Effects of Resistance Exercise on Glycated Hemoglobin and Functional Performance in Older Patients with Comorbid Diabetes Mellitus and Knee Osteoarthritis: A Randomized Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Interventions
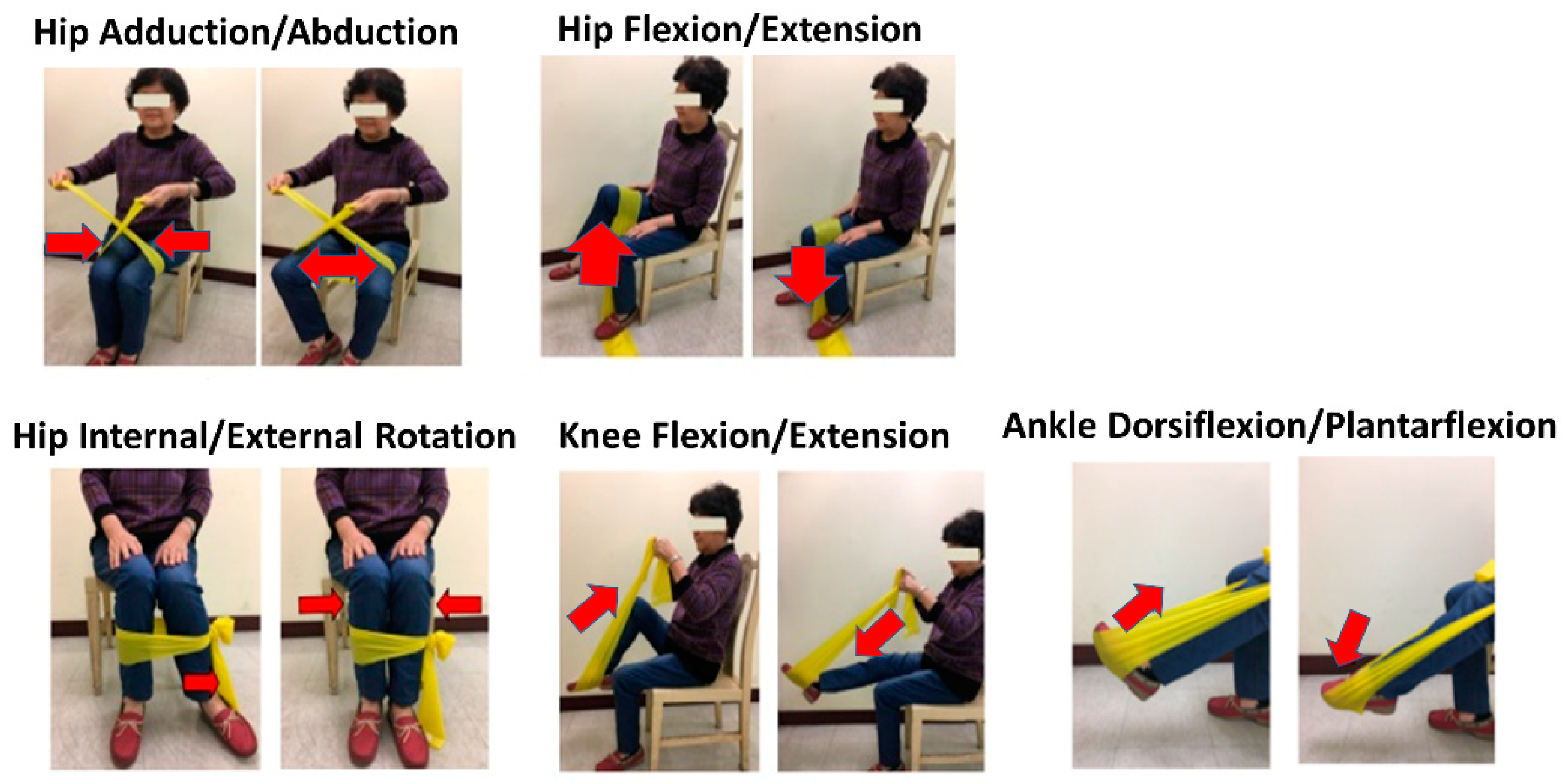
2.3.1. Dynamic Resistance Exercise Group
2.3.2. Isometric Resistance Exercise Group
2.4. Outcome Measures
2.4.1. HbA1c Analysis
2.4.2. Chair Stand Test (CST) of 30 Seconds
2.4.3. Timed Up and Go (TUG) Test
2.4.4. Western Ontario and McMaster Universities Osteoarthritis (WOMAC) Scale Scores
2.5. Statistical Analyses
3. Results
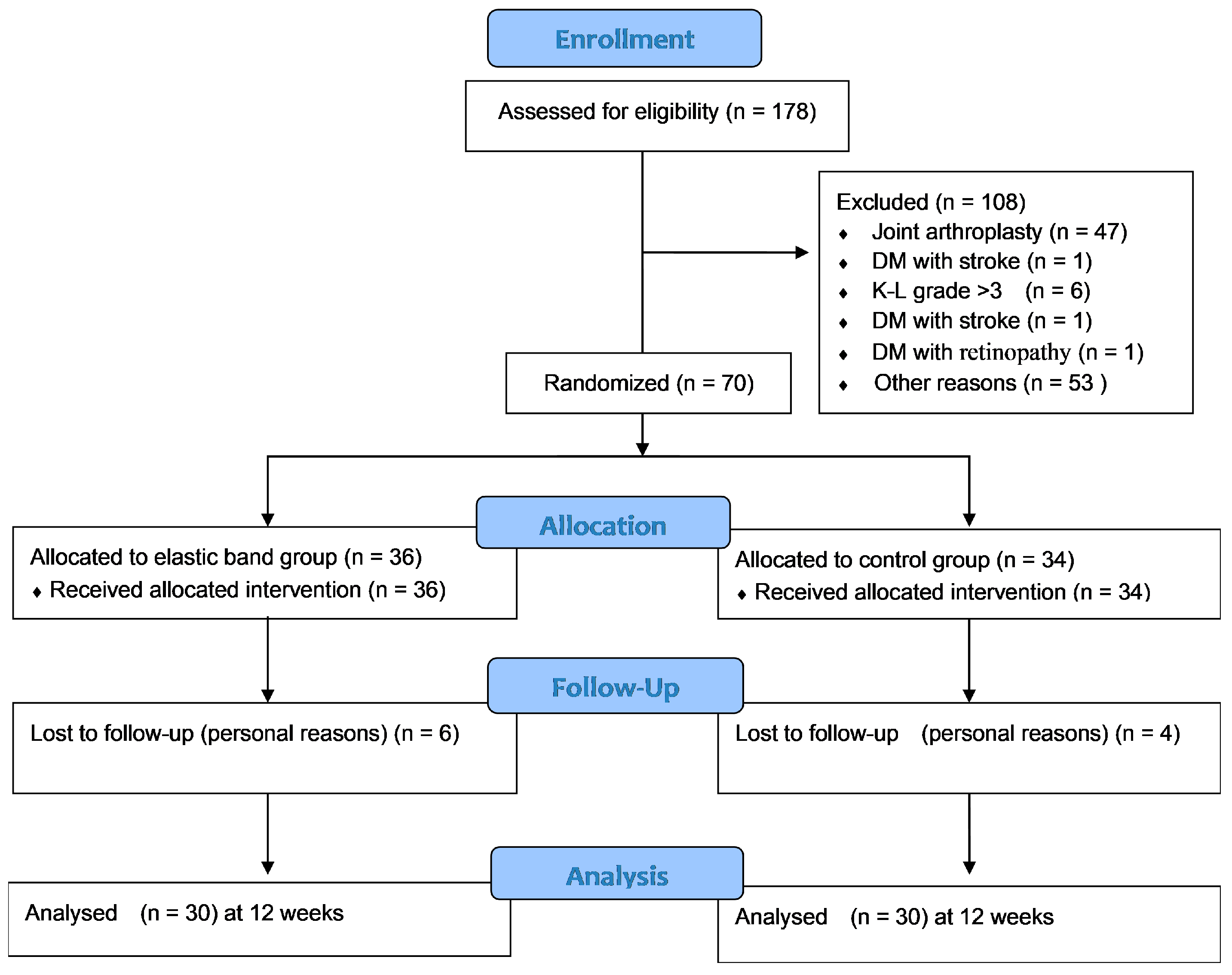
3.1. Flow of Participants through the Study
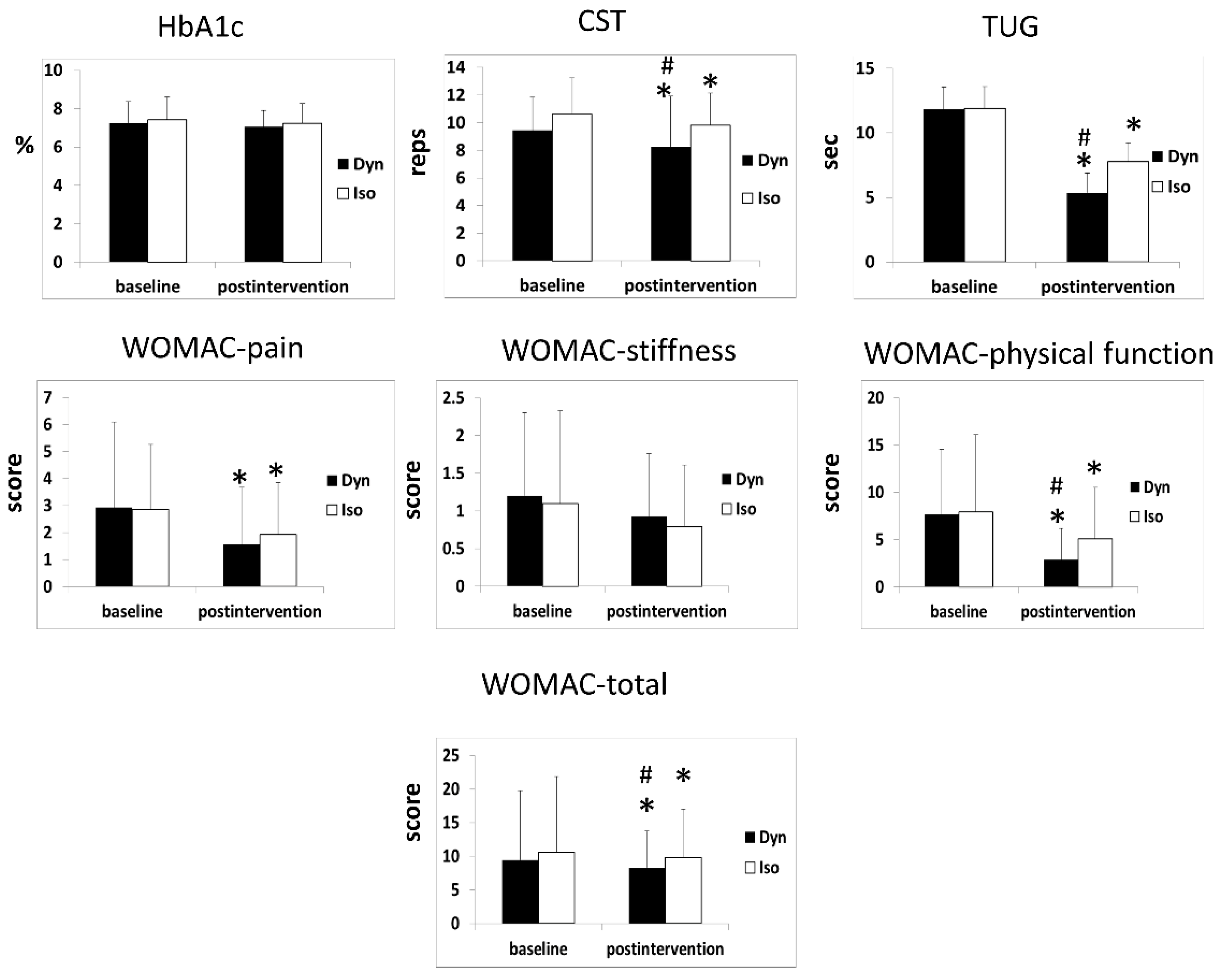
3.2. HbA1c Outcomes
3.3. CST 30-s Outcomes
3.4. TUG Test Outcomes
3.5. WOMAC Scores
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chung, I.M.; Rajakumar, G.; Subramanian, U.; Venkidasamy, B.; Khanna, V.G.; Thiruvengadam, M. Insights on the current status and advancement of diabetes mellitus type 2 and to avert complications—An overview. Biotechnol. Appl. Biochem. 2019. [Google Scholar] [CrossRef]
- Raveendran, A.V.; Chacko, E.C.; Pappachan, J.M. Non-pharmacological Treatment Options in the Management of Diabetes Mellitus. Eur. Endocrinol. 2018, 14, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Jorge, M.L.; de Oliveira, V.N.; Resende, N.M.; Paraiso, L.F.; Calixto, A.; Diniz, A.L.; Resende, E.S.; Ropelle, E.R.; Carvalheira, J.B.; Espindola, F.S.; et al. The effects of aerobic, resistance, and combined exercise on metabolic control, inflammatory markers, adipocytokines, and muscle insulin signaling in patients with type 2 diabetes mellitus. Metab. Clin. Exp. 2011, 60, 1244–1252. [Google Scholar] [CrossRef]
- Chodzko-Zajko, W.J.; Proctor, D.N.; Fiatarone Singh, M.A.; Minson, C.T.; Nigg, C.R.; Salem, G.J.; Skinner, J.S. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med. Sci. Sports Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef] [PubMed]
