Multiple Sclerosis in the Campania Region (South Italy): Algorithm Validation and 2015–2017 Prevalence
Abstract
1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Population
- Hospital Discharge Record database, which included all admissions in the study period with an ICD-9 CM code of MS as one of the discharges diagnoses.
- Regional Drug Prescription database, which included all MS-specific DMTs prescribed in the study period (e.g., Alemtuzumab, Dimethyl Fumarate, Fingolimod, Glatiramer Acetate, Interferon Beta-1a, Interferon Beta-1b, Natalizumab, Peg-Interferon Beta-1a, Teriflunomide).
- Outpatient database, which included all outpatient consultations with an MS-specific exemption from co-payment records (as defined in the Healthcare Co-payment Database).
2.3. Clinical Dataset
2.4. Statistical Analysis
2.5. Data Availability
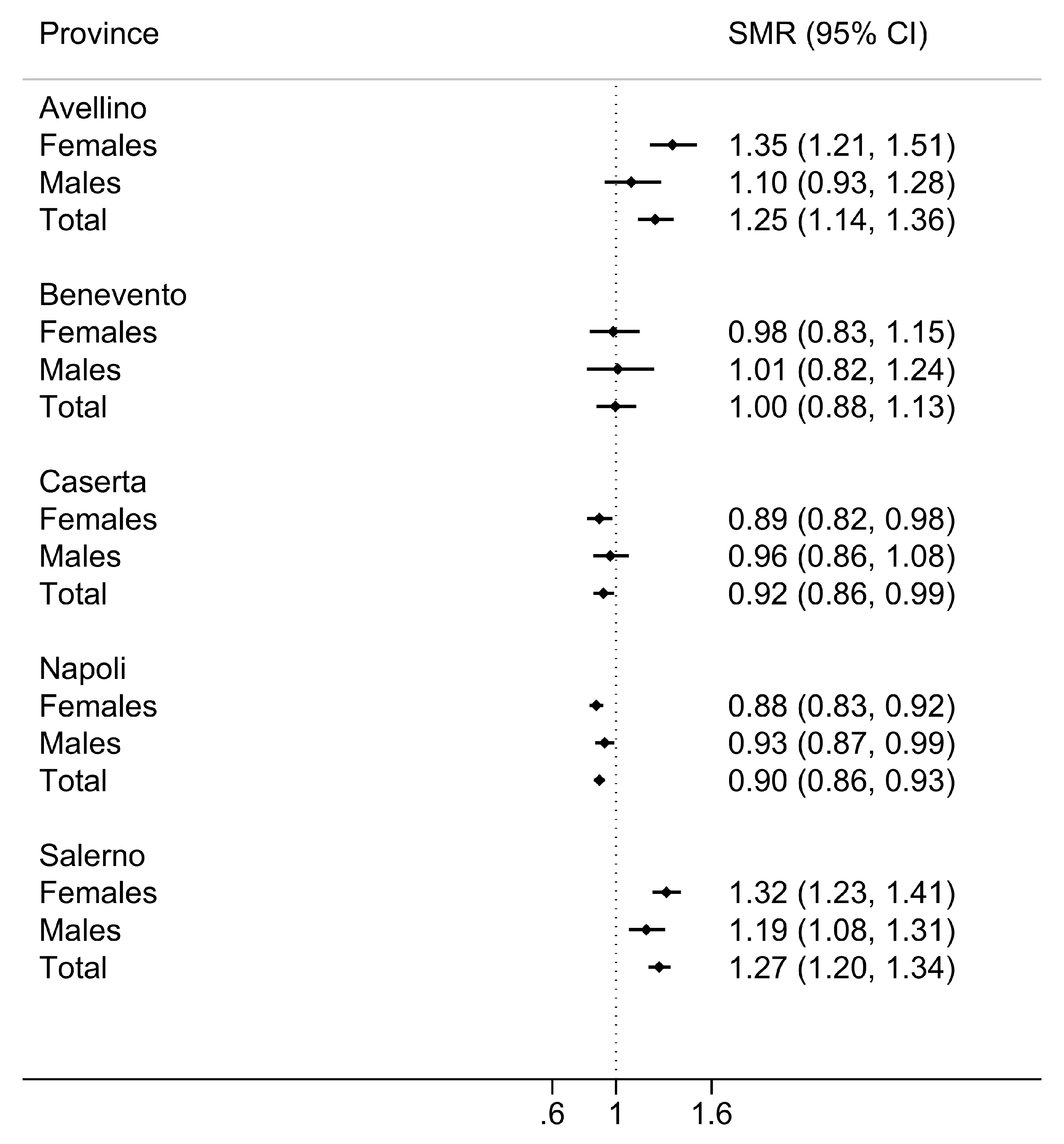
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wallin, M.T.; Culpepper, W.J.; Nichols, E.; Bhutta, Z.A.; Gebrehiwot, T.T.; Hay, S.I.; Khalil, I.A.; Krohn, K.J.; Liang, X.; Naghavi, M.; et al. Global, regional, and national burden of multiple sclerosis 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 269–285. [Google Scholar] [CrossRef]
- Thompson, A.J.; Baranzini, S.E.; Geurts, J.; Hemmer, B.; Ciccarelli, O. Multiple sclerosis. Lancet 2018, 391, 1622–1636. [Google Scholar] [CrossRef]
- De Angelis, F.; John, N.; Brownlee, W. Disease-modifying therapies for multiple sclerosis. BMJ 2018, 363, k4674. [Google Scholar] [CrossRef] [PubMed]
- Tur, C.; Moccia, M.; Barkhof, F.; Chataway, J.; Sastre-Garriga, J.; Thompson, A.J.; Ciccarelli, O. Assessing treatment outcomes in multiple sclerosis trials and in the clinical setting. Nat. Rev. Neurol. 2018, 14, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Thalheim, C. Pooling real-world multiple sclerosis patient data on a European level: A true story of success. Neurodegener. Dis. Manag. 2015, 5, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Glaser, A.; Stahmann, A.; Meissner, T.; Flachenecker, P.; Horáková, D.; Zaratin, P.; Brichetto, G.; Pugliatti, M.; Rienhoff, O.; Vukusic, S.; et al. Multiple Sclerosis Registries in Europe—An Updated Mapping Survey. Mult. Scler. Relat. Disord. 2019, 27, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Trojano, M.; Bergamaschi, R.; Amato, M.P.; Comi, G.; Ghezzi, A.; Lepore, V.; Marrosu, M.G.; Mosconi, P.; Patti, F.; Ponzio, M.; et al. The Italian multiple sclerosis register. Neurol. Sci. 2019, 40, 155–165. [Google Scholar] [CrossRef]
- Moccia, M.; Tajani, A.; Acampora, R.; Signoriello, E.; Corbisiero, G.; Vercellone, A.; Sergianni, P.; Pennino, F.; Lanzillo, R.; Palladino, R.; et al. Healthcare resource utilization and costs for multiple sclerosis management in the Campania Region of Italy: Comparison between centre-based and local service healthcare delivery. PLoS ONE 2019, 14, e0222012. [Google Scholar] [CrossRef]
