Predominant Mechanisms in the Treatment of Wastewater Due to Interaction of Benzaldehyde and Iron Slag Byproduct
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sorbent and Contaminant
2.2. Batch Experiments
2.3. Kinetic Studies
2.4. Adsorption Isotherm Models
3. Results
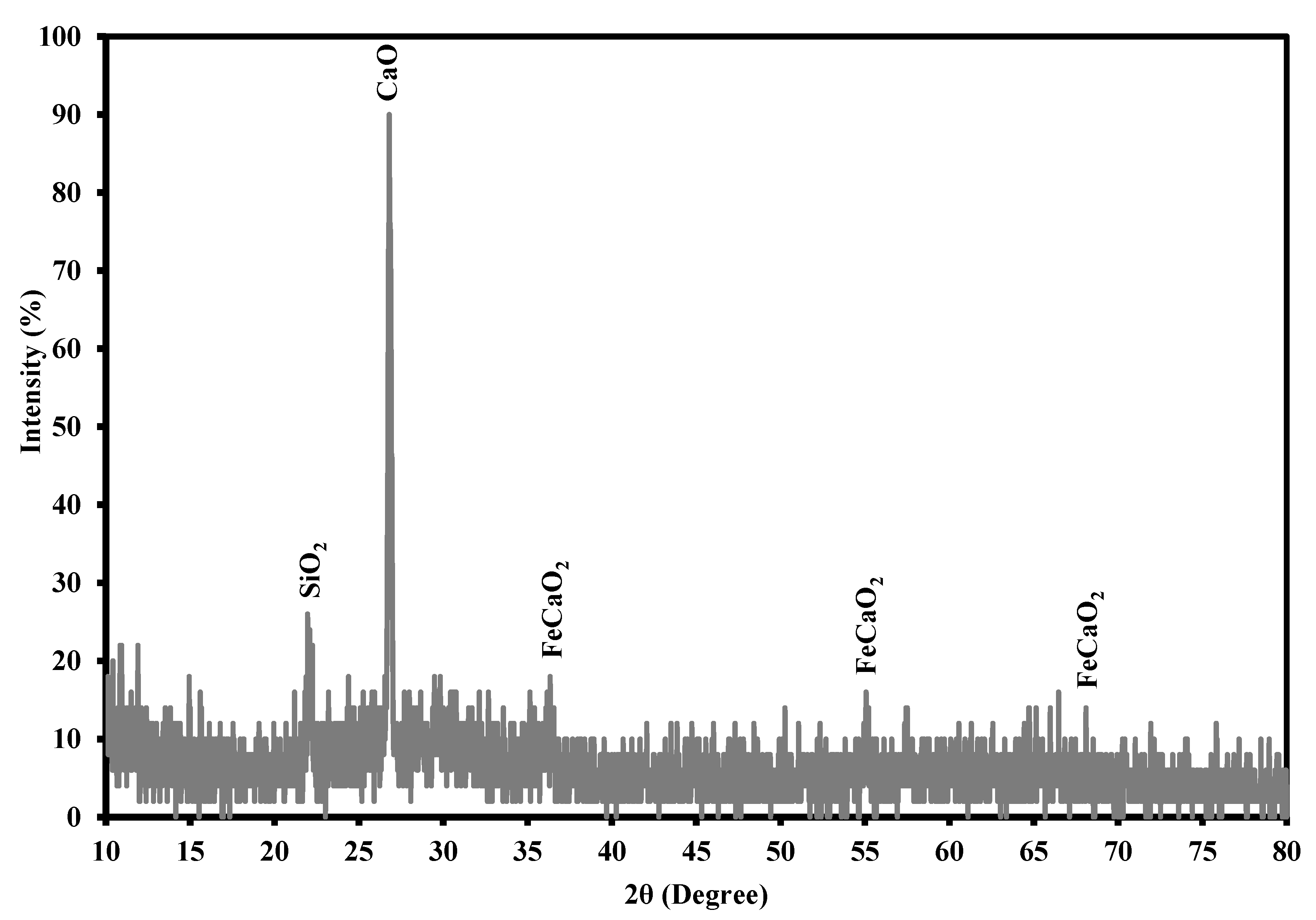
3.1. Description of Iron Slag
3.2. Operational Conditions
3.2.1. Equilibrium Time and Initial pH of the Solution
3.2.2. Slag Dosage and Initial Concentration
3.2.3. Agitation Speed
3.3. Models of Kinetic Studies
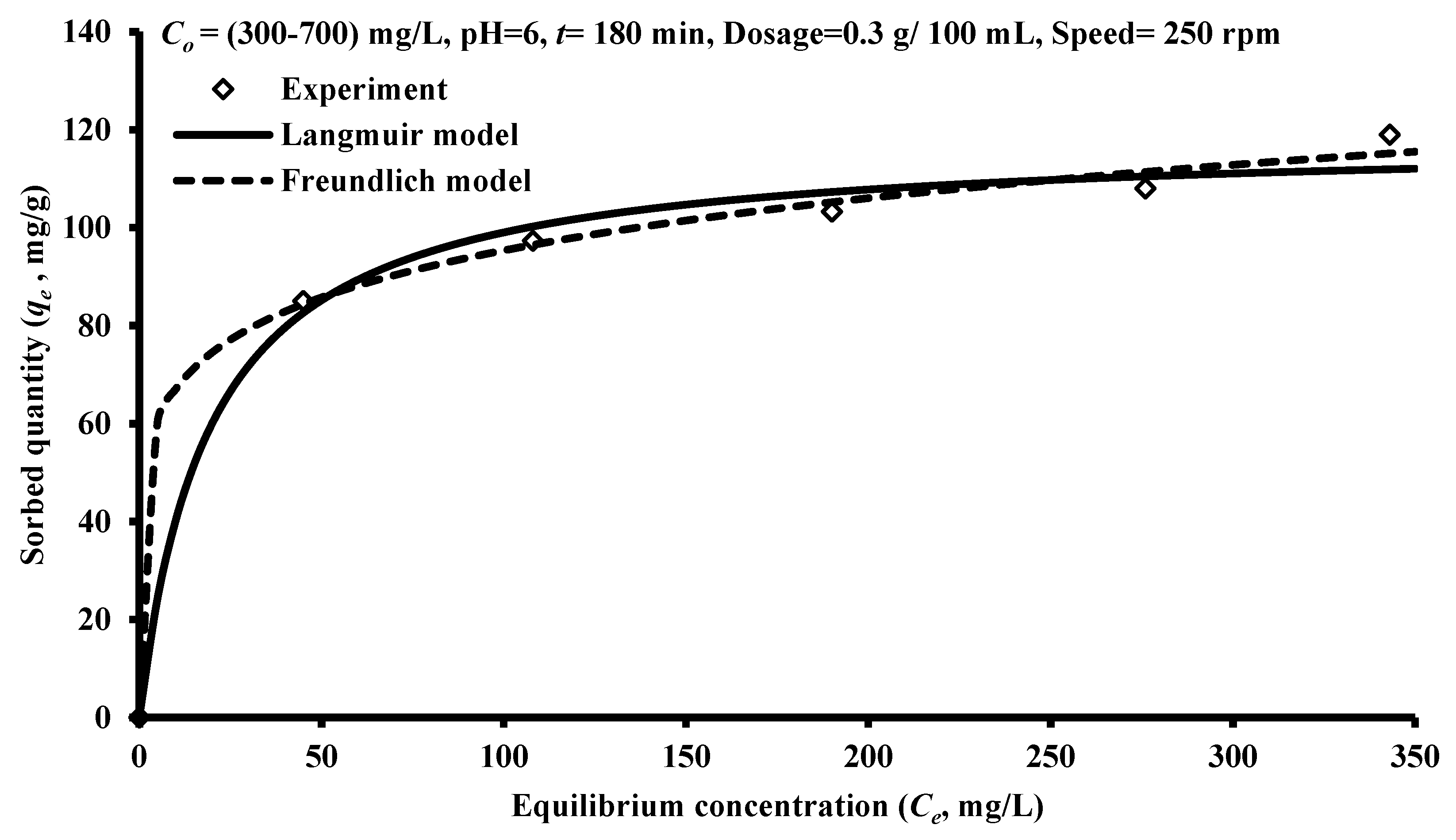
3.4. Sorption Isotherms
3.5. Thermodynamics of Adsorption Process
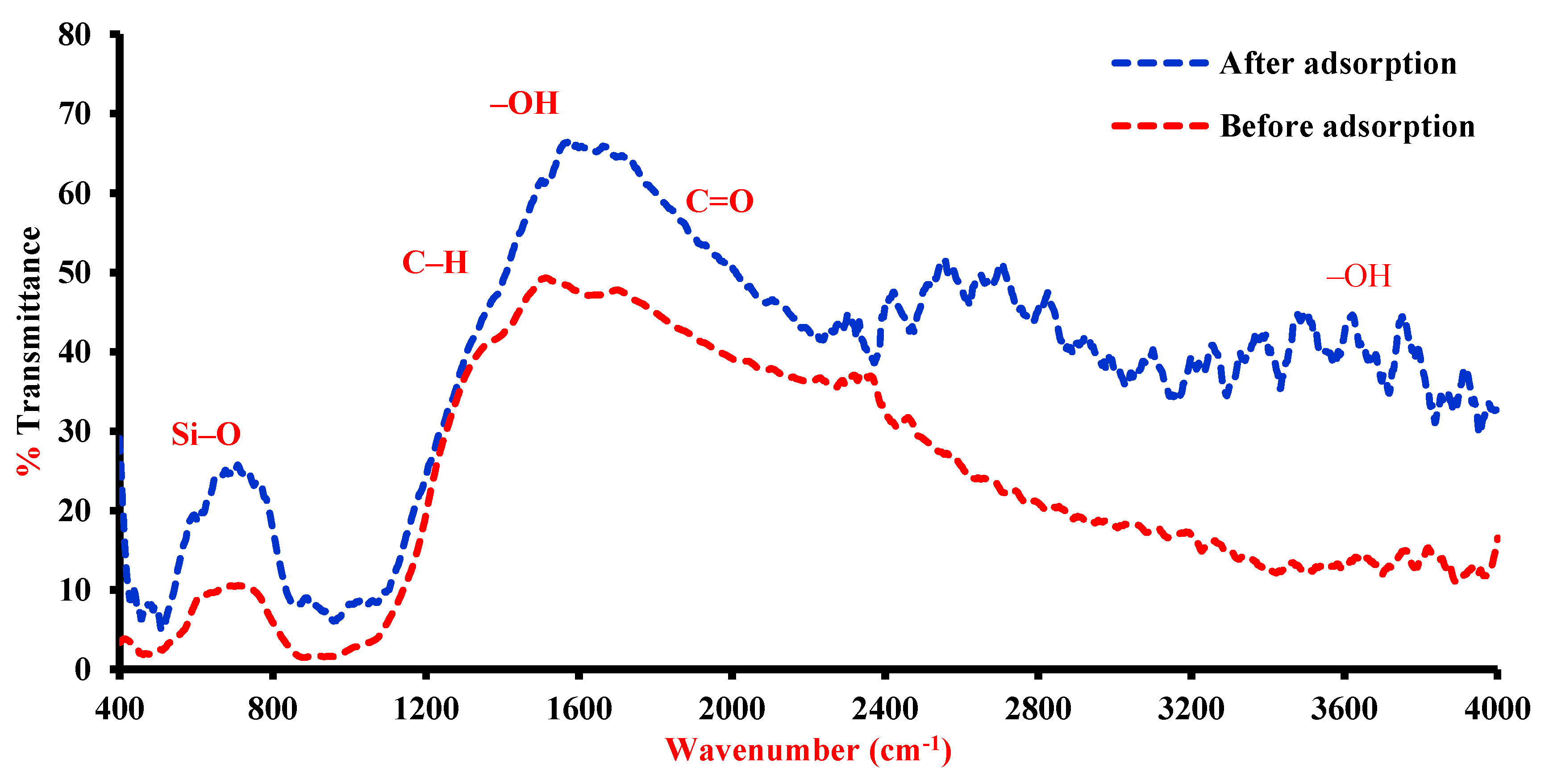
4. Predominant Mechanisms
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rajoriya, R.K.; Prasad, B.; Mishra, I.M.; Wasewar, K.L. Adsorption of benzaldehyde on granular activated carbon: Kinetics, equilibrium, and thermodynamic. Chem. Biochem. Eng. Q. 2007, 3, 219–226. [Google Scholar]
- Opgrande, J.L.; Dobratz, C.J.; Brown, E.; Liang, J.; Conn, G.S.; Shelton, F.J.; With, J. Benzaldehyde. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000. [Google Scholar]
- Shahbeig, H.; Bagheri, N.; Ghorbanian, S.A.; Hallajisani, A.; Poorkarimi, S. A new adsorption isotherm model of aqueous solutions on granular activated carbon. World J. Model. Simul. 2013, 9, 243–254. [Google Scholar]
- Saritha, P.; Aparna, C.; Himabindu, V.; Anjaneyulu, Y. Comparison of various advanced oxidation processes for the degradation of 4-chloro-2 nitrophenol. J. Hazard. Mater. 2007, 149, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Murthy, H.M.R.; Manonmani, H.K. Aerobic degradation of technical hexachlorocyclohexane by a defined microbial consortium. J. Hazard. Mater. 2007, 149, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.L.; Tan, L.S.; Shukor, S.R.A. Dimethoate and atrazine retention from aqueous solution by nanofiltration membranes. J. Hazard. Mater. 2008, 151, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.; Malato, S.; Perezestrada, L.; Gernjak, W.; Oller, I.; Domenech, X.; Peral, J. Partial degradation of five pesticides and an industrial pollutant by ozonation in a pilot-plant scale reactor. J. Hazard. Mater. 