- Alikhani, M.; Alikhani, Z.; Boyd, C.; MacLellan, C.M.; Raptis, M.; Liu, R.; Pischon, N.; Trackman, P.C.; Gerstenfeld, L.; Graves, D.T. Advanced glycation end products stimulate osteoblast apoptosis via the MAP kinase and cytosolic apoptotic pathways. Bone 2007, 40, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Louati, K.; Vidal, C.; Berenbaum, F.; Sellam, J. Association between diabetes mellitus and osteoarthritis: Systematic literature review and meta-analysis. RMD Open 2015, 1, e000077. [Google Scholar] [CrossRef]
- Williams, M.F.; London, D.A.; Husni, E.M.; Navaneethan, S.; Kashyap, S.R. Type 2 diabetes and osteoarthritis: A systematic review and meta-analysis. J. Diabetes Complicat. 2016, 30, 944–950. [Google Scholar] [CrossRef]
- Clynes, M.A.; Jameson, K.A.; Edwards, M.H.; Cooper, C.; Dennison, E.M. Impact of osteoarthritis on activities of daily living: Does joint site matter? Aging Clin. Exp. Res. 2019, 31, 1049–1056. [Google Scholar] [CrossRef]
- Slemenda, C.; Brandt, K.D.; Heilman, D.K.; Mazzuca, S.; Braunstein, E.M.; Katz, B.P.; Wolinsky, F.D. Quadriceps weakness and osteoarthritis of the knee. Ann. Intern. Med. 1997, 127, 97–104. [Google Scholar] [CrossRef]
- Fransen, M.; McConnell, S.; Harmer, A.R.; Van der Esch, M.; Simic, M.; Bennell, K.L. Exercise for osteoarthritis of the knee. Cochrane Database Syst. Rev. 2015, 1, CD004376. [Google Scholar] [CrossRef]
- Johnson, E.J.; Niles, B.L.; Mori, D.L. Targeted recruitment of adults with type 2 diabetes for a physical activity intervention. Diabetes Spectr. Publ. Am. Diabetes Assoc. 