Adsorption of Lead (II) from Aqueous Solution with High Efficiency by Hydrothermal Biochar Derived from Honey
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Materials
2.2. Preparation of HHTB
2.3. Characterization of HHTB
2.4. Batch Adsorption
3. Results and Discussion
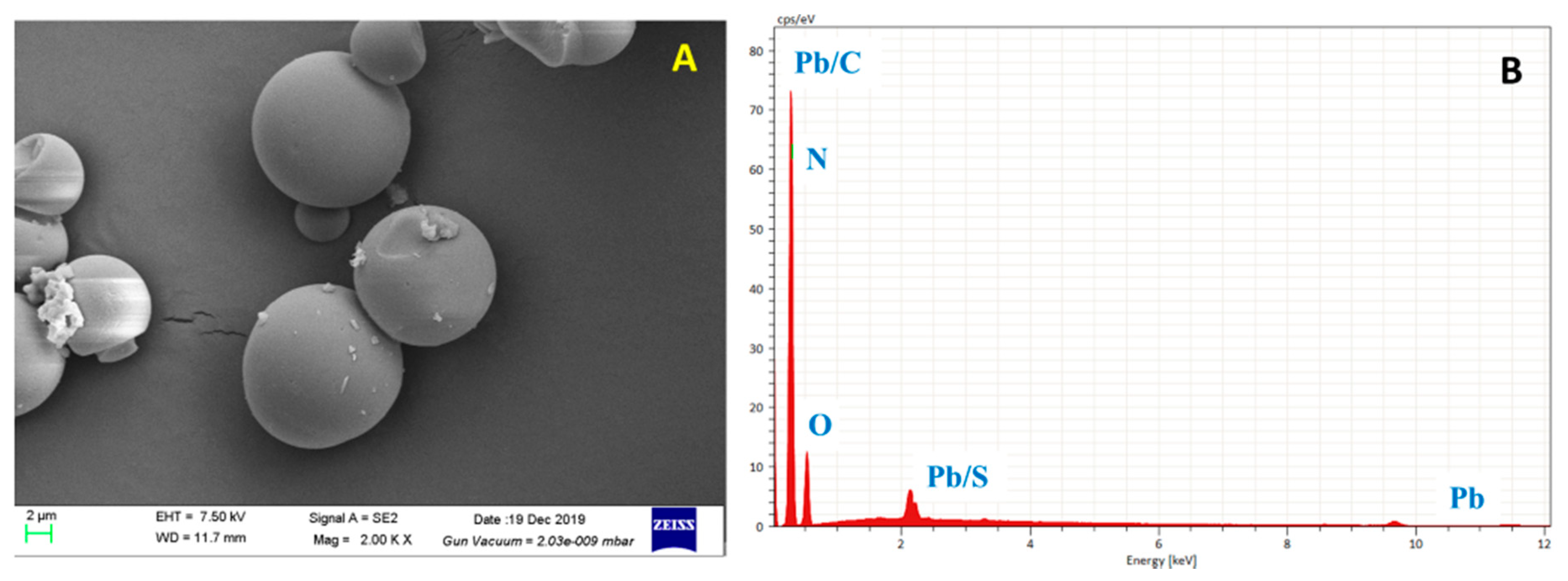
3.1. Characterization of HHTB
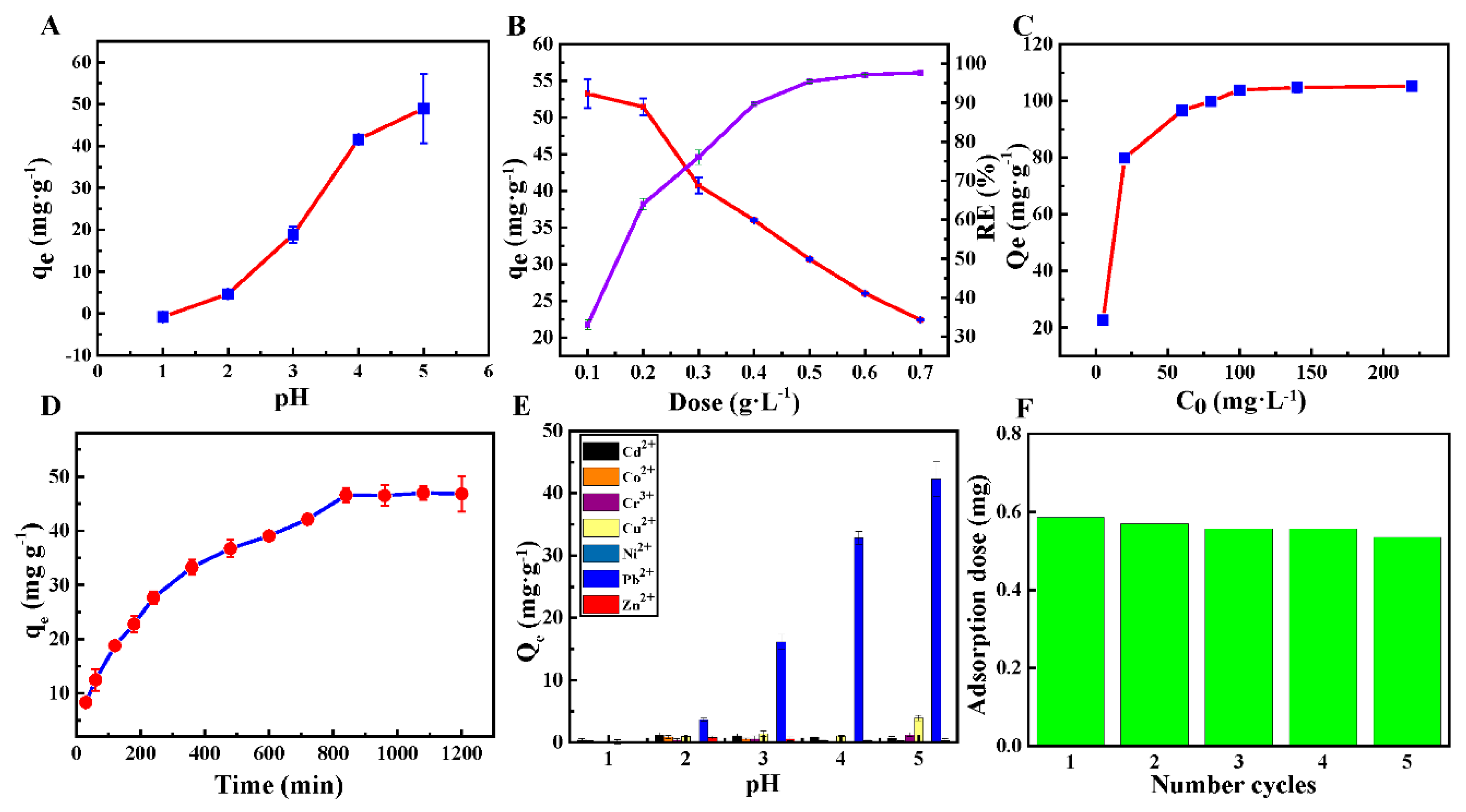
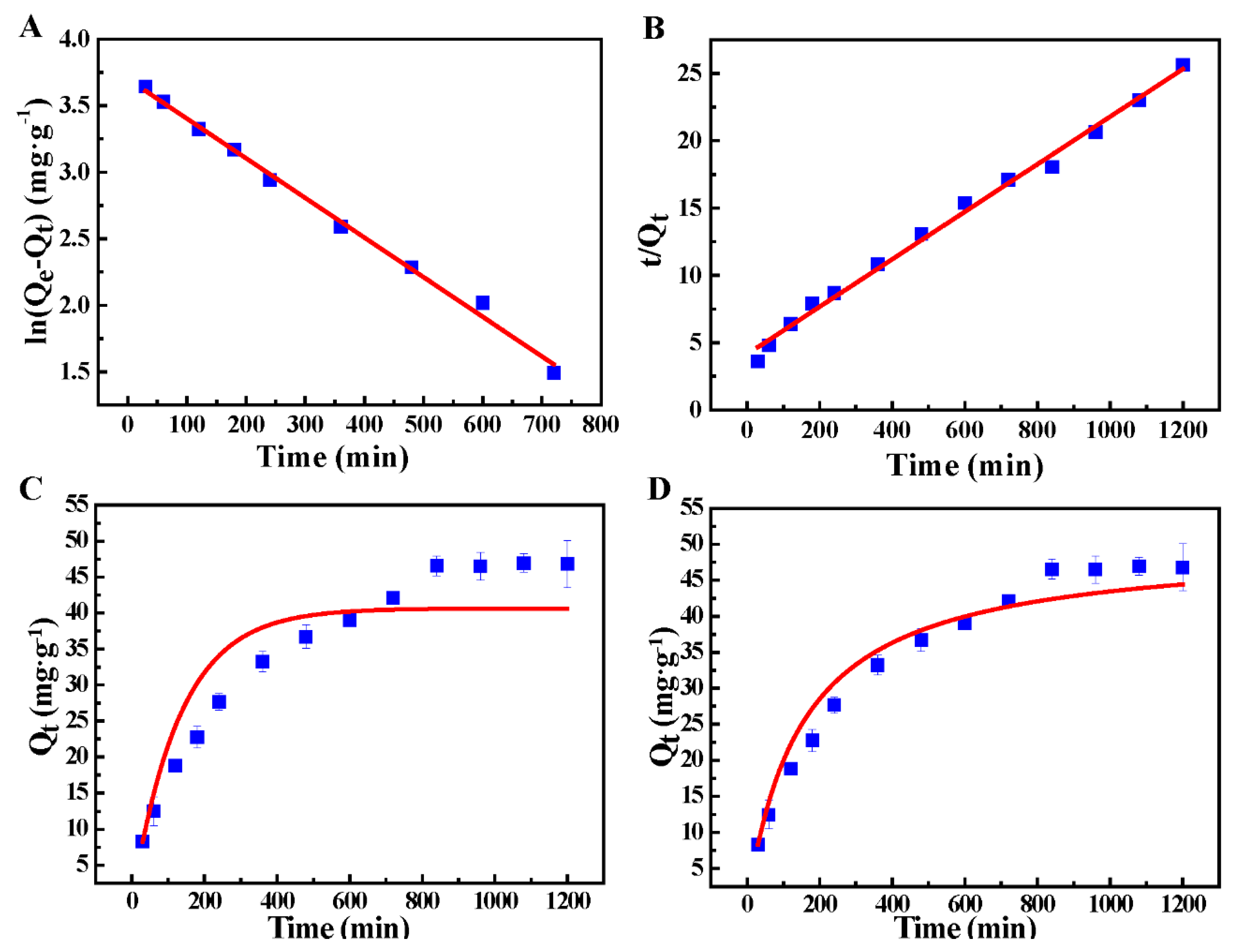
3.2. Bath Adsorption
3.3. Thermodynamic Analysis
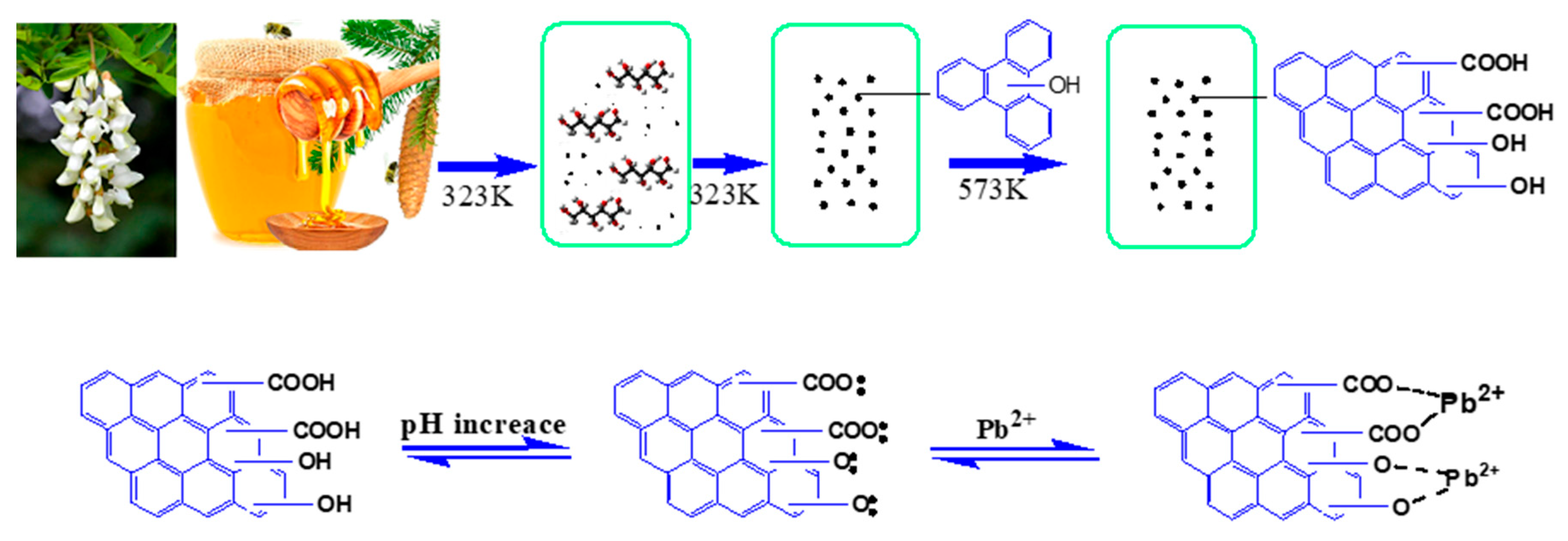
3.4. Preparation and Adsorption Mechanisms
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xue, S.G.; Shi, L.Z.; Wu, C.; Wu, H.; Qin, Y.Y.; Pan, W.S.; Hartley, W.; Cui, M.Q. Cadmium, lead, and arsenic contamination in paddy soils of a mining area and their exposure effects on human HEPG2 and keratinocyte cell-lines. Environ. Res. 2017, 156, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Saha, N.; Rahman, M.S.; Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.S. Industrial metal pollution in water and probabilistic assessment of human health risk. J. Environ. Manag. 2017, 185, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Z.; Singh, V.P. The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environ. Monit. Assess. 2019, 191, 21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Huo, X.; Xu, L.; Cheng, Z.H.; Cong, X.W.; Lu, X.L.; Xu, X.J. Elevated lead levels from e-waste exposure are linked to decreased olfactory memory in children. Environ. Pollut 2017, 231, 1112–1121. [Google Scholar] [CrossRef]
- Zeng, G.; Wan, J.; Huang, D.; Hu, L.; Huang, C.; Cheng, M.; Xue, W.; Gong, X.; Wang, R.; Jiang, D. Precipitation, adsorption and rhizosphere effect: The mechanisms for Phosphate-induced Pb immobilization in soils-A review. J. Hazard. Mater. 2017, 339, 354–367. [Google Scholar] [CrossRef]