2006, 138, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Naushad, M.; ALOthman, Z.A. Separation of toxic Pb2+ metal from aqueous solution using strongly acidic cation-exchange resin: Analytical applications for the removal of metal ions from pharmaceutical formulation. Desalin. Water Treat. 2015, 53, 2158–2166. [Google Scholar] [CrossRef]
- Mironyuk, I.; Tatarchuk, T.; Naushad, M.; Vasylyeva, H.; Mykytyn, I. Highly efficient adsorption of strontium ions by carbonated mesoporous TiO 2. J. Mol. Liq. 2019, 285, 742–753. [Google Scholar] [CrossRef]
- Naushad, M.; Mittal, A.; Rathore, M.; Gupta, V. Ion-exchange kinetic studies for Cd(II), Co(II), Cu(II), and Pb(II) metal ions over a composite cation exchanger. Desalin. Water Treat. 2015, 54, 2883–2890. [Google Scholar] [CrossRef]
- Naushad, M.; Sharma, G.; Kumar, A.; Sharma, S.; Ghfar, A.A.; Bhatnagar, A.; Stadler, F.J.; Khan, M.R. Efficient removal of toxic phosphate anions from aqueous environment using pectin based quaternary amino anion exchanger. Int. J. Biol. Macromol. 2018, 106, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Awual, M.R.; Hasan, M.M.; Naushad, M.; Shiwaku, H.; Yaita, T. Preparation of new class composite adsorbent for enhanced palladium(II) detection and recovery. Sensors Actuators B Chem. 2015, 209, 790–797. [Google Scholar] [CrossRef]
- Awual, R.; Eldesoky, G.E.; Yaita, T.; Naushad, M.; Shiwaku, H.; Alothman, Z.A.; Suzuki, S. Schiff based ligand containing nano-composite adsorbent for optical copper (II) ions removal from aqueous solutions. Chem. Eng. J. 2015, 279, 639–647. [Google Scholar] [CrossRef]
- Meghea, A.; Rehner, H.H.; Peleanu, I.; Mihalache, R. Test-fitting on adsorption isotherms of organic pollutants from waste waters on activated carbon. J. Radioanal. Nucl. Chem. 1998, 229, 105–110. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Paliychuk, N.; Bitra, R.B.; Shyichuk, A.; Naushad, M.; Mironyuk, I.; Ziółkowska, D. Adsorptive removal of toxic Methylene Blue and Acid Orange 7 dyes from aqueous medium using cobalt-zinc ferrite nanoadsorbents. Desalin. Water Treat. 2019, 150, 374–385. [Google Scholar] [CrossRef]
