Neglected Urban Villages in Current Vector Surveillance System: Evidences in Guangzhou, China
Abstract
1. Introduction
2. Materials and Methods
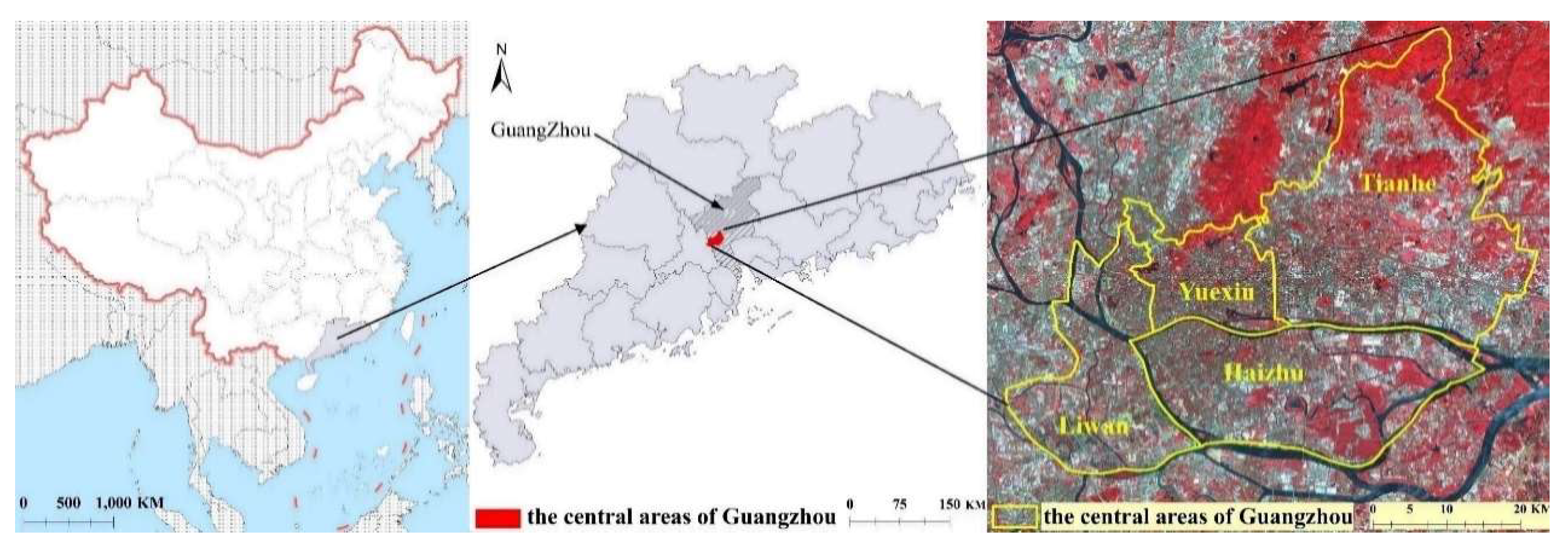
2.1. Study Area
2.2. Data Collection
2.3. Spatial and Statistical Analysis
2.4. Geodetector Model
3. Results
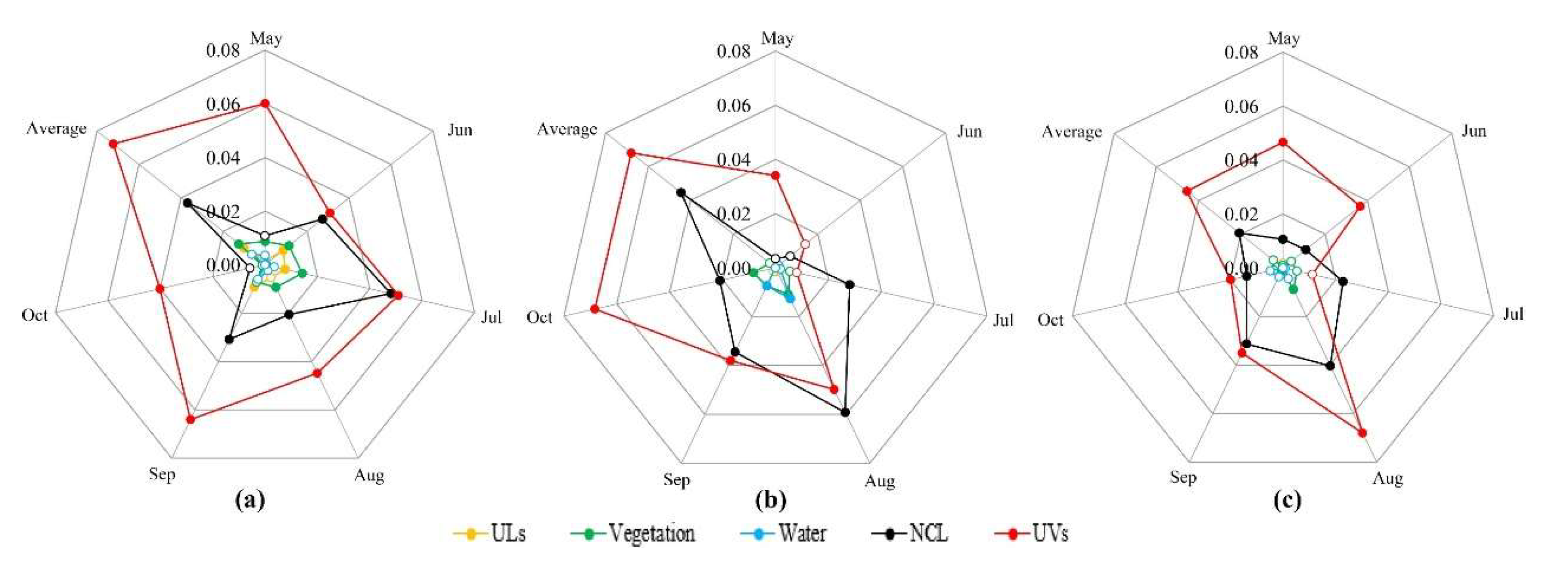
3.1. Current Surveillance Systems of Ae. albopictus
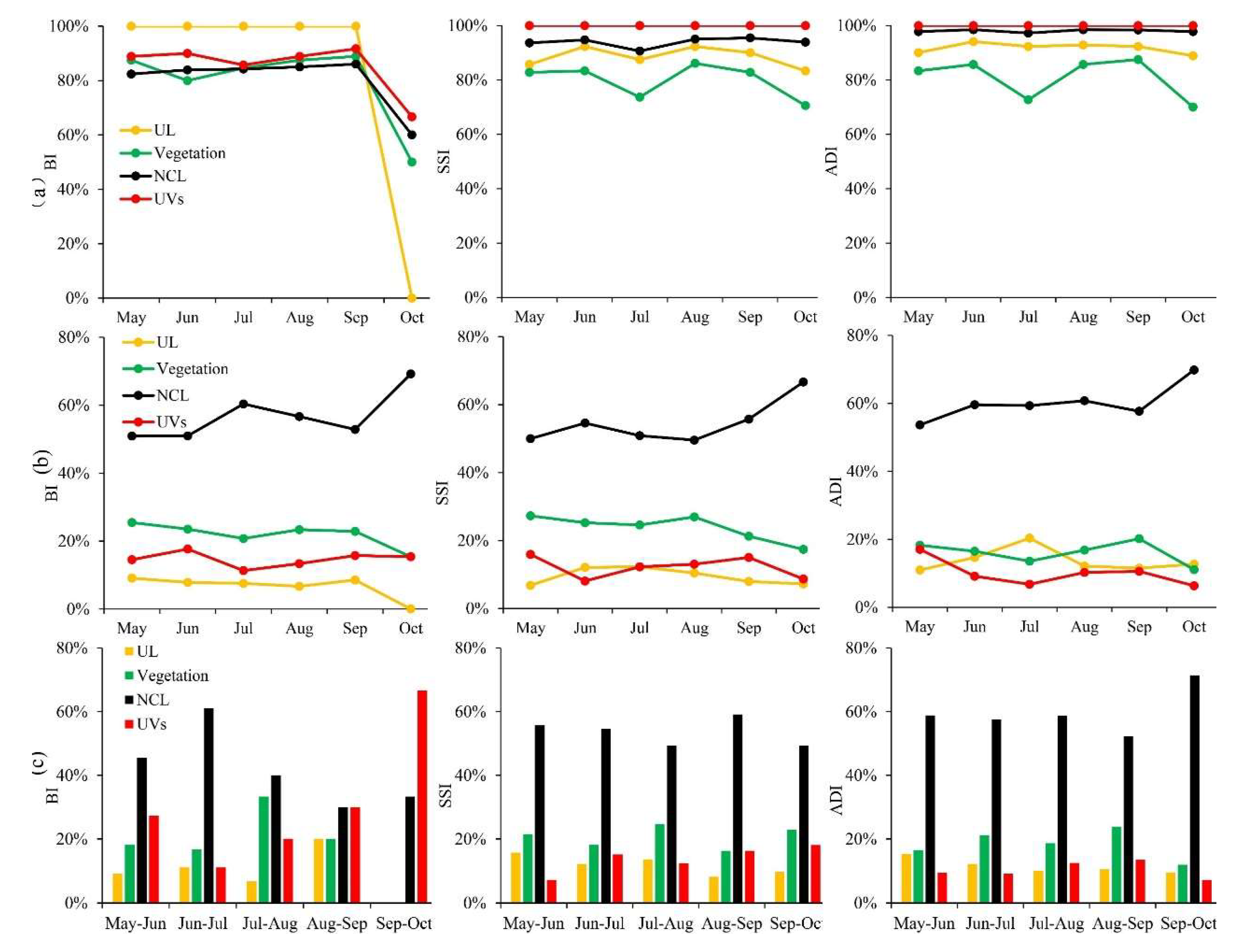
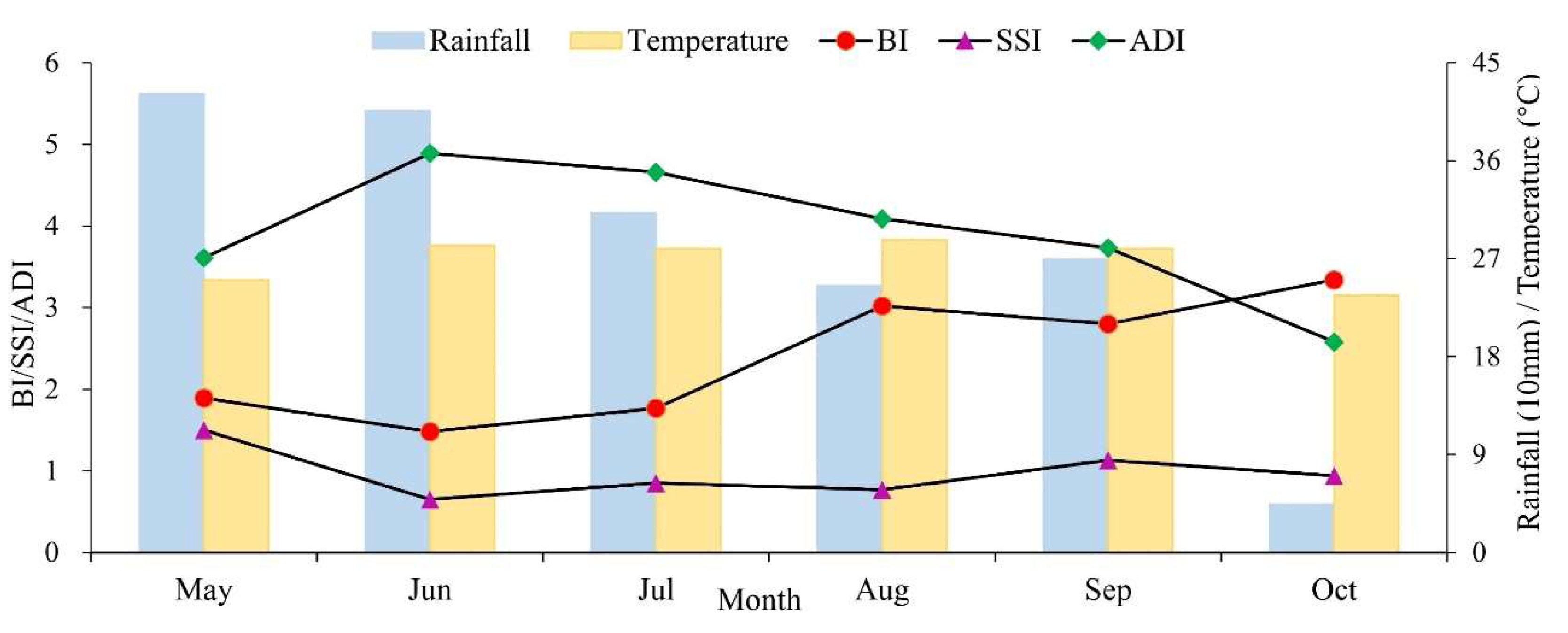
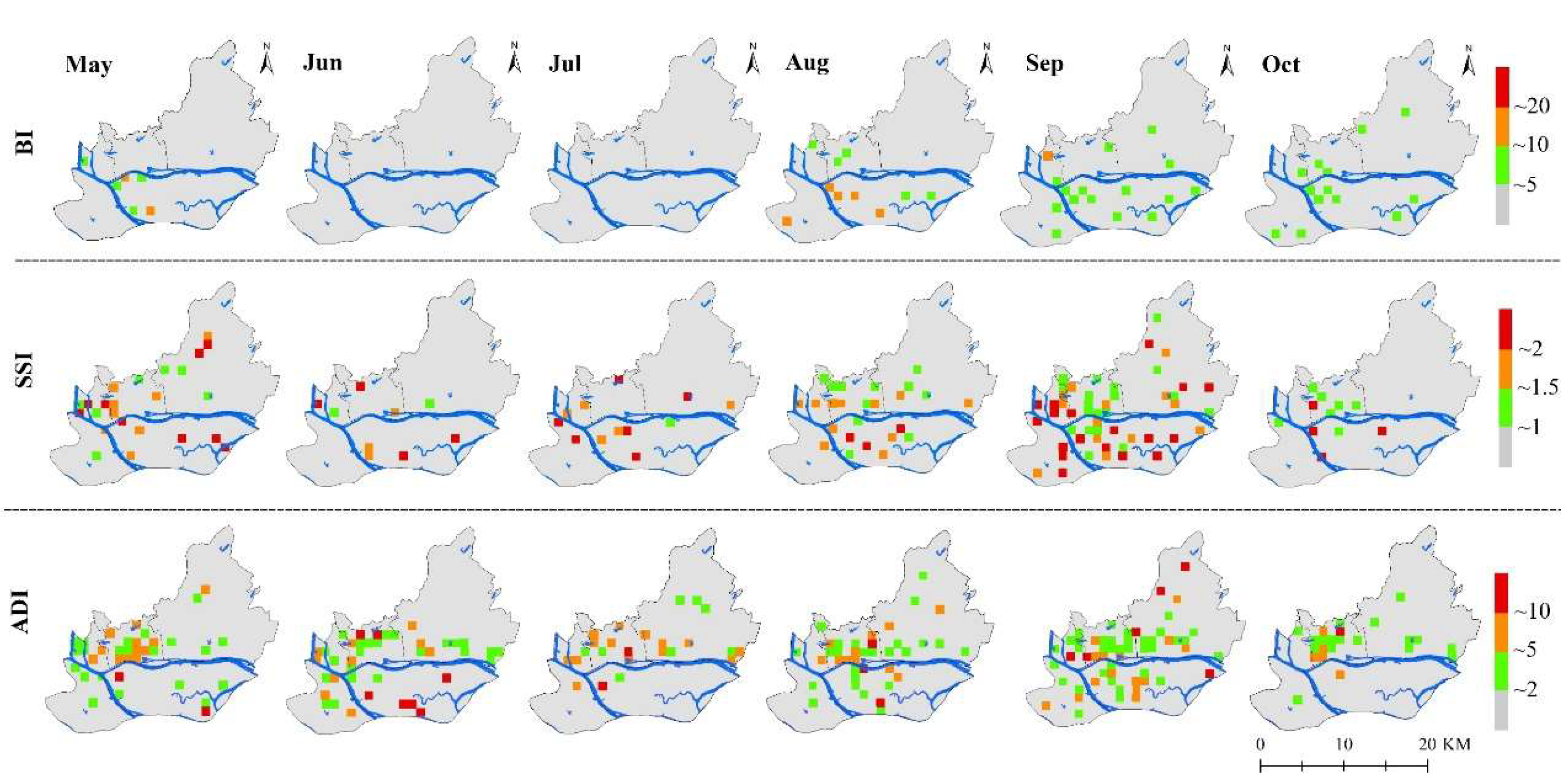
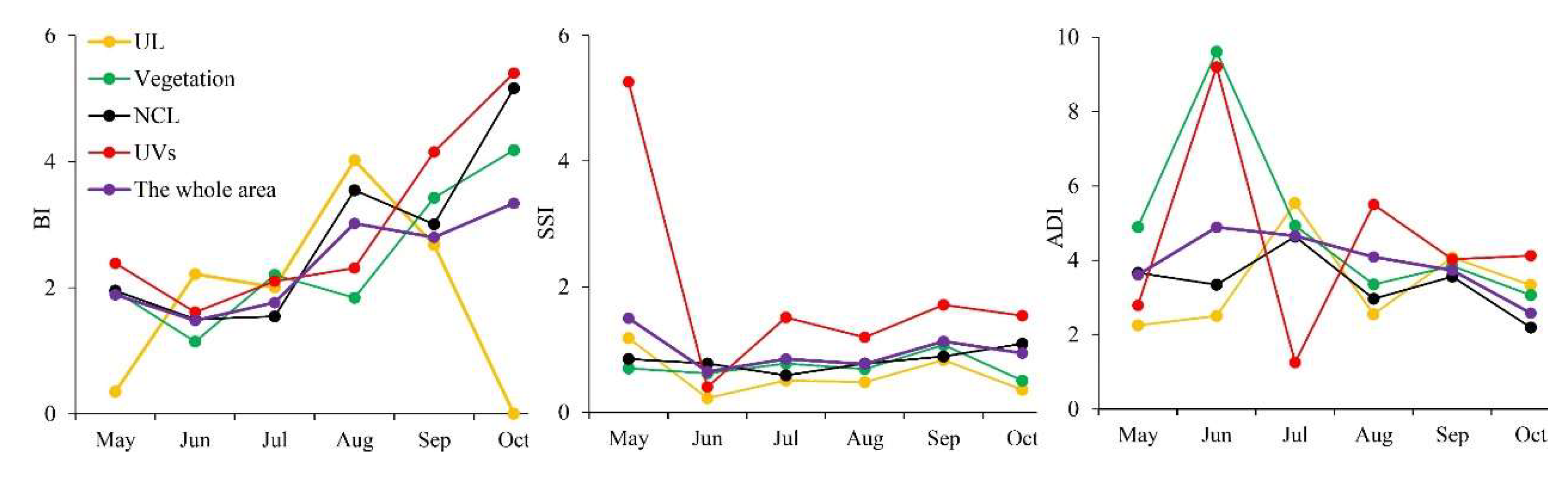
3.2. Temporal and Spatial Changes of Ae. albopictus Density
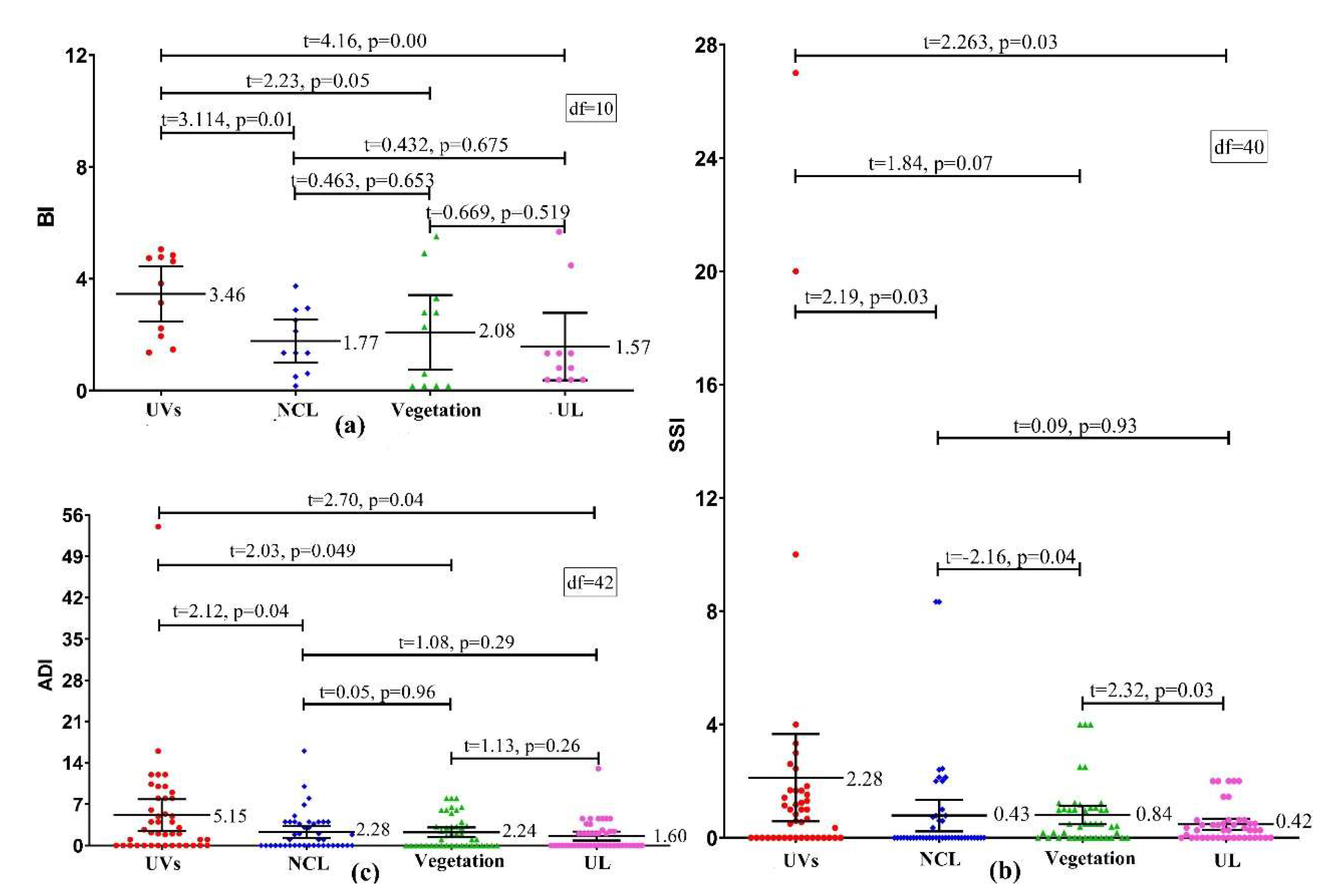
3.3. Differences of Vector Density in Various land-use Types
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jing, L.H.; Hans, S.; Annelies, W.S.; Joacim, R. Vectorial Capacity of Aedes aegypti: Effects of Temperature and Implications for Global Dengue Epidemic Potential. PLoS ONE 2014, 9, e89783. [Google Scholar] [CrossRef]
- Telle, O.; Vaguet, A.; Yadav, N.K.; Lefebvre, B.; Daudé, E.; Paul, R.E.; Cebeillac, A.; Nagpal, B.N. The Spread of Dengue in an Endemic Urban Milieu-The Case of Delhi, India. PLoS ONE 2016, 11, e0152847. [Google Scholar] [CrossRef] [PubMed]