- Sorensen, P.S.; Giovannoni, G.; Montalban, X.; Thalheim, C.; Zaratin, P.; Comi, G. The Multiple Sclerosis Care Unit. Mult. Scler. J. 2019, 25, 627–636. [Google Scholar] [CrossRef]
- Kobelt, G.; Thompson, A.; Berg, J.; Gannedahl, M.; Eriksson, J. New insights into the burden and costs of multiple sclerosis in Europe. Mult. Scler. J. 2017, 23, 1123–1136. [Google Scholar] [CrossRef]
- Trojano, M.; Tintore, M.; Montalban, X.; Hillert, J.; Kalincik, T.; Iaffaldano, P.; Spelman, T.; Sormani, M.; Butzkueven, H. Treatment decisions in multiple sclerosis - insights from real-world observational studies. Nat. Rev. Neurol. 2017, 13, 105–118. [Google Scholar] [CrossRef]
- Kalincik, T.; Butzkueven, H. Observational data: Understanding the real MS world. Mult. Scler. J. 2016, 22, 1642–1648. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.A.; Trojano, M.; Mowry, E.M.; Uitdehaag, B.M.J.; Reingold, S.C.; Marrie, R.A. Leveraging real-world data to investigate multiple sclerosis disease behavior, prognosis, and treatment. Mult. Scler. J. 2020, 26, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Bezzini, D.; Ulivelli, M.; Gualdani, E.; Razzanelli, M.; Ferretti, F.; Meucci, G.; Francesconi, P.; Battaglia, M.A. Increasing prevalence of multiple sclerosis in Tuscany, Italy. Neurol. Sci. 2020, 41, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, H.G.; Cremieux, P.Y.; Greenberg, P.E.; LeLorier, J.; Ostrander, J.A.; Venditti, L. Using healthcare claims data for outcomes research and pharmacoeconomic analyses. Pharmacoeconomics 1999, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Roux, J.; Guilleux, A.; Lefort, M.; Leray, E. Use of healthcare services by patients with multiple sclerosis in France over 2010–2015: A nationwide population-based study using health administrative data. Mult. Scler. J. Exp. Transl. Clin. 2019, 5, 1–11. [Google Scholar] [CrossRef]
- Fox, E.; Vieira, M.C.; Johnson, K.; Peeples, M.; Bensimon, A.G.; Signorovitch, J.; Herrera, V. Real-world durability of relapse rate reduction in patients with multiple sclerosis receiving fingolimod for up to 3 years: A retrospective US claims database analysis. J. Neurol. Sci. 2019, 398, 163–170. [Google Scholar] [CrossRef]
- Signorelli, C.; Odone, A.; Oradini-Alacreu, A.; Pelissero, G. Universal Health Coverage in Italy: Lights and shades of the Italian National Health Service which celebrated its 40th anniversary. Health Policy 2019, 124, 69–74. [Google Scholar] [CrossRef]
- Agenzia Italiana del Farmaco (AIFA). Banca Dati Farmaci. Available online: https://www.farmaci.agenziafarmaco.gov.it/ (accessed on 8 May 2019).
- European Medicine Agency (EMA). European Public Assessment Reports. Available online: https://www.ema.europa.eu/en/medicines (accessed on 8 May 2019).