2015, 28, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Jan, M.H.; Lin, J.J.; Liau, J.J.; Lin, Y.F.; Lin, D.H. Investigation of clinical effects of high- and low-resistance training for patients with knee osteoarthritis: A randomized controlled trial. Phys. Ther. 2008, 88, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.D.; Tsauo, J.Y.; Huang, S.W.; Ku, J.W.; Hsiao, D.J.; Liou, T.H. Effects of elastic band exercise on lean mass and physical capacity in older women with sarcopenic obesity: A randomized controlled trial. Sci. Rep. 2018, 8, 2317. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.W.; Tamulevicius, N.; Semple, S.J.; Krkeljas, Z. Efficacy of home-based kinesthesia, balance & agility exercise training among persons with symptomatic knee osteoarthritis. J. Sports Sci. Med. 2012, 11, 751–758. [Google Scholar] [PubMed]
- Umpierre, D.; Ribeiro, P.A.; Kramer, C.K.; Leitao, C.B.; Zucatti, A.T.; Azevedo, M.J.; Gross, J.L.; Ribeiro, J.P.; Schaan, B.D. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: A systematic review and meta-analysis. Jama 2011, 305, 1790–1799. [Google Scholar] [CrossRef]
- Teo, S.Y.M.; Kanaley, J.A.; Guelfi, K.J.; Cook, S.B.; Hebert, J.J.; Forrest, M.R.L.; Fairchild, T.J. Exercise Timing in Type 2 Diabetes Mellitus: A Systematic Review. Med. Sci. Sports Exerc. 2018, 50, 2387–2397. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42 (Suppl. 1), S13–S28. [Google Scholar] [CrossRef]
- Kohn, M.D.; Sassoon, A.A.; Fernando, N.D. Classifications in Brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin. Orthop. Relat. Res. 2016, 474, 1886–1893. [Google Scholar] [CrossRef]
- American College of Sports Medicine. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med. Sci. Sports Exerc. 2009, 41, 687–708. [Google Scholar] [CrossRef]
- Chang, T.F.; Liou, T.H.; Chen, C.H.; Huang, Y.C.; Chang, K.H. Effects of elastic-band exercise on lower-extremity function among female patients with osteoarthritis of the knee. Disabil. Rehabil. 2012, 34, 1727–1735. [Google Scholar] [CrossRef]