- Mariam, O.D.B.; Simard, F.; Caprara, A. Supporting and strengthening research on urban health interventions for the prevention and control of vector-borne and other infectious diseases of poverty: Scoping reviews and research gap analysis. Infect. Dis. Poverty 2018, 7, 94. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Lai, S.J.; Huang, Z.J.; Zhou, H.; Anders, K.L.; Perkins, T.A.; Yin, W.W.; Li, Y.; Mu, D.; Chen, Q.L.; Zhang, Z. The changing epidemiology of dengue in China, 1990-2014: A descriptive analysis of 25 years of nationwide surveillance data. BMC Med. 2015, 13, 100. [Google Scholar] [CrossRef]
- Gao, J.R.; Liu, J.H.; Liu, J.H.; Liu, S.Z. Research and control strategies for Aedes albopictus in Guangzhou, China, during recent five years. Chin. J. Vector Biol. Control 2014, 25, 483–485. [Google Scholar] [CrossRef]
- Stéphanie, D.; Kate, Z.; Valéry, R. Interventions for vector-borne diseases focused on housing and hygiene in urban areas: A scoping review. Infect. Dis. Poverty 2018, 7, 96. [Google Scholar] [CrossRef]
- Huang, J.H.; Su, C.L.; Yang, C.F.; Liao, T.L. Molecular characterization and phylogenetic analysis of dengue viruses imported into Taiwan during 2008–2010. Am. J. Trop. Med. Hyg. 2012, 87, 349. [Google Scholar] [CrossRef]
- Tandina, F.; Doumbo, O.K.; Yaro, A.S.; Traoré, S.F.; Parola, P.; Robert, V. Mosquitoes (Diptera: Culicidae) and mosquito-borne diseases in Mali, West Africa. Parasites Vectors 2018, 11, 467. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Wang, T.; Liu, K.K.; Xia, Y.; Yi, L.; Jing, Q.L.; Yang, Z.C.; Hu, W.B.; Lu, J.H. Developing a Time Series Predictive Model for Dengue in Zhongshan, China Based on Weather and Guangzhou Dengue Surveillance Data. PLoS Negl. Trop. Dis. 2016, 10, e0004473. [Google Scholar] [CrossRef]