- Moosavirad, S.M.; Sarikhani, R.; Mohammadi, S.Z. Removal of Some Heavy Metals from Inorganic Industrial Wastewaters by Ion Exchange Method (vol 37, pg 191, 2015). J. Water Chem. Technol. 2015, 37, 264. [Google Scholar] [CrossRef] [Green Version]
- Abou-Shady, A.; Peng, C.; Almeria, O.J.; Xu, H. Effect of pH on separation of Pb (II) and NO3− from aqueous solutions using electrodialysis. Desalination 2012, 285, 46–53. [Google Scholar] [CrossRef]
- Lu, K.P.; Yang, X.; Gielen, G.; Bolan, N.; Ok, Y.S.; Niazi, N.K.; Xu, S.; Yuan, G.D.; Chen, X.; Zhang, X.K.; et al. Effect of bamboo and rice straw biochars on the mobility and redistribution of heavy metals (Cd, Cu, Pb and Zn) in contaminated soil. J. Environ. Manag. 2017, 186, 285–292. [Google Scholar] [CrossRef]
- Chen, H.; Wang, X.X.; Li, J.X.; Wang, X.K. Cotton derived carbonaceous aerogels for the efficient removal of organic pollutants and heavy metal ions. J. Mater. Chem. A 2015, 3, 6073–6081. [Google Scholar] [CrossRef]
- Filote, C.; Volf, I.; Santos, S.C.R.; Botelho, C.M.S. Bioadsorptive removal of Pb(II) from aqueous solution by the biorefinery waste of Fucus spiralis. Sci. Total Environ. 2019, 648, 1201–1209. [Google Scholar] [CrossRef]
- Thitame, P.V.; Shukla, S.R. Removal of lead (II) from synthetic solution and industry wastewater using almond shell activated carbon. Environ. Prog. Sustain. Energy 2017, 36, 1628–1633. [Google Scholar] [CrossRef]
- Zhao, D.D.; Yu, Y.; Chen, J.P. Treatment of lead contaminated water by a PVDF membrane that is modified by zirconium, phosphate and PVA. Water Res. 2016, 101, 564–573. [Google Scholar] [CrossRef]
- Teh, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent Advancement of Coagulation-Flocculation and Its Application in Wastewater Treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Ma, Y.; Lv, L.; Guo, Y.; Fu, Y.; Shao, Q.; Wu, T.; Guo, S.; Sun, K.; Guo, X.; Wujcik, E.K.; et al. Porous lignin based poly (acrylic acid)/organo-montmorillonite nanocomposites: Swelling behaviors and rapid removal of Pb (II) ions. Polymer 2017, 128, 12–23. [Google Scholar] [CrossRef]