- Kwon, H.R.; Min, K.W.; Ahn, H.J.; Seok, H.G.; Lee, J.H.; Park, G.S.; Han, K.A. Effects of Aerobic Exercise vs. Resistance Training on Endothelial Function in Women with Type 2 Diabetes Mellitus. Diabetes Metab. J. 2011, 35, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.R.; Han, K.A.; Ku, Y.H.; Ahn, H.J.; Koo, B.K.; Kim, H.C.; Min, K.W. The effects of resistance training on muscle and body fat mass and muscle strength in type 2 diabetic women. Korean Diabetes J. 2010, 34, 101–110. [Google Scholar] [CrossRef]
- Higgins, T. HbA(1c)—An analyte of increasing importance. Clin. Biochem. 2012, 45, 1038–1045. [Google Scholar] [CrossRef]
- Dobson, F.; Hinman, R.S.; Hall, M.; Marshall, C.J.; Sayer, T.; Anderson, C.; Newcomb, N.; Stratford, P.W.; Bennell, K.L. Reliability and measurement error of the Osteoarthritis Research Society International (OARSI) recommended performance-based tests of physical function in people with hip and knee osteoarthritis. Osteoarthr. Cartil. 2017, 25, 1792–1796. [Google Scholar] [CrossRef] [PubMed]
- Dobson, F.; Hinman, R.S.; Roos, E.M.; Abbott, J.H.; Stratford, P.; Davis, A.M.; Buchbinder, R.; Snyder-Mackler, L.; Henrotin, Y.; Thumboo, J.; et al. OARSI recommended performance-based tests to assess physical function in people diagnosed with hip or knee osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Alghadir, A.; Anwer, S.; Brismee, J.M. The reliability and minimal detectable change of Timed Up and Go test in individuals with grade 1-3 knee osteoarthritis. BMC Musculoskelet. Disord. 2015, 16, 174. [Google Scholar] [CrossRef]
- Wright, A.A.; Cook, C.E.; Baxter, G.D.; Dockerty, J.D.; Abbott, J.H. A comparison of 3 methodological approaches to defining major clinically important improvement of 4 performance measures in patients with hip osteoarthritis. J. Orthop. Sports Phys. Ther. 2011, 41, 319–327. [Google Scholar] [CrossRef]