- Yukiko, H. Dengue Vectors and their Spatial Distribution. Trop. Med. Health 2011, 39, 17–27. [Google Scholar] [CrossRef]
- Medley, K.A. Niche shifts during the global invasion of the Asian tiger mosquito, Aedes albopictus Skuse (Culicidae), revealed by reciprocal distribution models. Glob. Ecol. Biogeogr. 2010, 19, 122–133. [Google Scholar] [CrossRef]
- Åström, C.; Rocklöv, J.; Hales, S.; Béguin, A.; Louis, V.; Sauerborn, R. Potential Distribution of Dengue Fever Under Scenarios of Climate Change and Economic Development. Ecohealth 2012, 9, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Proestos, Y.; Christophides, G.K.; Ergüler, K.; Tanarhte, M.; Waldock, J.; Lelieveld, J. Present and future projections of habitat suitability of the Asian tiger mosquito, a vector of viral pathogens, from global climate simulation. Philos. Trans. R. Soc. Lond. 2015, 370, 219–222. [Google Scholar] [CrossRef]
- Li, Y.; Kamara, F.; Zhou, G.; Puthiyakunnon, S.; Li, C.; Liu, Y.; Zhou, Y.; Yao, L.; Yan, G.; Chen, X.G. Urbanization increases Aedes albopictus larval habitats and accelerates mosquito development and survivorship. PLoS Negl. Trop. Dis. 2014, 8, e3301. [Google Scholar] [CrossRef]