- Gu, P.C.; Xing, J.L.; Wen, T.; Zhang, R.; Wang, J.; Zhao, G.X.; Hayat, T.; Ai, Y.J.; Lin, Z.; Wang, X.K. Experimental and theoretical calculation investigation on efficient Pb(II) adsorption on etched Ti3AlC2 nanofibers and nanosheets. Environ.-Sci. Nano 2018, 5, 946–955. [Google Scholar] [CrossRef]
- Awual, M.R. Innovative composite material for efficient and highly selective Pb(II) ion capturing from wastewater. J. Mol. Liq. 2019, 284, 502–510. [Google Scholar] [CrossRef]
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol. 2016, 46, 406–433. [Google Scholar] [CrossRef]
- Liao, H.; Yu, J.; Zhu, W.; Kuang, M.; Duan, T.; Zhang, Y.; Lin, X.; Luo, X.; Zhou, J. Nano-zero-valent Fe/Ni particles loaded on collagen fibers immobilized by bayberry tannin as an effective reductant for uranyl in aqueous solutions. Appl. Surf. Sci. 2020, 507. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Zhu, Z.Y.; Shen, B.X.; Liu, L.N. Insights into biochar and hydrochar production and applications: A review. Energy 2019, 171, 581–598. [Google Scholar] [CrossRef]
- Li, B.; Yang, L.; Wang, C.Q.; Zhang, Q.P.; Liu, Q.C.; Li, Y.D.; Xiao, R. Adsorption of Cd(II) from aqueous solutions by rape straw biochar derived from different modification processes. Chemosphere 2017, 175, 332–340. [Google Scholar] [CrossRef]
- Laili, Z.; Yasir, M.S.; Yusof, M.A.W. Influence of Water-to-Cement Ratio on the Compressive Strength of Cement-Biochar-Spent Ion Exchange Resins Matrix. Sains Malays. 2017, 46, 1617–1623. [Google Scholar] [CrossRef]
- Wen, G.D.; Wang, B.L.; Wang, C.X.; Wang, J.; Tian, Z.J.; Schlogl, R.; Su, D.S. Hydrothermal Carbon Enriched with Oxygenated Groups from Bio-mass Glucose as an Efficient Carbocatalyst. Angew. Chem. Int. Ed. 2017, 56, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.B.; Liu, Y.C.; Xie, Z.L. Biomass derived 2D carbons via a hydrothermal carbonization method as efficient bifunctional ORR/HER electrocatalysts. J. Mater. Chem. A 2017, 5, 23481–23488. [Google Scholar] [CrossRef]