- Naushad, M.; Sharma, G.; Alothman, Z.A. Photodegradation of toxic dye using Gum Arabic-crosslinked-poly(acrylamide)/Ni(OH)2/FeOOH nanocomposites hydrogel. J. Clean. Prod. 2019, 241, 118263. [Google Scholar] [CrossRef]
- Sharma, G.; Naushad, M.; Pathania, D.; Mittal, A.; El-desoky, G.E. Modification of Hibiscus cannabinus fiber by graft copolymerization: Application for dye removal. Desalin. Water Treat. 2015, 54, 3114–3121. [Google Scholar] [CrossRef]
- Duman, O.; Ayranci, E. Adsorption Characteristics of Benzaldehyde, Sulphanilic acid, and p-Phenolsulfonate from Water, Acid, or Base Solutions onto Activated Carbon Cloth. Sep. Sci. Technol. 2006, 41, 3673–3692. [Google Scholar] [CrossRef]
- Zaitan, H.; Mohamed, E.F.; Valdés, H.; Nawdali, M.; Rafqah, S.; Manero, M.H. Toluene, Methanol and Benzaldehyde Removal from Gas Streams by Adsorption onto Natural Clay and Faujasite-Y type Zeolite. Acta Chim. Slov. 2016, 63, 798–808. [Google Scholar] [CrossRef] [Green Version]
- Proctor, D.M.; Fehling, K.A.; Shay, E.C.; Wittenborn, J.L.; Green, J.J.; Avent, C.; Bigham, R.D.; Connolly, M.; Lee, B.; Shepker, T.O.; et al. Physical and Chemical Characteristics of Blast Furnace, Basic Oxygen Furnace, and Electric Arc Furnace Steel Industry Slags. Environ. Sci. Technol. 2000, 34, 1576–1582. [Google Scholar] [CrossRef]
- Tsakiridis, P.E.; Papadimitriou, G.D.; Tsivilis, S.; Koroneos, C. Utilization of steel slag for Portland cement clinker production. J. Hazard. Mater. 2008, 152, 805–811. [Google Scholar] [CrossRef]
- Naji, L.A.; Jassam, S.H.; Yaseen, M.J.; Faisal, A.A.H.; Al-Ansari, N. Modification of Langmuir model for simulating initial pH and temperature effects on sorption process. Sep. Sci. Technol. 2019, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Weber, W.; Morris, J. Advances in Water Pollution Research: Removal of Biologically Resistant Pollutant from Waste Water by adsorption. In Proceedings of the International Conference on Water Pollution Symposium; Pergamon Press: Oxford, UK, 1962; pp. 231–266. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Comparisons of porous and adsorption properties of carbons activated by steam and KOH. J. Colloid Interface Sci. 2005, 283, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Faisal, A.A.H.; Naji, L.A. Simulation of Ammonia Nitrogen Removal from Simulated Wastewater by Sorption onto Waste Foundry Sand Using Artificial Neural Network. Assoc. Arab Univ. J. Eng. Sci. 2019, 26, 28–34. [Google Scholar] [CrossRef]
- Saad, N.; Abd Ali, Z.T.; Naji, L.A.; AAH Faisal, A. Development of Bi-Langmuir model for description initial pH and temperature effects on the sorption of cadmium onto waste foundry sand. Environ. Eng. Res. 2019. [Google Scholar] [CrossRef]
- Gheju, M.; Miulescu, A. Sorption Equilibrium of Hexavalent Chromium on Granular Activated Carbon. Chem. Bull. POLITEHNICA Univ. 2007, 52, 1–2. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Nguyen, T.C.; Loganathan, P.; Nguyen, T.V.; Kandasamy, J.; Naidu, R.; Vigneswaran, S. Adsorptive removal of five heavy metals from water using blast furnace slag and fly ash. Environ. Sci. Pollut. Res. 2018, 25, 20430–20438. [Google Scholar] [CrossRef] [Green Version]
- Berthod, A.; Carda-Broch, S.; Garcia-Alvarez-Coque, M.C. Hydrophobicity of Ionizable Compounds. A Theoretical Study and Measurements of Diuretic Octanol–Water Partition Coefficients by Countercurrent Chromatography. Anal. Chem. 1999, 71, 879–888. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, W.; Xia, W.; Yang, K.; Xing, B. Influence of pH and surface oxygen-containing groups on multiwalled carbon nanotubes on the transformation and adsorption of 1-naphthol. J. Colloid Interface Sci. 2012, 374, 226–231. [Google Scholar] [CrossRef]
- Ho, Y. The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res. 2000, 34, 735–742. [Google Scholar] [CrossRef]
- Drweesh, S.A.; Fathy, N.A.; Wahba, M.A.; Hanna, A.A.; Akarish, A.I.M.; Elzahany, E.A.M.; El-Sherif, I.Y.; Abou-El-Sherbini, K.S. Equilibrium, kinetic and thermodynamic studies of Pb(II) adsorption from aqueous solutions on HCl-treated Egyptian kaolin. J. Environ. Chem. Eng. 2016, 4, 1674–1684. [Google Scholar] [CrossRef]