- Canova, C.; Danieli, S.; Amidei, C.B.; Simonato, L.; Di Domenicantonio, R.; Cappai, G.; Bargagli, A.M. A systematic review of case-identification algorithms based on Italian healthcare administrative databases for three relevant diseases of the nervous system: Parkinson’s disease, multiple sclerosis, and epilepsy. Epidemiol. Prev. 2019, 43, 62–74. [Google Scholar]
- Moccia, M.; Lanzillo, R.; Palladino, R.; Chang, K.K.C.M.K.C.-M.; Costabile, T.; Russo, C.; De Rosa, A.; Carotenuto, A.; Saccà, F.; Maniscalco, G.T.G.T.; et al. Cognitive impairment at diagnosis predicts 10-year multiple sclerosis progression. Mult. Scler. J. 2016, 22, 659–667. [Google Scholar] [CrossRef]
- Moccia, M.; Capacchione, A.; Lanzillo, R.; Carbone, F.; Micillo, T.; Perna, F.; De Rosa, A.; Carotenuto, A.; Albero, R.; Matarese, G.; et al. Coenzyme Q10 supplementation reduces peripheral oxidative stress and inflammation in Interferon-Beta1a treated multiple sclerosis. Ther. Adv. Neurol. Disord. 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Claflin, S.B.; Broadley, S.; Taylor, B.V. The effect of disease modifying therapies on disability progression in multiple sclerosis: A systematic overview of meta-analyses. Front. Neurol. 2019, 10, 101374. [Google Scholar] [CrossRef]
- Moccia, M.; Palladino, R.; Carotenuto, A.; Saccà, F.; Russo, C.V.; Lanzillo, R.; Brescia Morra, V. A 8-year retrospective cohort study comparing Interferon-β formulations for relapsing-remitting multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 19, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Palladino, R.; Marrie, R.; Majeed, A.; Chataway, J. Evaluating the Risk of Macrovascular Events and Mortality Among People With Multiple Sclerosis in England. JAMA Neurol. 2020. [Google Scholar] [CrossRef]
- Bezzini, D.; Policardo, L.; Meucci, G.; Ulivelli, M.; Bartalini, S.; Profili, F.; Battaglia, M.A.; Francesconi, P. Prevalence of multiple sclerosis in tuscany (central Italy): A study based on validated administrative data. Neuroepidemiology 2016, 46, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Culpepper, W.J.; Marrie, R.A.; Langer-Gould, A.; Wallin, M.T.; Campbell, J.D.; Nelson, L.M.; Kaye, W.E.; Wagner, L.; Tremlett, H.; Chen, L.H.; et al. Validation of an algorithm for identifying MS cases in administrative health claims datasets. Neurology 2019, 92, e1016–e1028. [Google Scholar] [CrossRef]
- Disanto, G.; Zecca, C.; MacLachlan, S.; Sacco, R.; Handunnetthi, L.; Meier, U.C.; Simpson, A.; McDonald, L.; Rossi, A.; Benkert, P.; et al. Prodromal symptoms of multiple sclerosis in primary care. Ann. Neurol. 2018, 83, 1162–1173. [Google Scholar] [CrossRef]