- Huynh, N.T.; Smagghe, G.; Gonzales, G.B.; Van Camp, J.; Raes, K. Extraction and bioconversion of kaempferol metabolites from cauliflower outer leaves through fungal fermentation. Biochem. Eng. J. 2016, 116, 27–33. [Google Scholar] [CrossRef]
- Branca, C.; Di Blasi, C. A lumped kinetic model for banana peel combustion. Thermochim. Acta 2015, 614, 68–75. [Google Scholar] [CrossRef]
- Leng, L.J.; Huang, H.J.; Li, H.; Li, J.; Zhou, W.G. Biochar stability assessment methods: A review. Sci. Total Environ. 2019, 647, 210–222. [Google Scholar] [CrossRef]
- Cha, J.S.; Park, S.H.; Jung, S.-C.; Ryu, C.; Jeon, J.-K.; Shin, M.-C.; Park, Y.-K. Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Bartoli, M.; Giorcelli, M.; Jagdale, P.; Rovere, M.; Tagliaferro, A. A Review of Non-Soil Biochar Applications. Materials 2020, 13, 261. [Google Scholar] [CrossRef] [Green Version]
- Zhong, C.; Wei, X. A comparative experimental study on the liquefaction of wood. Energy 2004, 29, 1731–1741. [Google Scholar] [CrossRef]
- Jiang, T.Y.; Jiang, J.; Xu, R.K.; Li, Z. Adsorption of Pb(II) on variable charge soils amended with rice-straw derived biochar. Chemosphere 2012, 89, 249–256. [Google Scholar] [CrossRef]
- Xu, X.; Cao, X.; Zhao, L.; Zhou, H.; Luo, Q. Interaction of organic and inorganic fractions of biochar with Pb(ii) ion: Further elucidation of mechanisms for Pb(ii) removal by biochar. RSC Adv. 2014, 4, 44930–44937. [Google Scholar] [CrossRef]
- Prabhu, S.M.; Meenakshi, S. Enriched fluoride sorption using chitosan supported mixed metal oxides beads: Synthesis, characterization and mechanism. J. Water Process. Eng. 2014, 2, 96–104. [Google Scholar] [CrossRef]
