper, D.; Curtis, J.L.; Gouskova, N.A.; Hansel, N.N.; Hoffman, E.A.; Kanner, R.E.; et al. Clinical significance of symptoms in smokers with preserved pulmonary function. N. Engl. J. Med. 2016, 374, 1811–1821. [Google Scholar] [CrossRef]
- Nambu, A.; Zach, J.; Schroeder, J.; Jin, G.; Kim, S.S.; Kim, Y.I.; Schnell, C.; Bowler, R.; Lynch, D.A. Quantitative computed tomography measurements to evaluate airway disease in chronic obstructive pulmonary disease: Relationship to physiological measurements, clinical index and visual assessment of airway disease. Eur. J. Radiol. 2016, 85, 2144–2151. [Google Scholar] [CrossRef]
- Elbehairy, A.F.; Ciavaglia, C.E.; Webb, K.A.; Guenette, J.A.; Jensen, D.; Mourad, S.M.; Neder, J.A.; O’Donnell, D.E.; Canadian Respiratory Research Network. Pulmonary gas exchange abnormalities in mild chronic obstructive pulmonary disease. Implications for dyspnea and exercise intolerance. Am. J. Respir. Crit. Care Med. 2015, 191, 1384–1394. [Google Scholar] [CrossRef]
- Bhatt, S.P.; Soler, X.; Wang, X.; Murray, S.; Anzueto, A.R.; Beaty, T.H.; Boriek, A.M.; Casaburi, R.; Criner, G.J.; Diaz, A.A.; et al. Association between functional small airway disease and fev1 decline in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2016, 194, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Boes, J.L.; Hoff, B.A.; Bule, M.; Johnson, T.D.; Rehemtulla, A.; Chamberlain, R.; Hoffman, E.A.; Kazerooni, E.A.; Martinez, F.J.; Han, M.K.; et al. Parametric response mapping monitors temporal changes on lung ct scans in the subpopulations and intermediate outcome measures in copd study (spiromics). Acad. Radiol. 2015, 22, 186–194. [Google Scholar] [CrossRef]
- Friedman, S.M.; Maslow, C.B.; Reibman, J.; Pillai, P.S.; Goldring, R.M.; Farfel, M.R.; Stellman, S.D.; Berger, K.I. Case-control study of lung function in world trade center health registry area residents and workers. Am. J. Respir. Crit. Care Med. 2011, 184, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Jordan, H.T.; Friedman, S.M.; Reibman, J.; Goldring, R.M.; Miller Archie, S.A.; Ortega, F.; Alper, H.; Shao, Y.; Maslow, C.B.; Cone, J.E.; et al. Risk factors for persistence of lower respiratory symptoms among community members exposed to the 2001 world trade center terrorist attacks. Occup. Env. Med. 2017, 74, 449–455. [Google Scholar] [CrossRef]
- Crisafulli, E.; Pisi, R.; Aiello, M.; Vigna, M.; Tzani, P.; Torres, A.; Bertorelli, G.; Chetta, A. Prevalence of small-airway dysfunction among copd patients with different gold stages and its role in the impact of disease. Respiration 2017, 93, 32–41. [Google Scholar] [CrossRef] [PubMed]



| Variable | Population without Chest CT N = 5361 | Chest CT Study Population N = 4277 |
|---|---|---|
| Age on 9/11 | 39.4 ± 7.5 | 41.2 ± 7.2 |
| BMI ‡ | 28.7 ± 3.4 | 29.0 ± 3.5 |
| Smoking Status, N (%) | ||
| Never | 3804 (71.0) | 2653 (62.0) |
| Ever | 1557 (29.0) | 1624 (38.0) |
| Race, N (%) | ||
| White | 5026 (93.8) | 4053 (94.8) |
| Black | 139 (2.6) | 86 (2.0) |
| Hispanic | 180 (3.4) | 128 (3.0) |
| Other | 16 (0.3) | 10 (0.2) |
| World Trade Center exposure, N (%) | ||
| Morning of 9/11 | 463 (8.6) | 1113 (26.0) |
| Afternoon on 9/11–9/12 | 4125 (76.9) | 2781 (65.0) |
| 9/13–9/24 | 773 (14.4) | 383 (9.0) |
| First Post-9/11 Spirometry | ||
| FEV1 % predicted | 98.3 ± 13.1 | 95.4 ± 14.2 |
| FVC % predicted | 93.4 ± 11.8 | 91.1± 12.3 |
| FEV1/FVC | 0.84 ± 0.05 | 0.83 ± 0.06 |
| Post-9/11: FEV1 slope (mL/year) | −34.6 ± 25.6 | −38.9 ± 30.5 |
| Report of respiratory symptoms within 6 months of 9/11 | ||
| Shortness of breath | 1004 (22.3) § | 1289 (35.7) ‖ |
| Wheeze | 797 (17.7) § | 989 (27.4) ‖ |