- Pang, S.T.; Lei, X.G.; Chen, B.Z.; Wang, X.; Xue, W.; Wu, P.B. Analysis of Aedes albopictus larvae surveillance and its influence factors in Xi’an City. Chin. J. Hyg. Insectic. Equip. 2017, 23, 454–456. [Google Scholar]
- Yan, Z.Q.; Hu, Z.G.; Jiang, Y.M. The larva distribution characteristics of Aedes albopictus population in Guangzhou. Chin. J. Vector Biol. Control 2007, 18, 193–195. [Google Scholar]
- Zahouli, J.B.Z.; Koudou, B.G.; Müller, P.; Malone, D.; Tano, Y.; Utzinger, J. Urbanization is a main driver for the larval ecology of Aedes mosquitoes in arbovirus-endemic settings in south-eastern Côte d’Ivoire. PLoS Negl. Trop. Dis. 2017, 11, e0005751. [Google Scholar] [CrossRef]
- Chung, H. Building an image of Villages-in-the-City: A clarification of China’s distinct urban spaces. Int. J. Urban Reg. Res. 2010, 34, 421. [Google Scholar] [CrossRef]
- Hao, P.; Hooimeijer, P.; Sliuzas, R.; Geertman, S. What Drives the Spatial Development of Urban Villages in China? Urban Stud. 2013, 50, 3394–3411. [Google Scholar] [CrossRef]
- Taubenböck, H.; Kraff, N.J. The physical face of slums: A structural comparison of slums in Mumbai, India, based on remotely sensed data. J. Hous. Built Environ. 2014, 29, 15–38. [Google Scholar] [CrossRef]
- Huang, X.; Liu, H.; Zhang, L.P. Spatiotemporal Detection and Analysis of Urban Villages in Mega City Regions of China Using High-Resolution Remotely Sensed Imagery. IEEE Trans. Geosci. Remote Sens. 2015, 53, 3639–3657. [Google Scholar] [CrossRef]
