Impact of Recreational Sports Activities on Metabolic Syndrome Components in Adolescents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Ethical Aspects
2.2. Procedure
2.3. MetS Components
2.4. Anthropometry and Body Composition
2.5. Maturational Evaluation
2.6. Resting Blood Pressure
2.7. Resting Heart Rate
2.8. Eating Habits
2.9. Physical Activity and Sedentary Behavior
2.10. Intervention
2.11. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zimmet, P.; Alberti, A.G.; Kaufman, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennett, P.; Shaw, J.; Caprio, S.; et al. The metabolic syndrome in children and adolescents. Lancet 2007, 369, 2059–2061. [Google Scholar] [CrossRef]
- Cook, S.; Weitzman, M.; Auinger, P.; Nguyen, M.; Dietz, W.H. Prevalence of a metabolic syndrome phenotype in adolescents: Findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch. Pediatr. Adolesc. Med. 2003, 157, 821–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed] [Green Version]
- Kuschnir, M.C.; Bloch, K.V.; Szklo, M.; Klein, C.H.; Barufaldi, L.A.; Abreu, G.A.; Schaan, B.; Veiga, G.V.; Silva, T.L.N.; Vasconcellos, M.T.L.; et al. ERICA: Prevalence of metabolic syndrome in Brazilian adolescents. Rev. Saude Publica 2016, 50, 11. [Google Scholar] [CrossRef] [Green Version]
- Stabelini Neto, A.; Bozza, R.; Ulbrich, A.; Mascarenhas, L.P.; Boguszewski, M.C.; Campos, W. Metabolic syndrome in adolescents of different nutritional status. Arq. Bras. Endocrinol. Metabol. 2012, 56, 104–109. [Google Scholar] [CrossRef] [Green Version]
- Caranti, D.A.; de Mello, M.T.; Prado, W.L.; Tock, L.; Siqueira, K.O.; de Piano, A.; Lofrano, M.C.; Cristofalo, D.M.; Lederman, H.; Tufik, S.; et al. Short- and long-term beneficial effects of a multidisciplinary therapy for the control of metabolic syndrome in obese adolescents. Metabolism 2007, 56, 1293–1300. [Google Scholar] [CrossRef]
- Araki, M.V.R.; Martins, I.C.R.; Barros, C.; Santos, E.G. Non-HDL-cholesterol in children and adolescents. Sci. Plena 2013, 9, 1–8. [Google Scholar]
- Conceicao-Machado, M.E.; Silva, L.R.; Santana, M.L.; Pinto, E.J.; Silva, R.D.; Moraes, L.T.; Couto, R.D.; Assis, A.M. Hypertriglyceridemic waist phenotype: Association with metabolic abnormalities in adolescents. J. Pediatr. 2013, 89, 56–63. [Google Scholar] [CrossRef] [Green Version]
- De las Fuentes, L.; Brown, A.L.; Mathews, S.J.; Waggoner, A.D.; Soto, P.F.; Gropler, R.J.; Dávila-Román, V.G. Metabolic syndrome is associated with abnormal left ventricular diastolic function independent of left ventricular mass. Eur. Heart J. 2007, 28, 553–559. [Google Scholar] [CrossRef] [Green Version]
- Yin, Q.; Chen, X.; Li, L.; Zhou, R.; Huang, J.; Yang, D. Apolipoprotein B/apolipoprotein A1 ratio is a good predictive marker of metabolic syndrome and pre-metabolic syndrome in Chinese adolescent women with polycystic ovary syndrome. J. Obstet. Gynaecol. Res. 2013, 39, 203–209. [Google Scholar] [CrossRef]