- Topp, R.; Woolley, S.; Hornyak, J., 3rd; Khuder, S.; Kahaleh, B. The effect of dynamic versus isometric resistance training on pain and functioning among adults with osteoarthritis of the knee. Arch. Phys. Med. Rehabil. 2002, 83, 1187–1195. [Google Scholar] [CrossRef]
- Zhuang, J.; Huang, L.; Wu, Y.; Zhang, Y. The effectiveness of a combined exercise intervention on physical fitness factors related to falls in community-dwelling older adults. Clin. Interv. Aging 2014, 9, 131–140. [Google Scholar] [CrossRef]
- White, D.; Waugh, N.; Elliott, J.; Lawton, J.; Barnard, K.; Campbell, M.J.; Dixon, S.; Heller, S. The Relative Effectiveness of Pumps Over MDI and Structured Education (REPOSE): Study protocol for a cluster randomised controlled trial. BMJ Open 2014, 4, e006204. [Google Scholar] [CrossRef]
- Jansons, P.; Robins, L.; O’Brien, L.; Haines, T. Gym-based exercise and home-based exercise with telephone support have similar outcomes when used as maintenance programs in adults with chronic health conditions: A randomised trial. J. Physiother. 2017, 63, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, D.W.; Daly, R.M.; Owen, N.; Jolley, D.; Vulikh, E.; Shaw, J.; Zimmet, P. Home-based resistance training is not sufficient to maintain improved glycemic control following supervised training in older individuals with type 2 diabetes. Diabetes Care 2005, 28, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Tanimoto, M.; Oba, N.; Sanada, K.; Miyachi, M.; Ishii, N. Effect of resistance training using bodyweight in the elderly: Comparison of resistance exercise movement between slow and normal speed movement. Geriatr. Gerontol. Int. 2015, 15, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Krieger, J.W. Single vs. multiple sets of resistance exercise for muscle hypertrophy: A meta-analysis. J. Strength Cond. Res. 2010, 24, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Juhl, C.; Christensen, R.; Roos, E.M.; Zhang, W.; Lund, H. Impact of exercise type and dose on pain and disability in knee osteoarthritis: A systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol. 2014, 66, 622–636. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Dyn (n = 30) | Iso (n = 30) | p |
