Response of Scenedesmus quadricauda (Chlorophyceae) to Salt Stress Considering Nutrient Enrichment and Intracellular Proline Accumulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Sample Preparation
2.2. Experimental Setup
2.3. Determination of Algal Growth Rate
2.4. Proline Analysis
2.5. Data Analyses
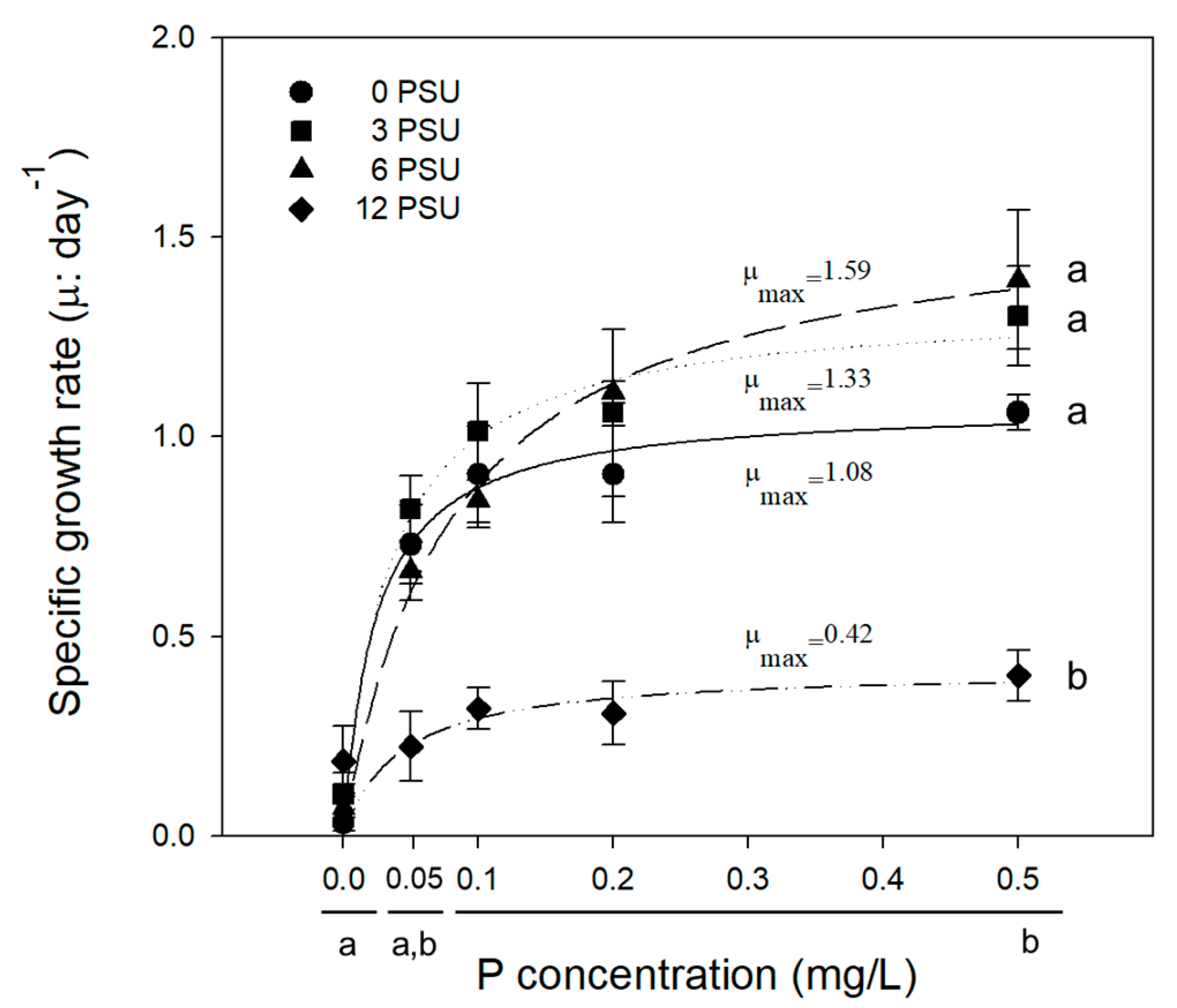
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nielsen, D.L.; Brock, M.A.; Rees, G.N.; Baldwin, D.S. Effects of increasing salinity on freshwater ecosystems in Australia. Aust. J. Bot. 2003, 51, 655–665. [Google Scholar] [CrossRef]
- Velasco, J.; Gutiérrez-Cánovas, C.; Botella-Cruz, M.; Sánchez-Fernández, D.; Arribas, P.; Carbonell, J.A.; Millán, A.; Pallarés, S. Effects of salinity changes on aquatic organisms in a multiple stressor context. Phil. Trans. R. Soc. B 2019, 374, 20180011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, D.L.; Brock, M.A.; Crosslé, K.; Harris, K.; Healey, M.; Jarosinski, I. The effects of salinity on aquatic plant germination and zooplankton hatching from two wetland sediments. Freshwater Biol. 2003, 48, 2214–2223. [Google Scholar] [CrossRef] [Green Version]
- Hintz, W.D.; Relyea, R.A. A salty landscape of fear: Responses of fish and zooplankton to freshwater salinization and predatory stress. Oecologia 2017, 185, 147–156. [Google Scholar] [CrossRef]
- Moisnder, P.H.; McClinton, E.; Paer, H.W. Salinity effects on growth, photosynthetic parameters, and nitrogenase activity in estuarine planktonic cyanobacteria. Microb. Ecol. 2002, 43, 432–442. [Google Scholar] [CrossRef]
- Lartigue, J.; Neill, A.; Hayden, B.L.; Pulfer, J.; Cebrian, J. The impact of salinity fluctuations on net oxygen production and inorganic nitrogen uptake by Ulva lactuca (Chlorophyceae). Aquat. Bot. 2003, 75, 339–350. [Google Scholar] [CrossRef]
- Demetriou, G.; Neonaki, C.; Navakoudis, E.; Kotzabasis, K. Salt stress impact on the molecular structure and function of the photosynthetic apparatus – the protective role of polyamines. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Affenzeller, M.J.; Darehshouri, A.; Andosch, A.; Lutz, C.; Lutz-Meindl, U. Salt stress-induced cell death in the unicellular green alga Micrasterias denticulata. J. Exp. Bot. 2009, 60, 939–954. [Google Scholar] [CrossRef] [Green Version]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef] [Green Version]
- Krell, A.; Funck, D.; Plettner, I.; John, U.; Dieckmann, G. Regulation of proline metabolism under salt stress in the psychrophilic diatom Fragilariopsis cylindrus (Bacillariophyceae). J. Phycol. 2007, 43, 753–762. [Google Scholar] [CrossRef] [Green Version]