- Ren, H.Y.; Wu, W.; Li, T.G.; Yang, Z.C. Urban villages as transfer stations for dengue fever epidemic: A case study in the Guangzhou, China. PLoS Negl. Trop. Dis. 2019, 13. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.Y.; Zheng, L.; Li, Q.X.; Wu, Y.; Lu, L. Exploring Determinants of Spatial Variations in the Dengue Fever Epidemic Using Geographically Weighted Regression Model: A Case Study in the Joint Guangzhou-Foshan Area, China, 2014. Int. J. Environ. Res. Public Health 2017, 14, 1518. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.B.; Ma, X.G.; Li, G.C. Formation and Governance of Informality in Urban Village Under the Rapid Urbanization Process. Econ. Geogr. 2014, 34, 162–168. [Google Scholar] [CrossRef]
- Li, Z.G.; Wu, F.L. Residential Satisfaction in China’s Informal Settlements: A Case Study of Beijing, Shanghai, and Guangzhou. Urban Geogr. 2013, 34, 923–949. [Google Scholar] [CrossRef]
- Wekesa, B.W.; Steyn, G.S.; Otieno, F.A.O. A review of physical and socio-economic characteristics and intervention approaches of informal settlements. Habitat Int. 2011, 35, 238–245. [Google Scholar] [CrossRef]
- Cunze, S.; Koch, L.K.; Kochmann, J.; Klimpel, S. Aedes albopictus and Aedes japonicus- two invasive mosquito species with different temperature niches in Europe. Parasites Vectors 2016, 9, 573. [Google Scholar] [CrossRef]