|---|---|---|---|
| Gender | |||
| Male/female, n (%) | 11 (37%)/19 (63%) | 18 (60%)/12 (40%) | 0.325 |
| Age (year) | 65.9 (2.9) | 65.0 (3.1) | 0.233 |
| Body height (cm) | 159.16 (9.75) | 157.29 (7.30) | 0.404 |
| Body weight (kg) | 67.65(12.02) | 69.68(13.53) | 0.542 |
| Body mass index (kg/m2) | 26.68 (4.17) | 28.11 (4.93) | 0.231 |
| Duration of T2DM (year) | 9.13 (7.40) | 11.46 (7.77) | 0.239 |
| HbA1c (%) | 7.24 (1.1) | 7.43 (1.2) | 0.522 |
| Kellgren–Lawrence grade | 1.66 (0.80) | 1.47 (0.82) | 0.343 |
| I, n (%) | 16 (53) | 22 (73) | |
| II, n (%) | 8 (27) | 2 (7) | |
| III, n (%) | 6 (20) | 6 (20) | |
| Taking NSAIDs n (%) | 1 (3.3%) | 1 (3.3%) | 1 |
| Outcomes | Group | Baseline | Post-Intervention | Change (95% CI) | Effect Size (Cohen’s d) | Within-Group, p | Between Group, p | MDC |
|---|---|---|---|---|---|---|---|---|
| HbA1c (%) | Dyn | 7.24 ± 1.14 | 7.06 ± 0.83 | −0.18 (−0.47; −0.11) | 0.18 | 0.212 | 0.462 | N |
| Iso | 7.43 ± 1.17 | 7.24 ± 1.04 | −0.19 (−0.55; 0.17) | 0.17 | 0.29 | N | ||
| CST (reps) # | Dyn | 12.08 ± 2.39 | 13.38 ± 3.68 | 1.3 (0.46; 2.14) | 0.42 | 0.004 | 0.011 | Y |
| Iso | 10.55 ± 2.64 | 11.30 ± 2.33 | 0.75 (0.25; 1.25) | 0.39 | 0.004 | N | ||
| TUG (sec) # | Dyn | 9.45 ± 1.73 | 8.25 ± 1.50 | −1.2 (1.65; −0.74) | 0.74 | <0.001 | <0.001 | Y |
| Iso | 10.61 ± 1.69 | 9.8 ± 1.42 | −0.82 (−1.31; −0.32) | 0.51 | 0.002 | N | ||
| WOMAC (score) | ||||||||
| Pain | Dyn | 2.93 ± 3.16 | 1.56 ± 2.12 | −1.37 (−2.14; −0.61) | 0.51 | 0.001 | 0.48 | Y |
| Iso | 2.86 ± 2.40 | 1.93 ± 1.91 | −0.93 (−1.54; 0.32) | 0.43 | 0.004 | N | ||
| Stiffness | Dyn | 1.2±1.10 | 0.93 ± 0.83 | −0.29 (-0.6; 0.1) | 0.29 | 0.058 | 0.549 | N |
| Iso | 1.1 ± 1.23 | 0.79±0.82 | −0.31 (−0.77; 1.45) | 0.29 | 0.174 | N | ||