- Machado-Rodrigues, A.M.; Leite, N.; Coelho e Silva, M.J.; Valente-dos-Santos, J.; Martins, R.A.; Mascarenhas, L.P.; Boguszewski, M.C.; Padez, C.; Malina, R.M. Relationship between metabolic syndrome and moderate-to-vigorous physical activity in youth. J. Phys. Act. Health 2015, 12, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Durstine, J.L.; Gordon, B.; Wang, Z.; Luo, X. Chronic disease and the link to physical activity. J. Sport Health Sci. 2013, 2, 3–11. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Tremblay, M.S.; LeBlanc, A.G.; Kho, M.E.; Saunders, T.J.; Larouche, R.; Colley, R.C.; Goldfield, G.; Connor Gober, S. Systematic review of sedentary behaviour and health indicators in school-aged children and youth. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farias Júnior, J.C. (In) Atividade física e comportamento sedentário: Estamos caminhando para uma mudança de paradigma? Rev. Bras. Ativ. Fis. Saúde 2011, 16, 279–280. [Google Scholar]
- Bianchini, J.A.; da Silva, D.F.; Nardo, C.C.; Carolino, I.D.; Hernandes, F.; Nardo, N., Jr. Multidisciplinary therapy reduces risk factors for metabolic syndrome in obese adolescents. Eur. J. Pediatr. 2013, 172, 215–221. [Google Scholar] [CrossRef]
- Gronbaek, H.; Lange, A.; Birkebaek, N.H.; Holland-Fischer, P.; Solvig, J.; Horlyck, A.; Kristensen, K.; Rittig, S.; Vilstrup, H. Effect of a 10-week weight loss camp on fatty liver disease and insulin sensitivity in obese Danish children. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 223–228. [Google Scholar] [CrossRef]
- Lee, S.; Bacha, F.; Hannon, T.; Kuk, J.L.; Boesch, C.; Arslanian, S. Effects of aerobic versus resistance exercise without caloric restriction on abdominal fat, intrahepatic lipid, and insulin sensitivity in obese adolescent boys: A randomized, controlled trial. Diabetes 2012, 61, 2787–2795. [Google Scholar] [CrossRef] [Green Version]
- De Ferranti, S.D.; Gauvreau, K.; Ludwig, D.S.; Neufeld, E.J.; Newburger, J.W.; Rifai, N. Prevalence of the metabolic syndrome in American adolescents: Findings from the Third National Health and Nutrition Examination Survey. Circulation 2004, 110, 2494–2497. [Google Scholar] [CrossRef] [Green Version]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar]
- Srinivasan, S.R.; Myers, L.; Berenson, G.S. Distribution and correlates of non-high-density lipoprotein cholesterol in children: The Bogalusa Heart Study. Pediatrics 2002, 110, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Brazilian Society of Cardiology. VII Brazilian Guidelines on Hypertension. Arq. Bras. Cardiol. 2016, 107, 1–83. [Google Scholar]
- Ten, S.; Maclaren, N. Insulin resistance syndrome in children. J. Clin. Endocrinol. Metab. 2004, 89, 2526–2539. [Google Scholar] [CrossRef] [PubMed]
- International Society for the Advancement of Kinanthropometry. International Standards for Anthropometric Assessment; National Library of Australia: Adelaide, Australia, 2001. [Google Scholar]