- Serpa, L.L.N.; Gisela, R.A.M.M.; Lima, A.P.D.; Voltolini, J.C.; Arduino, M.D.B.; Barbosa, G.L.; Andrade, V.B.; Lima, V.L.C. Study of the distribution and abundance of the eggs of Aedes aegypti and Aedes albopictus according to the habitat and meteorological variables, municipality of São Sebastião, São Paulo State, Brazil. Parasites Vectors 2013, 6, 321. [Google Scholar] [CrossRef]
- Tsai, C.H.; Chen, T.H.; Lin, C.; Shu, P.Y.; Su, C.L.; Teng, H.J. The impact of temperature and Wolbachia infection on vector competence of potential dengue vectors Aedes aegypti and Aedes albopictus in the transmission of dengue virus serotype 1 in southern Taiwan. Parasites Vectors 2017, 10, 551. [Google Scholar] [CrossRef]
- Duan, J.H.; Li, R.B.; Lin, W.B.; Cai, S.W.; Lu, W.C.; Li, J.Q.; Lin, H.B.; Yi, J.R.; Liu, W.H. Study on the effect of urbanization on the breeding characteristics of Aedes albopictus in residential area. Chin. J. Vector Biol. Control 2008, 19, 279–282. [Google Scholar]
- Sang, S.W.; Chen, B.; Wu, H.X.; Yang, Z.C.; Di, B.; Wang, L.; Tao, X.Y.; Liu, X.B.; Liu, Q.Y. Dengue is still an imported disease in China: A case study in Guangzhou. Infect. Genet. Evol. 2015, 32, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Guangzhou Economic and Social Development Statistics Bulletin 2017. Statistics Bureau of Guangzhou Municipality. Available online: http://www.gdstats.gov.cn/tjzl/tjgb/ (accessed on 14 March 2018).