- Maliyekkal, S.M.; Lisha, K.P.; Pradeep, T. A novel cellulose–manganese oxide hybrid material by in situ soft chemical synthesis and its application for the removal of Pb (II) from water. J. Hazard. Mater. 2010, 181, 986–995. [Google Scholar] [CrossRef]
- Zhang, S.J.; Li, X.Y.; Chen, J.P. An XPS study for mechanisms of arsenate adsorption onto a magnetite-doped activated carbon fiber. J. Colloid Interface Sci. 2010, 343, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Li, X.; Liu, Y.; Zeng, G.; Liang, J.; Song, B.; Wei, X. Alginate-modified biochar derived from Ca(II)-impregnated biomass: Excellent anti-interference ability for Pb(II) removal. Ecotoxicol. Environ. Saf. 2018, 165, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liang, W.; Wang, J.J.; Gaston, L.A.; Huang, D.; Huang, H.; Lei, S.; Awasthi, M.K.; Zhou, B.; Xiao, R.; et al. Facilitative capture of As(V), Pb(II) and methylene blue from aqueous solutions with MgO hybrid sponge-like carbonaceous composite derived from sugarcane leafy trash. J. Environ. Manag. 2018, 212, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Lu, X.; Li, X.-Y. Selective removals of heavy metals (Pb2+, Cu2+, and Cd2+) from wastewater by gelation with alginate for effective metal recovery. J. Hazard. Mater. 2016, 308, 75–83. [Google Scholar] [CrossRef]
- Pam, A.A.; Abdullah, A.H.; Ping, T.Y.; Zainal, Z. Batch and Fixed Bed Adsorption of Pb(II) from Aqueous Solution using EDTA Modified Activated Carbon Derived from Palm Kernel Shell. BioResources 2018, 13, 1235–1250. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Li, J.; Lan, T.; Muller, K.; Niazi, N.K.; Chen, X.; Xu, S.; Zheng, L.; Chu, Y.; Li, J.; et al. Unraveling sorption of lead in aqueous solutions by chemically modified biochar derived from coconut fiber: A microscopic and spectroscopic investigation. Sci. Total Environ. 2017, 576, 766–774. [Google Scholar] [CrossRef]