| CT Abnormality | Percentage of Chest CT Scans with Abnormality |
|---|---|
| Air Trapping | 20.9 |
| Bronchial Wall Thickening | 19.6 |
| Nodules ≥ 5 mm | 14.6 |
| Ground Glass Opacities | 12.2 |
| Emphysema | 5.9 |
| Bronchiectasis | 3.6 |
| Pleural Thickening | 3.0 |
| Pulmonary Fibrosis | 0.6 |
| Variables | Emphysema | Bronchial Wall Thickening | Air Trapping | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| WTC exposure morning of 9/11 | 1.83 | 1.36–2.45 | <0.001 | 2.33 | 2.00–2.72 | <0.001 | 2.34 | 1.95–2.80 | <0.001 |
| Ever-smoker c | 7.04 | 4.94–10.04 | <0.001 | 1.25 | 1.08–1.44 | 0.003 | 0.73 | 0.61–0.88 | 0.001 |
| Variables | Emphysema | Bronchial Wall Thickening | Air Trapping | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| Either shortness of breath or wheeze | 1.43 | 1.04–1.97 | 0.03 | 1.38 | 1.16–1.63 | <0.001 | 1.27 | 1.08–1.49 | 0.005 |
| Both shortness of breath and wheeze | 1.62 | 1.12–2.33 | 0.01 | 1.49 | 1.22–1.83 | <0.001 | 1.41 | 1.16–1.70 | <0.001 |
| CT Diagnosis | OR | 95% CI |
|---|---|---|
| Emphysema | 1.89 | 1.37–2.60 |
| Bronchial Wall Thickening | 1.55 | 1.25–1.92 |
| Air Trapping | 0.77 | 0.61–0.97 |
| CT Diagnosis | Two Consecutive Screening Spirometric FEV1/FVC < 0.70 N = 4277 | Post-BD $ FEV1/FVC < 0.70 N = 1226 | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Emphysema | 2.03 | 1.44–2.88 | 2.63 | 1.56–4.42 |
| Bronchial Wall Thickening | 2.25 | 1.77–2.87 | 2.67 | 1.84–3.88 |
| Air Trapping | 0.40 | 0.29–0.55 | 0.36 | 0.22–0.58 |
| CT Abnormality | Total Lung Capacity c | Functional Residual Capacity c | Expiratory Reserve Volume c | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Beta | 95% CI | p | Beta | 95% CI | p | Beta | 95% CI | p | |
| Air Trapping | 57 | −76, 189 | 0.40 | −71 | −169, 27 | 0.16 | −205 | −281, −129 | <0.001 |
| Emphysema | 571 | 357, 786 | <0.001 | 478 | 319, 637 | <0.001 | 161 | 38, 285 | 0.01 |
| Bronchial Wall Thickening | 300 | 169, 432 | <0.001 | 236 | 139, 334 | <0.001 | −46 | −122, 30 | 0.23 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Putman, B.; Singh, A.; Zeig-Owens, R.; Hall, C.B.; Schwartz, T.; Webber, M.P.; Cohen, H.W.; Fazzari, M.J.; Prezant, D.J.; et al. Abnormalities on Chest Computed Tomography and Lung Function Following an Intense Dust Exposure: A 17-Year Longitudinal Study. Int. J. Environ. Res. Public Health 2019, 16, 1655. https://doi.org/10.3390/ijerph16091655
Liu C, Putman B, Singh A, Zeig-Owens R, Hall CB, Schwartz T, Webber MP, Cohen HW, Fazzari MJ, Prezant DJ, et al. Abnormalities on Chest Computed Tomography and Lung Function Following an Intense Dust Exposure: A 17-Year Longitudinal Study. International Journal of Environmental Research and Public Health. 2019; 16(9):1655. https://doi.org/10.3390/ijerph16091655
Chicago/Turabian StyleLiu, Charles, Barbara Putman, Ankura Singh, Rachel Zeig-Owens, Charles B. Hall, Theresa Schwartz, Mayris P. Webber, Hillel W. Cohen, Melissa J. Fazzari, David J. Prezant, and et al. 2019. "Abnormalities on Chest Computed Tomography and Lung Function Following an Intense Dust Exposure: A 17-Year Longitudinal Study" International Journal of Environmental Research and Public Health 16, no. 9: 1655. https://doi.org/10.3390/ijerph16091655
APA StyleLiu, C., Putman, B., Singh, A., Zeig-Owens, R., Hall, C. B., Schwartz, T., Webber, M. P., Cohen, H. W., Fazzari, M. J., Prezant, D. J., & Weiden, M. D. (2019). Abnormalities on Chest Computed Tomography and Lung Function Following an Intense Dust Exposure: A 17-Year Longitudinal Study. International Journal of Environmental Research and Public Health, 16(9), 1655. https://doi.org/10.3390/ijerph16091655