- Liang, L.L.; Li, X.N.; Luo, L.; Xia, Y. Analysis on the density of Aedes albopictus and the risk of dengue fever transmission in Guangzhou City from 2016 to 2017. Chin. J. Hyg. Insectic. Equip. 2019, 25, 148–152. [Google Scholar]
- Lin, C.H.; Wen, T.H. Using Geographically Weighted Regression (GWR) to Explore Spatial Varying Relationships of Immature Mosquitoes and Human Densities with the Incidence of Dengue. Int. J. Environ. Res. Public Health 2011, 8, 2798–2815. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.L.; Zhang, J.Y.; Li, X.N.; Luo, L. A comparative analysis of Aedes albopictus surveillance between Guangzhou emergency mosquito vector control team and district CDCs in 2016–2017. Chin. J. Vector Biol. Control 2019, 30, 464–468. [Google Scholar]
- Yue, Y.J.; Lu, L.; Liu, Q.Y. Relationship between mosquito density and land cover types in Guangzhou, China. Chin. J. Vector Biol. Control 2016, 27, 99–102. [Google Scholar]
- Yan, J.; He, L.H. Advances in research on impacts of geographical landscape factors on mosquito density. Chin. J. Vector Biol. Control 2017, 28, 193–196. [Google Scholar]
- Wu, W.; Ren, H.Y.; Yu, M.; Wang, Z. Distinct Influences of Urban Villages on Urban Heat Islands: A Case Study in the Pearl River Delta, China. Int. J. Environ. Res. Public Health 2018, 15, 1666. [Google Scholar] [CrossRef]
- Bowman, L.R.; Runge-Ranzinger, S.; Mccall, P.J. Assessing the relationship between vector indices and dengue transmission: A systematic review of the evidence. PLoS Negl. Trop. Dis. 2014, 8, e2848. [Google Scholar] [CrossRef]
- Roiz, D.; Eritja, R.; Molina, R.; Melero-Alcibar, R.; Lucientes, J. Initial distribution assessment of Aedes albopictus (Diptera: Culicidae) in the Barcelona, Spain, area. J. Med Entomol. 2008, 45, 347–352. [Google Scholar] [CrossRef]
- Wang, J.F.; Li, X.H.; George, C.; Liao, Y.L.; Zhang, T.; Gu, X.; Zheng, X.Y. Geographical Detectors-Based Health Risk Assessment and its Application in the Neural Tube Defects Study of the Heshun Region, China. Int. J. Geogr. Inf. Sci. 2010, 24, 107–127. [Google Scholar] [CrossRef]
- Wang, J.F.; Xu, C.D. Geodetector: Principle and prospective. Acta Geogr. Sin. 2017, 72, 116–134. [Google Scholar] [CrossRef]
- Xin, X.; Yuan, Z.; Zhang, X.L.; Xia, S.Y. Identifying the Impacts of Social, Economic, and Environmental Factors on Population Aging in the Yangtze River Delta Using the Geographical Detector Technique. Sustainability 2018, 10, 1528. [Google Scholar] [CrossRef]
- Pan, J.Y.; Ling, Y.L.; Zhou, Y.; Cai, Z.L.; Liu, Q.L. Correlation between dengue fever epidemic and community spatial factors in Haizhu District of Guangzhou. J. Med. Pest Control 2017, 33, 614–616. [Google Scholar] [CrossRef]
- Brady, O.J.; Golding, N.; Pigott, D.M.; Kraemer, M.U.G.; Messina, J.P.; Jr, R.C.R.; Scott, T.W.; Smith, D.L.; Gething, P.W.; Hay, S.I. Global temperature constraints on Aedes aegypti and Ae. albopictus persistence and competence for dengue virus transmission. Parasites Vectors 2014, 7, 338. [Google Scholar] [CrossRef]
- Zhang, B. Study on the Characteristics of Mosquito Breeding in Urbanization and Its Relationship with Landscapes and Water Quality (Taking Shanghai for Example); East China Normal University: Shanghai, China, 2014. [Google Scholar]
- Yan, Z.Q.; Hu, Z.G.; Jiang, Y.M.; Wu, H.Y.; Pan, Z.M. Study on the application of standard space index in the population dynamic surveillance of Aedes albopictus. Chin. J. Hyg. Insectic. Equip. 2006, 12, 352–354. [Google Scholar]
- Cheong, Y.L.; Burkart, K.; Leitão, P.J.; Lakes, T. Assessing weather effects on dengue disease in Malaysia. Int. J. Environ. Res. Public Health 2013, 10, 6319–6334. [Google Scholar] [CrossRef]
- Jiang, Y.M.; Yan, Z.Q.; Hu, Z.G.; Li, C.L.; Xu, J.M.; Liang, X.Y. Predicting the population density of Aedes albopictus according to meteorological data. J. Trop. Med. 2014, 14, 235–237. [Google Scholar]