- De Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Technical Report Series 894 Obesity: Preventing and Managing the Global Epidemic; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Slaughter, M.H.; Lohman, T.G.; Boileau, R.A.; Horswill, C.A.; Stillman, R.J.; Van Loan, M.D.; Bemben, D.A. Skinfold equations for estimation of body fatness in children and youth. Hum. Biol. 1988, 60, 709–723. [Google Scholar] [PubMed]
- Going, S.B.; Lohman, T.G.; Cussler, E.C.; Williams, D.P.; Morrison, J.A.; Horn, P.S. Percent body fat and chronic disease risk factors in U.S. children and youth. Am. J. Prev. Med. 2011, 41, 77–86. [Google Scholar] [CrossRef]
- Mirwald, R.L.; Baxter-Jones, A.D.; Bailey, D.A.; Beunen, G.P. An assessment of maturity from anthropometric measurements. Med. Sci. Sports Exerc. 2002, 34, 689–694. [Google Scholar]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef] [Green Version]
- Rich, C.; Geraci, M.; Griffiths, L.; Sera, F.; Dezateux, C.; Cortina-Borja, M. Quality control methods in accelerometer data processing: Defining minimum wear time. PLoS ONE 2013, 8, 67206. [Google Scholar] [CrossRef]
- Puyau, M.R.; Adolph, A.L.; Vohra, F.A.; Butte, N.F. Validation and calibration of physical activity monitors in children. Obes. Res. 2002, 10, 150–157. [Google Scholar] [CrossRef]
- Brooks, G.A.; Butte, N.F.; Rand, W.M.; Flatt, J.P.; Caballero, B. Chronicle of the Institute of Medicine physical activity recommendation: How a physical activity recommendation came to be among dietary recommendations. Am. J. Clin. Nutr. 2004, 79, 921–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schofield, W.N. Predicting basal metabolic rate, new standards and review of previous work. Hum. Nutr. Clin. Nutr. 1985, 39, 5–41. [Google Scholar] [PubMed]
- Khan, L.K.; Sobush, K.; Keener, D.; Goodman, K.; Lowry, A.; Kakietek, J.; Zaro, S.; Centers for Disease Control and Prevention. Recommended community strategies and measurements to prevent obesity in the United States. MMWR Recomm. Rep. 2009, 58, 1–26. [Google Scholar] [PubMed]
- Moraes, A.C.; Fulaz, C.S.; Netto-Oliveira, E.R.; Reichert, F.F. Prevalence of metabolic syndrome in adolescents: A systematic review. Cad. Saude Publica 2009, 25, 1195–1202. [Google Scholar]
- Wickham, E.P.; Stern, M.; Evans, R.K.; Bryan, D.L.; Moskowitz, W.B.; Clore, J.N.; Laver, J.H. Prevalence of the metabolic syndrome among obese adolescents enrolled in a multidisciplinary weight management program: Clinical correlates and response to treatment. Metab. Syndr. Relat. Disord. 2009, 7, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Andaki, A.C.; Tinoco, A.L.; Mendes, E.L.; Andaki Junior, R.; Hills, A.P.; Amorim, P.R. Different waist circumference measurements and prediction of cardiovascular risk factors and metabolic syndrome in children. Obes. Res. Clin. Pract. 2012, 6, 91–174. [Google Scholar] [CrossRef]