- Sim, J.; Wright, C.C. The Kappa Statistic in Reliability Studies: Use, Interpretation, and Sample Size Requirements. Phys. Ther. 2005, 85, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Barros, A.J.D.; Hirakata, V.N. Alternatives for logistic regression in cross-sectional studies: An empirical comparison of models that directly estimate the prevalence ratio. BMC Med. Res. Methodol. 2003, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- El Adssi, H.; Debouverie, M.; Guillemin, F. Estimating the prevalence and incidence of multiple sclerosis in the Lorraine region, France, by the capture-recapture method. Mult. Scler. J. 2012, 18, 1244–1250. [Google Scholar] [CrossRef]
- Farcomeni, A. Population size estimation with interval censored counts and external information: Prevalence of multiple sclerosis in Rome. Biom. J. 2020. [Google Scholar] [CrossRef] [PubMed]
- International Working Group for Disease Monitoring and Forecasting. Capture-recapture and Multiple-Record Systems Estimation I: History and Theoretical Development. Am. J. Epidemiol. 1995, 142, 1047–1058. [Google Scholar]
- International Working Group for Disease Monitoring and Forecasting. Capture-recapture and multiple-record systems estimation II: Applications in human diseases. Am. J. Epidemiol. 1995, 142, 1059–1068. [Google Scholar]
- Hook, E.; Regal, R. Capture-recapture Methods in Epidemiology: Methods and Limitations. Epidemiol. Rev. 1995, 17, 243–264. [Google Scholar] [CrossRef] [PubMed]
- Regal, R.R.; Hook, E.B. Goodness-of-fit based confidence intervals for estimates of the size of a closed population. Stat. Med. 1984, 3, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, M.A.; Bezzini, D. Estimated prevalence of multiple sclerosis in Italy in 2015. Neurol. Sci. 2017, 38, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Bargagli, A.M.; Colais, P.; Agabiti, N.; Mayer, F.; Buttari, F.; Centonze, D.; Di Folco, M.; Filippini, G.; Francia, A.; Galgani, S.; et al. Prevalence of multiple sclerosis in the Lazio region, Italy: Use of an algorithm based on health information systems. J. Neurol. 2016, 263, 751–759. [Google Scholar] [CrossRef]
- Grassivaro, F.; Puthenparampil, M.; Pengo, M.; Saiani, M.; Venturini, M.; Stropparo, E.; Perini, P.; Rinaldi, F.; Freddi, N.; Cadaldini, M.; et al. Multiple sclerosis incidence and prevalence trends in the Province of Padua, Northeast Italy, 1965-2018. Neuroepidemiology 2019, 52, 41–46. [Google Scholar] [CrossRef]
- Solaro, C.; Ponzio, M.; Moran, E.; Tanganelli, P.; Pizio, R.; Ribizzi, G.; Venturi, S.; Mancardi, G.L.; Battaglia, M.A. The changing face of multiple sclerosis: Prevalence and incidence in an aging population. Mult. Scler. J. 2015, 21, 1244–1250. [Google Scholar] [CrossRef]
- Steri, M.; Orrù, V.; Idda, M.L.; Pitzalis, M.; Pala, M.; Zara, I.; Sidore, C.; Faà, V.; Floris, M.; Deiana, M.; et al. Overexpression of the Cytokine BAFF and Autoimmunity Risk. N. Engl. J. Med. 2017, 376, 1615–1626. [Google Scholar] [CrossRef]