- Kalhori, E.M.; Al-Musawi, T.J.; Ghahramani, E.; Kazemian, H.; Zarrabi, M. Enhancement of the adsorption capacity of the light-weight expanded clay aggregate surface for the metronidazole antibiotic by coating with MgO nanoparticles: Studies on the kinetic, isotherm, and effects of environmental parameters. Chemosphere 2017, 175, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Nashine, A.L.; Tembhurkar, A.R. Equilibrium, kinetic and thermodynamic studies for adsorption of As(III) on coconut (Cocos nucifera L.) fiber. J. Environ. Chem. Eng. 2016, 4, 3267–3273. [Google Scholar] [CrossRef]
- Konggidinata, M.I.; Chao, B.; Lian, Q.; Subramaniam, R.; Zappi, M.; Gang, D.D. Equilibrium, kinetic and thermodynamic studies for adsorption of BTEX onto Ordered Mesoporous Carbon (OMC). J. Hazard. Mater. 2017, 336, 249–259. [Google Scholar] [CrossRef]
- Bedin, K.C.; Martins, A.C.; Cazetta, A.L.; Pezoti, O.; Almeida, V.C. KOH-activated carbon prepared from sucrose spherical carbon: Adsorption equilibrium, kinetic and thermodynamic studies for Methylene Blue removal. Chem. Eng. J. 2016, 286, 476–484. [Google Scholar] [CrossRef]
- Fierro, V.; Torné-Fernández, V.; Montané, D.; Celzard, A. Adsorption of phenol onto activated carbons having different textural and surface properties. Microporous Mesoporous Mater. 2008, 111, 276–284. [Google Scholar] [CrossRef]
- Rahman, A.; Kumar, S.; Bafana, A.; Dahoumane, S.; Jeffryes, C. Individual and Combined Effects of Extracellular Polymeric Substances and Whole Cell Components of Chlamydomonas reinhardtii on Silver Nanoparticle Synthesis and Stability. Molecules 2019, 24, 956. [Google Scholar] [CrossRef] [Green Version]
- Silverstein, R.M.; Bassler, G.C. Spectrometric identification of organic compounds. J. Chem. Educ. 1962, 39, 546. [Google Scholar] [CrossRef]
- Hoffman, R.V. Structure Determination of Organic Compounds. In Organic Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 332–394. [Google Scholar]
- Tolstorozhev, G.B.; Skornyakov, I.V.; Bel’kov, M.V.; Shadyro, O.I.; Brinkevich, S.D.; Samovich, S.N. IR spectra of benzaldehyde and its derivatives in different aggregate states. Opt. Spectrosc. 2012, 113, 179–183. [Google Scholar] [CrossRef]