- Barzin, M.; Asghari, G.; Hosseinpanah, F.; Mirmiran, P.; Azizi, F. The association of anthropometric indices in adolescence with the occurrence of the metabolic syndrome in early adulthood: Tehran Lipid and Glucose Study (TLGS). Pediatr. Obes. 2013, 8, 170–177. [Google Scholar] [CrossRef]
- Sangun, O.; Dundar, B.; Kosker, M.; Pirgon, O.; Dundar, N. Prevalence of metabolic syndrome in obese children and adolescents using three different criteria and evaluation of risk factors. J. Clin. Res. Pediatr. Endocrinol. 2011, 3, 70–76. [Google Scholar] [CrossRef]
- Andaki, A.C.; Tinoco, A.L.; Mendes, E.L.; Andaki Junior, R.; Hills, A.P.; Amorim, P.R. Anthropometry and physical activity level in the prediction of metabolic syndrome in children. Public. Health Nutr. 2014, 17, 2287–2294. [Google Scholar] [CrossRef] [Green Version]
- Leite, N.; Milano, G.E.; Cieslak, F.; Lopes, W.A.; Rodacki, A.; Radominski, R.B.I. Effects of physical exercise and nutritional guidance on metabolic syndrome in obese adolescents. Rev. Bras. Fisioter. 2009, 13, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Thomas, N.E.; Cooper, S.M.; Williams, S.P.; Baker, J.S.; Davies, B. Relationship of fitness, fatness, and coronary-heart-disease risk factors in 12- to 13-year-olds. Pediatr. Exerc. Sci. 2007, 19, 93–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichert, F.F.; Hallal, P.C.; Wells, J.C.; Horta, B.L.; Ekelund, U.; Menezes, A.M. Objectively measured physical activity in the 1993 Pelotas (Brazil) birth cohort. Med. Sci. Sports Exerc. 2012, 44, 2369–2375. [Google Scholar] [CrossRef] [PubMed]
- Guinhouya, B.C.; Samouda, H.; de Beaufort, C. Level of physical activity among children and adolescents in Europe: A review of physical activity assessed objectively by accelerometry. Public Health 2013, 127, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Herman, K.M.; Chaput, J.P.; Sabiston, C.M.; Mathieu, M.E.; Tremblay, A.; Paradis, G. Combined physical activity/sedentary behaviour associations with indices of adiposity in 8- to 10-year-old children. J. Phys. Act. Health 2015, 12, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Vaisto, J.; Eloranta, A.M.; Viitasalo, A.; Tompuri, T.; Lintu, N.; Karjalainen, P.; Lampinen, E.K.; Ågren, J.; Laaksonen, D.E.; Lakka, H.M.; et al. Physical activity and sedentary behaviour in relation to cardiometabolic risk in children: Cross-sectional findings from the Physical Activity and Nutrition in Children (PANIC) Study. Int. J. Behav. Nutr. Phys. Act. 2014, 11, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cliff, D.P.; Jones, R.A.; Burrows, T.L.; Morgan, P.J.; Collins, C.E.; Baur, L.A.; Okely, A.D. Volumes and bouts of sedentary behavior and physical activity: Associations with cardiometabolic health in obese children. Obesity 2014, 22, 112–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faria, F.R.; Canabrava, K.L.R.; Amorim, P.R.S. Nível de Atividade Física durante o recreio escolar em escola pública e particular. Revisita Bras. Cienc. E Mov. 2013, 21, 90–97. [Google Scholar] [CrossRef]