- Nicoletti, A.; Rascunà, C.; Boumediene, F.; Vasta, R.; Cicero, C.E.; Lo Fermo, S.; Ferrante, M.; Marziolo, R.; Maimone, D.; Grimaldi, L.M.; et al. Incidence of multiple sclerosis in the province of Catania. A geo-epidemiological study. Environ. Res. 2020, 182, 109022. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, R.; Monti, M.C.; Trivelli, L.; Introcaso, V.P.; Mallucci, G.; Borrelli, P.; Gerosa, L.; Montomoli, C. Increased prevalence of multiple sclerosis and clusters of different disease risk in Northern Italy. Neurol. Sci. 2020, 41, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Höer, A.; Schiffhorst, G.; Zimmermann, A.; Fischaleck, J.; Gehrmann, L.; Ahrens, H.; Carl, G.; Sigel, K.; Osowski, U.; Klein, M.; et al. Multiple sclerosis in Germany: Data analysis of administrative prevalence and healthcare delivery in the statutory health system. BMC Health Serv. Res. 2014, 14, 381. [Google Scholar] [CrossRef] [PubMed]
- Dilokthornsakul, P.; Valuck, R.J.; Nair, K.V.; Corboy, J.R.; Allen, R.R.; Campbell, J.D. Multiple sclerosis prevalence in the United States commercially insured population. Neurology 2016, 86, 1–8. [Google Scholar] [CrossRef]
- Wallin, M.T.; Culpepper, W.J.; Campbell, J.D.; Nelson, L.M.; Langer-Gould, A.; Marrie, R.A.; Cutter, G.R.; Kaye, W.E.; Wagner, L.; Tremlett, H.; et al. The prevalence of MS in the United States: A population-based estimate using health claims data. Neurology 2019, 92, e1029–e1040. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moccia, M.; Brescia Morra, V.; Lanzillo, R.; Loperto, I.; Giordana, R.; Fumo, M.G.; Petruzzo, M.; Capasso, N.; Triassi, M.; Sormani, M.P.; et al. Multiple Sclerosis in the Campania Region (South Italy): Algorithm Validation and 2015–2017 Prevalence. Int. J. Environ. Res. Public Health 2020, 17, 3388. https://doi.org/10.3390/ijerph17103388
Moccia M, Brescia Morra V, Lanzillo R, Loperto I, Giordana R, Fumo MG, Petruzzo M, Capasso N, Triassi M, Sormani MP, et al. Multiple Sclerosis in the Campania Region (South Italy): Algorithm Validation and 2015–2017 Prevalence. International Journal of Environmental Research and Public Health. 2020; 17(10):3388. https://doi.org/10.3390/ijerph17103388
Chicago/Turabian StyleMoccia, Marcello, Vincenzo Brescia Morra, Roberta Lanzillo, Ilaria Loperto, Roberta Giordana, Maria Grazia Fumo, Martina Petruzzo, Nicola Capasso, Maria Triassi, Maria Pia Sormani, and et al. 2020. "Multiple Sclerosis in the Campania Region (South Italy): Algorithm Validation and 2015–2017 Prevalence" International Journal of Environmental Research and Public Health 17, no. 10: 3388. https://doi.org/10.3390/ijerph17103388
APA StyleMoccia, M., Brescia Morra, V., Lanzillo, R., Loperto, I., Giordana, R., Fumo, M. G., Petruzzo, M., Capasso, N., Triassi, M., Sormani, M. P., & Palladino, R. (2020). Multiple Sclerosis in the Campania Region (South Italy): Algorithm Validation and 2015–2017 Prevalence. International Journal of Environmental Research and Public Health, 17(10), 3388. https://doi.org/10.3390/ijerph17103388