- Vanhengstum, A. Infrared study of the selective oxidation of toluene and o-xylene on vanadium oxide/TiO2. J. Catal. 1986, 101, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Besselmann, S.; Löffler, E.; Muhler, M. On the role of monomeric vanadyl species in toluene adsorption and oxidation on V2O5/TiO2 catalysts: A Raman and in situ DRIFTS study. J. Mol. Catal. A Chem. 2000, 162, 401–411. [Google Scholar] [CrossRef]
- Lampert, H.; Mikenda, W.; Karpfen, A. Molecular Geometries and Vibrational Spectra of Phenol, Benzaldehyde, and Salicylaldehyde: Experimental versus Quantum Chemical Data. J. Phys. Chem. A 1997, 101, 2254–2263. [Google Scholar] [CrossRef]
- Palomar, J.; De Paz, J.L.G.; Catalán, J. Vibrational study of intramolecular hydrogen bonding in o-hydroxybenzoyl compounds. Chem. Phys. 1999, 246, 167–208. [Google Scholar] [CrossRef]
- Kung, K.-H.; McBride, M.B. Adsorption of Para-substituted Benzoates on Iron Oxides. Soil Sci. Soc. Am. J. 1989, 53, 1673. [Google Scholar] [CrossRef]
- Naushad, M. Surfactant assisted nano-composite cation exchanger: Development, characterization and applications for the removal of toxic Pb2+ from aqueous medium. Chem. Eng. J. 2014, 235, 100–108. [Google Scholar] [CrossRef]
- Díez, V. Effect of the chemical composition on the catalytic performance of MgyAlOx catalysts for alcohol elimination reactions. J. Catal. 2003, 215, 220–233. [Google Scholar] [CrossRef]




| Element | Concentration (mg/L) | |
|---|---|---|
| pH 3 | pH 6 | |
| Pb | 0.01 | 0.008 |
| Cr | n.d. | n.d. |
| Cu | 0.072 | 0.02 |
| Zn | n.d. | n.d. |
| n.d.: not detected | ||
| Kinetic Model | Parameter | Co (mg/L) | |||
|---|---|---|---|---|---|
| 300 | 400 | 500 | 600 | ||
| Pseudo First-Order | k1 (min−1) | 0.0058 | 0.0067 | 0.0075 | 0.0084 |
| qe (mg/g) | 134.5 | 144.6 | 147.6 | 147.0 | |
| R2 | 0.999 | 0.999 | 0.999 | 0.999 | |
| Pseudo Second-Order | k2 (g/mg min) | 0.000016 | 0.00002 | 0.000022 | 0.000026 |
| qe (mg/g) | 225.0 | 225.9 | 230.3 | 224.5 | |
| h (mg/g min) | 0.796 | 1.029 | 1.165 | 1.314 | |
| R2 | 0.999 | 0.999 | 0.999 | 0.999 | |