- Reiner, R.C.; Perkins, T.A.; Barker, C.M.; Niu, T.; Chaves, L.F.; Ellis, A.M.; George, D.B.; Menach, A.L.; Pulliam, J.R.C.; Bisanzio, D.; et al. A systematic review of mathematical models of mosquito-borne pathogen transmission: 1970–2010. J. R. Soc. Interface 2013, 10. [Google Scholar] [CrossRef]
- Hassan, A.N.; Nogoumy, N.E.; Kassem, H.A. Characterization of landscape features associated with mosquito breeding in urban Cairo using remote sensing. Egypt. J. Remote Sens. Space Sci. 2013, 16, 63–69. [Google Scholar] [CrossRef]
- Vikram, K.; Nagpal, B.N.; Pande, V.; Srivastava, A.; Saxena, R.; Anvikar, A.; Das, A.; Singh, H.; Tuli, N.R.; Gupta, S.K.; et al. An epidemiological study of Dengue in Delhi, India. Acta Trop. 2016, 153, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.S.; Tseng, Y.T.; Hsu, P.S.; Chen, C.-D.; Lian, I.B.; Chao, D.Y. Re-assess Vector Indices Threshold as an Early Warning Tool for Predicting Dengue Epidemic in a Dengue Non-endemic Country. PLoS Negl. Trop. Dis. 2015, 9, e0004043. [Google Scholar] [CrossRef] [PubMed]
- Thammapalo, S.; Chongsuvivatwong, V.; Geater, A.; Dueravee, M. Environmental factors and incidence of dengue fever and dengue haemorrhagic fever in an urban area, Southern Thailand. Epidemiol. Infect. 2008, 136, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Liao, C.M.; Chio, C.P.; Chou, H.H.; You, S.H.; Cheng, Y.H. Lagged temperature effect with mosquito transmission potential explains dengue variability in southern Taiwan: Insights from a statistical analysis. Sci. Total Environ. 2010, 408, 4069–4075. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.V.; Doan, H.T.; Phan, T.T.; Minh, N.N.T. Ecological factors associated with dengue fever in a central highlands Province, Vietnam. BMC Infect. Dis. 2011, 11, 172. [Google Scholar] [CrossRef] [PubMed]

| Month | No. of Monitoring Sites of BI | No. of Monitoring Sites of SSI | No. of Monitoring Sites of ADI |
|---|---|---|---|
| May | 64 | 97 | 87 |
| Jun | 59 | 108 | 114 |
| Jul | 62 | 66 | 64 |
| Aug | 69 | 124 | 112 |
| Sep | 79 | 122 | 109 |
| Oct | 22 | 78 | 68 |
| Indices | No. of Random Sites | The Proportion of Random Sites | No. of Fixed Sites | The Proportion of Fixed Sites |
|---|---|---|---|---|
| BI | 70 | 88.61% | 9 | 11.39% |
| SSI | 303 | 97.12% | 9 | 2.88% |
| ADI | 321 | 98.47% | 5 | 1.53% |
| Area | Indices | Minimum | Maximum | Mean | Standard Deviation | Coefficient of Variation |
|---|---|---|---|---|---|---|
| The total area | BI | 0 | 44 | 15.80 | 17.09 | 1.082 |
| SSI | 0 | 175 | 62.40 | 67.13 | 1.076 | |
| ADI | 0 | 184 | 65.20 | 70.21 | 1.077 | |
| The total area except water | BI | 6 | 44 | 19.75 | 14.64 | 0.741 |
| SSI | 31 | 175 | 78.00 | 57.35 | 0.735 | |
| ADI | 36 | 184 | 81.50 | 60.00 | 0.736 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Ren, H.; Chen, W.; Li, T. Neglected Urban Villages in Current Vector Surveillance System: Evidences in Guangzhou, China. Int. J. Environ. Res. Public Health 2020, 17, 2. https://doi.org/10.3390/ijerph17010002
Wu S, Ren H, Chen W, Li T. Neglected Urban Villages in Current Vector Surveillance System: Evidences in Guangzhou, China. International Journal of Environmental Research and Public Health. 2020; 17(1):2. https://doi.org/10.3390/ijerph17010002
Chicago/Turabian StyleWu, Sijia, Hongyan Ren, Wenhui Chen, and Tiegang Li. 2020. "Neglected Urban Villages in Current Vector Surveillance System: Evidences in Guangzhou, China" International Journal of Environmental Research and Public Health 17, no. 1: 2. https://doi.org/10.3390/ijerph17010002
APA StyleWu, S., Ren, H., Chen, W., & Li, T. (2020). Neglected Urban Villages in Current Vector Surveillance System: Evidences in Guangzhou, China. International Journal of Environmental Research and Public Health, 17(1), 2. https://doi.org/10.3390/ijerph17010002