- Martín-Lara, M.A.; Blázquez, G.; Calero, M.; Almendros, A.I.; Ronda, A. Binary biosorption of copper and lead onto pine cone shell in batch reactors and in fixed bed columns. Int. J. Mineral. Process. 2016, 148, 72–82. [Google Scholar] [CrossRef]
- Sahraei, R.; Sekhavat Pour, Z.; Ghaemy, M. Novel magnetic bio-sorbent hydrogel beads based on modified gum tragacanth/graphene oxide: Removal of heavy metals and dyes from water. J. Clean Prod. 2017, 142, 2973–2984. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Krukowska, J.; Thomas, P. Comparison of sorption and desorption studies of heavy metal ions from biochar and commercial active carbon. Chem. Eng. J. 2017, 307, 353–363. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffe. K. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y. McKay GPseudo-second order model for sorption processes. Process. Biochem 1999, 34, 451l465. [Google Scholar] [CrossRef]
- Yu, J.; Liao, H.; Zhu, W.; Duan, T.; Wang, S.; Kuang, M.; Zhang, Y.; Lin, X.; Luo, X.; Zhou, J. Marinobacter sp. Stable Hydrous Titanium Oxide-Functionalized Bovine Serum Albumin Nanospheres for Uranium Capture from Spiked Seawater. ACS Appl. Mater. Interfaces 2019, 11, 40898–40908. [Google Scholar] [CrossRef] [PubMed]


| Biomass Material | pH | T (K) | Q max (mg·g−1) | Literature |
|---|---|---|---|---|
| Palm Kernel Shell | 4.0 | 298 | 104.0 | [38] |
| HHTB | 5.0 | 328 | 107.17 | This article |
| Coconut fiber | — | 573 | 105.5 | [39] |
| Pine cone shell | 5.0 | 298 | 17.41 | [40] |
| Biopolymer GT | 5.0 | 298 | 101.74 | [41] |
| Biochar (Coaltec Energy, USA Inc) | 5.0 | 295 | 37.80 | [42] |
| Type | T / K | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|
| Qm/(mg·g−1) | KL/(L·mg−1) | R2 | Kf | n | R2 | ||
| Linear | 288 | 49.2368 | 3.7963 | 0.7525 | 28.1582 | 6.5249 | 0.7015 |
| 298 | 75.1880 | 3.2282 | 0.9762 | 35.9346 | 5.1562 | 0.9608 | |
| 308 | 82.9187 | 3.3972 | 0.9557 | 37.8413 | 4.7746 | 0.9233 | |