| Intra-Particle Diffusion | First Portion | ||||
| kint (mg/g min0.5) | 8.243 | 9.466 | 10.131 | 10.74 | |
| R2 | 0.997 | 0.998 | 0.999 | 0.999 | |
| Second Portion | |||||
| kint (mg/g min0.5) | 7.332 | 8.557 | 9.341 | 8.942 | |
| R2 | 0.990 | 0.989 | 1.00 | 0.999 | |
| Isotherm Model | Parameter | Value |
|---|---|---|
| Freundlich | KF (mg/g)(L/mg)1/n | 47.12 |
| 1/n | 0.153 | |
| R2 | 0.952 | |
| Langmuir | b (L/mg) | 0.052 |
| qmax (mg/g) | 118.25 | |
| R2 | 0.865 |
| Thermodynamic Parameters | Temperature (K) | ||
|---|---|---|---|
| 293.15 | 303.15 | 313.15 | |
| 1.84058 | 2.29825 | 3.2381 | |
| ΔG° (* 103 cal mol−1) | −0.3554 | −0.5013 | −0.7311 |
| ΔH° (* 103 cal mol−1) | 5.81494 | ||
| ΔS° (cal mol−1 K−1) | 18.69577 | ||
| Initial pH | Control Test | After Adsorption | ||
|---|---|---|---|---|
| Final pH | Ca+2 (mg/L) | Final pH | Ca+2 (mg/L) | |
| 3.17 | 4.39 | 3.4 | 3.76 | 1.3 |
| 5.9 | 6.49 | 3.2 | 6.3 | 2 |
| 6.25 | 8.20 | 10 | 6.75 | 4 |
| 6.98 | 8.5 | 7 | 7.24 | 5 |
| 8.87 | 6.5 | 2.6 | 7.4 | 0.5 |
| 11 | 10.7 | 0.5 | 10.6 | 0.54 |
| Band (cm−1) | Functional Group |
|---|---|
| 996,530 and 450 | Si–O |
| 450 | Ca–O |
| 1421 and 872 | C-O |
| 3622 | Si–Si–OH or Al–Al–OH |
| 1635 | A1MgOH |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faisal, A.A.H.; Alquzweeni, S.S.; Naji, L.A.; Naushad, M. Predominant Mechanisms in the Treatment of Wastewater Due to Interaction of Benzaldehyde and Iron Slag Byproduct. Int. J. Environ. Res. Public Health 2020, 17, 226. https://doi.org/10.3390/ijerph17010226
Faisal AAH, Alquzweeni SS, Naji LA, Naushad M. Predominant Mechanisms in the Treatment of Wastewater Due to Interaction of Benzaldehyde and Iron Slag Byproduct. International Journal of Environmental Research and Public Health. 2020; 17(1):226. https://doi.org/10.3390/ijerph17010226
Chicago/Turabian StyleFaisal, Ayad A. H., Saif S. Alquzweeni, Laith A. Naji, and Mu Naushad. 2020. "Predominant Mechanisms in the Treatment of Wastewater Due to Interaction of Benzaldehyde and Iron Slag Byproduct" International Journal of Environmental Research and Public Health 17, no. 1: 226. https://doi.org/10.3390/ijerph17010226