| Physical Function # | Dyn | 7.67 ± 6.89 | 2.89 ± 3.27 | −4.78 (−6.73; −2.84) | 0.89 | <0.001 | 0.033 | Y |
| Iso | 7.93 ± 8.19 | 5.07 ± 5.47 | −2.86 (−4.89; 0.84) | 0.41 | 0.007 | Y | ||
| Total # | Dyn | 11.81 ± 10.32 | 5.37 ± 5.52 | −6.44 (−8.97; -3.92) | 0.78 | <0.001 | 0.036 | Y |
| Iso | 11.90 ± 11.21 | 7.79 ± 7.22 | −4.11 (−6.79; 1.4) | 0.43 | 0.004 | Y |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-M.; Shen, F.-C.; Chen, J.-F.; Chang, W.-D.; Chang, N.-J. Effects of Resistance Exercise on Glycated Hemoglobin and Functional Performance in Older Patients with Comorbid Diabetes Mellitus and Knee Osteoarthritis: A Randomized Trial. Int. J. Environ. Res. Public Health 2020, 17, 224. https://doi.org/10.3390/ijerph17010224
Chen S-M, Shen F-C, Chen J-F, Chang W-D, Chang N-J. Effects of Resistance Exercise on Glycated Hemoglobin and Functional Performance in Older Patients with Comorbid Diabetes Mellitus and Knee Osteoarthritis: A Randomized Trial. International Journal of Environmental Research and Public Health. 2020; 17(1):224. https://doi.org/10.3390/ijerph17010224
Chicago/Turabian StyleChen, Shu-Mei, Feng-Chih Shen, Jung-Fu Chen, Wen-Dien Chang, and Nai-Jen Chang. 2020. "Effects of Resistance Exercise on Glycated Hemoglobin and Functional Performance in Older Patients with Comorbid Diabetes Mellitus and Knee Osteoarthritis: A Randomized Trial" International Journal of Environmental Research and Public Health 17, no. 1: 224. https://doi.org/10.3390/ijerph17010224
APA StyleChen, S.-M., Shen, F.-C., Chen, J.-F., Chang, W.-D., & Chang, N.-J. (2020). Effects of Resistance Exercise on Glycated Hemoglobin and Functional Performance in Older Patients with Comorbid Diabetes Mellitus and Knee Osteoarthritis: A Randomized Trial. International Journal of Environmental Research and Public Health, 17(1), 224. https://doi.org/10.3390/ijerph17010224