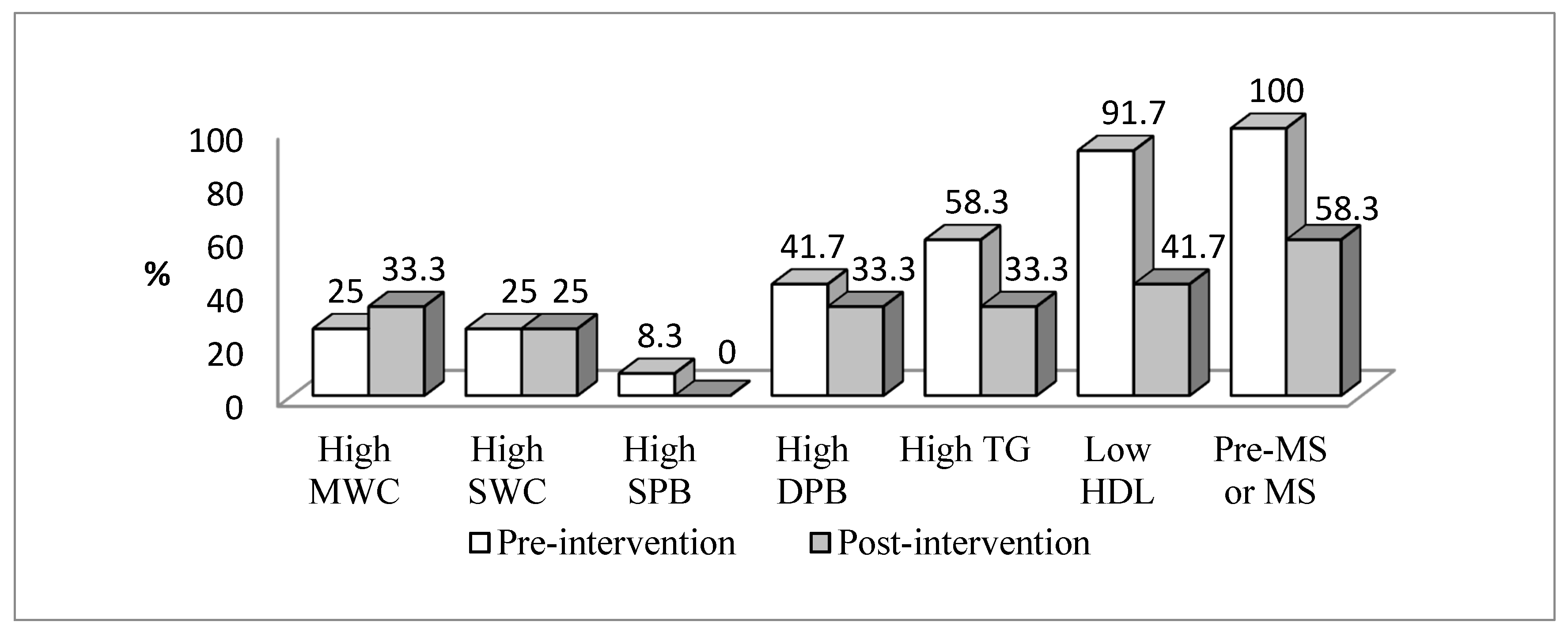
| Variables | Total (n = 92) | No Pre-MetS or MetS (n = 56) | Pre-MetS or MetS (n = 36) | p Value | ES |
|---|---|---|---|---|---|
| Age (years) * | 16.0 (14.0–18.0) | 16.0 (14.0–18.0) | 16.0 (14.0–18.0) | 0.287 | 0.11 |
| Weight (kg) * | 61.3 (39.1–105) | 60.6 (39.1–84.1) | 65.1 (48.8–105) | 0.004 † | 0.30 |
| Height (m) | 1.74 (±0.06) | 1.73 (±0.06) | 1.75 (±0.06) | 0.214 | 0.33 |
| BMI (kg/m2) * | 20.5 (15.1–33.9) | 20.2 (15.1–25.1) | 21.2 (16.8–33.9) | 0.021 † | 0.24 |
| WHtR * | 0.4 (0.36–0.56) | 0.4 (0.36–0.5) | 0.41 (0.36–0.56) | 0.139 | 0.15 |
| BF%* | 13.4 (7.2–41.4) | 13.5 (7.9–26.6) | 12.5 (7.2–41.4) | 0.914 | 0.01 |
| MWC (cm) * | 72.1 (63.0–99.4) | 71.7 (63.0–86.8) | 74.7 (64.5–99.4) | 0.030 † | 0.23 |
| SWC (cm) * | 71.4 (61.4–97.1) | 70.9 (61.4–84.0) | 73.0 (63.6–97.1) | 0.025 † | 0.23 |
| SBP (mmHg) | 111.6 (±10.8) | 109.1 (±10.2) | 115.5 (±10.7) | 0.005 † | 0.61 |
| DBP(mmHg) | 72.5 (±7.7) | 70.5 (±7.3) | 75.6 (±7.3) | 0.002 † | 0.70 |
| HDL (mg/dL) | 42.9 (±8.6) | 45.8 (±8.9) | 38.3 (±5.9) | 0.000 ‡ | 1.00 |
| TG (mg/dL) * | 85 (36–288) | 75.5 (36–110) | 111 (65–288) | 0.000 ‡ | 0.64 |
| FG (mg/dL) | 77.8 (±7.5) | 77.1 (±7.1) | 78.9 (±8.0) | 0.248 | 0.25 |
| Insulin (mU/L) * | 4.8 (1.4–28.5) | 4.4 (1.4–21.0) | 6.09 (1.9–28.5) | 0.049 † | 0.21 |
| HOMA-IR * | 0.98 (0.27–5.44) | 0.85 (0.27–4.58) | 1.13 (0.36–5.44) | 0.045 † | 0.21 |
| TC (mg/dL) | 159.6 (±23.6) | 155.4 (±22.8) | 166.2 (±23.5) | 0.031 † | 0.47 |
| LDL (mg/dL) | 97.6 (±19.4) | 94.4 (±18.3) | 102.6 (±20.4) | 0.048 † | 0.42 |
| Non-HDL | 116.7 (±22.6) | 109.5 (±19.4) | 127.8 (±23.1) | 0.000 ‡ | 0.86 |
| Variables | n | Total Sample | n | No Pre-MetS or MetS | n | Pre-MetS or MetS | p Value | ES |
|---|---|---|---|---|---|---|---|---|
| MVPA (min/week) | 83 | 70.3 (±19.8) | 51 | 71.4 (±21.3) | 32 | 68.6 (±17.2) | 0.54 | 0.14 |
| MVPA (min/weekday) | 83 | 76.5 (±20.8) | 51 | 77.3 (±22.0) | 32 | 75.3 (±19.2) | 0.67 | 0.10 |
| MVPA (min/weekend day) * | 80 | 48.5 (1.5–149.5) | 49 | 51.5 (1.5–149.5) | 31 | 48 (4.5–113) | 0.61 | 0.05 |
| TEE (kcal/day) | 83 | 3095 (±582) | 51 | 3004 (±472) | 32 | 3240 (±708) | 0.11 | 0.40 |
| BMR (kcal/day) * | 92 | 1743 (1350–2516) | 56 | 1730 (1350–2145) | 36 | 1810 (1521–2516) | 0.004 † | 0.30 |
| Variables | MWC | SWC | BF% | BMI | WHtR | |
|---|---|---|---|---|---|---|
| HDL | β R2 | −0.428 † 0.106 | −0.487 † 0.115 | −0.129 0.010 | −0.860 † 0.082 | 53.344 ‡ 0.048 |
| SBP | β R2 | 0.604 † 0.136 | 0.670 † 0.140 | 0.535 † 0.108 | 1.601 † 0.183 | 93.779 † 0.095 |
| DBP | β R2 | 0.335 † 0.082 | 0.391 † 0.094 | 0.234 ‡ 0.041 | 0.996 † 0.140 | 59.009 † 0.074 |
| BMI ** | β R2 | 0.007 † 0.722 | 0.008 † 0.741 | 0.006 † 0.458 | –– | 1.384 † 0.736 |