| 318 | 94.6074 | 4.4042 | 0.9897 | 44.1521 | 4.7540 | 0.9453 | |
| 328 | 107.1711 | 5.6545 | 0.9848 | 49.4781 | 4.7337 | 0.9210 | |
| Nonlinear | 288 | 53.5934 | 3.0680 | 0.3709 | 26.6208 | 5.7407 | 0.6375 |
| 298 | 77.7247 | 2.5439 | 0.8770 | 39.9690 | 6.1060 | 0.9447 | |
| 308 | 96.4243 | 0.3264 | 0.6488 | 38.4166 | 4.8303 | 0.8643 | |
| 318 | 105.6159 | 0.7035 | 0.8745 | 50.3250 | 5.8075 | 0.9467 | |
| 328 | 117.6933 | 0.8858 | 0.7989 | 55.6299 | 5.6401 | 0.8985 | |
| Type | Lagrangian Quasi-First-Order Kinetic Constant | Lagrangian Quasi-Secondary Kinetic Constant | ||||
|---|---|---|---|---|---|---|
| K1 (min−1) | Qe (mg·g−1) | R2 | K2 (g·mg−1·min−1) | Qe (mg·g−1) | R2 | |
| Linear | 0.0030 | 40.4368 | 0.9946 | 0.0177 | 56.6251 | 0.9933 |
| Nonlinear | 0.0076 | 40.6446 | 0.9685 | 1.3272 | 50.0091 | 0.9898 |
| T/K | ΔH0 (kJ·mol−1) | ΔG0 (kJ·mol− 1) | ΔS0 (J·mol−1· K−1) |
|---|---|---|---|
| 288 | 14.1578 | −9.3302 | 0.0824 |
| 298 | −10.7031 | ||
| 308 | −11.3129 | ||
| 318 | −12.0288 | ||
| 328 | −12.7474 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Yu, J.; Liao, H.; Zhu, W.; Ding, P.; Zhou, J. Adsorption of Lead (II) from Aqueous Solution with High Efficiency by Hydrothermal Biochar Derived from Honey. Int. J. Environ. Res. Public Health 2020, 17, 3441. https://doi.org/10.3390/ijerph17103441
Wang B, Yu J, Liao H, Zhu W, Ding P, Zhou J. Adsorption of Lead (II) from Aqueous Solution with High Efficiency by Hydrothermal Biochar Derived from Honey. International Journal of Environmental Research and Public Health. 2020; 17(10):3441. https://doi.org/10.3390/ijerph17103441
Chicago/Turabian StyleWang, Bo, Jie Yu, Hui Liao, Wenkun Zhu, Pingping Ding, and Jian Zhou. 2020. "Adsorption of Lead (II) from Aqueous Solution with High Efficiency by Hydrothermal Biochar Derived from Honey" International Journal of Environmental Research and Public Health 17, no. 10: 3441. https://doi.org/10.3390/ijerph17103441