| Variables | Pre- | Post- | p Value | ES |
|---|---|---|---|---|
| WHtR * | 0.42 (0.39–0.56) | 0.42 (0.37–0.53) | 0.071 | 0.36 |
| BF% | 18.11 (±10.25) | 18.31 (±9.72) | 0.771 | 0.02 |
| MWC (cm) * | 74.47 (67.5–99.0) | 72.57 (69.1–92.8) | 0.875 | 0.03 |
| SWC (cm) | 75.17 (±7.55) | 75.09 (±7.14) | 0.896 | 0.01 |
| SBP (mmHg) | 114.5 (±12.19) | 111.25 (±10.2) | 0.082 | 0.29 |
| DBP (mmHg) | 75.41 (±8.37) | 76.0 (±6.79) | 0.737 | 0.07 |
| HDL (mg/dL) | 38.53 (±6.19) | 52.10 (±14.25) | 0.004 † | 1.30 |
| TG (mg/dL) * | 101.0 (65.0–286.0) | 77.6 (49.0–112.5) | 0.117 | 0.31 |
| FG (mg/dL) | 78.02 (±8.55) | 83.86 (±5.92) | 0.005 | 0.81 |
| Insulin (mU/L) * | 5.91 (1.91–25.4) | 6.90 (2.58–19.7) | 0.347 | 0.19 |
| HOMA-IR * | 1.12 (0.36–4.35) | 1.35 (0.6–3.95) | 0.638 | 0.09 |
| TC (mg/dL) | 164.01 (±27.18) | 148.01 (±24.2) | 0.024 † | 0.62 |
| LDL (mg/dL) | 103.76 (±24.44) | 79.68 (±19.53) | 0.002 † | 1.1 |
| Non-HDL | 125.48 (±26.96) | 95.90 (±21.83) | 0.001 † | 1.2 |
| Variables | n | Pre- | Post- | p Value | ES |
|---|---|---|---|---|---|
| TEE (kcal/day) | 11 | 2963 (2511–4423) | 3494 (2886–4752) | 0.010 † | 0.55 |
| BMR (kcal/day) * | 12 | 1822 (±212) | 1837 (±203) | 0.164 | 0.07 |
| MVPA | |||||
| min/day * | 11 | 72.5 (56.5–108.5) | 81.4 (73–137) | 0.016† | 0.51 |
| min/weekday | 11 | 88 (±19.9) | 100.6 (± 19.5) | 0.070 | 0.64 |
| min/weekend day * | 10 | 50.4 (±27.6) | 39.9 (± 29.8) | 0.291 | 0.37 |
| SB | |||||
| min/week * | 11 | 598.4 (492.7–673.3) | 587.8 (498–922.6) | 0.929 | 0.01 |
| min/weekday * | 11 | 650.2 (464–682.6) | 596.7 (488.8–1054) | 0.929 | 0.01 |
| min/weekend day * | 9 | 520.5 (380–668.5) | 498 (265–552) | 0.594 | 0.13 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faria, F.; Howe, C.; Faria, R.; Andaki, A.; Marins, J.C.; Amorim, P.R. Impact of Recreational Sports Activities on Metabolic Syndrome Components in Adolescents. Int. J. Environ. Res. Public Health 2020, 17, 143. https://doi.org/10.3390/ijerph17010143
Faria F, Howe C, Faria R, Andaki A, Marins JC, Amorim PR. Impact of Recreational Sports Activities on Metabolic Syndrome Components in Adolescents. International Journal of Environmental Research and Public Health. 2020; 17(1):143. https://doi.org/10.3390/ijerph17010143
Chicago/Turabian StyleFaria, Fernanda, Cheryl Howe, Ricardo Faria, Alynne Andaki, João Carlos Marins, and Paulo Roberto Amorim. 2020. "Impact of Recreational Sports Activities on Metabolic Syndrome Components in Adolescents" International Journal of Environmental Research and Public Health 17, no. 1: 143. https://doi.org/10.3390/ijerph17010143
APA StyleFaria, F., Howe, C., Faria, R., Andaki, A., Marins, J. C., & Amorim, P. R. (2020). Impact of Recreational Sports Activities on Metabolic Syndrome Components in Adolescents. International Journal of Environmental Research and Public Health, 17(1), 143. https://doi.org/10.3390/ijerph17010143