- Szabados, L.; Savouré, A. Proline: a multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Matloob, A.; Khan, F.A.; Khaliq, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.; Ullah, N.; et al. Phytohormones and plant responses to salinity stress: a review. Plant Growth Regul. 2015, 75, 391–404. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, J.; Singh, V.P.; Prasad, S.M. Proline and salinity tolerance in plants. Biochem. Pharmacol. 2014, 3, e170. [Google Scholar] [CrossRef] [Green Version]
- Yildiz, M.; Terz, H. Effect of NaCl stress on chlorophyll biosynthesis, proline, lipid peroxidation and antioxidative enzymes in leaves of salt-tolerant and salt sensitive barley cultivars. J. Agric. Sci. 2013, 19, 79–88. [Google Scholar]
- Iqbala, N.; Umara, S.; Khan, N.A. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J. Plant Physiol. 2015, 178, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Delauney, A.; Verma, D.P.S. Proline biosynthesis and osmoregulation in plants. Plant J. 1993, 4, 215–223. [Google Scholar] [CrossRef]
- Jain, M.; Mathur, G.; Koul, S.; Sarin, N.B. Ameliorative effects of proline on salt stress-induced lipid peroxidation in cell lines of groundnut (Arachis hypogea L.). Plant Cell Rep. 2001, 20, 463–468. [Google Scholar] [CrossRef]
- Mbarki, S.; Sytar, O.; Zivcak, M.; Abdelly, C.; Cerda, A.; Brestic, M. Anthocyanins of coloured wheat genotypes in specific response to salt stress. Molecules 2018, 23, 1518. [Google Scholar] [CrossRef] [Green Version]
- Mbarki, S.; Sytar, O.; Cerda, A.; Zivcak, M.; Rastogi, A.; He, X.; Zoghlami, A.; Abdelly, C.; Brestic, M. Strategies to mitigate the salt stress effects on photosynthetic apparatus and productivity of crop plants. In Salinity Responses and Tolerance in Plants, Volume 1; Kumar, V., Wani, S., Suprasanna, P., Tran, L.S., Eds.; Springer: Cham, Switzerland, 2018; Chapter 4; pp. 85–136. [Google Scholar]
- Elser, J.J.; Marzolf, E.R.; Goldman, C.R. Phosphorus and nitrogen limitation of phytoplankton growth in freshwaters of North America: a review and critique of experimental enrichments. Can. J. Fish. Aquat. Sci. 1990, 47, 1468–1477. [Google Scholar] [CrossRef]
- Rai, A.K.; Sharma, N.K. Phosphate metabolism in the cyanobacterium Anabaena doliolum under salt stress. Curr. Microbiol. 2006, 52, 6–12. [Google Scholar] [CrossRef]
- Smith, V. Effects of eutrophication on maximum algal biomass in lake and river ecosystems. Inland Waters 2016, 6, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Shi, X.; Yang, Z.; Yu, Y.; Shi, L.; Qin, B. Long-term dynamics and drivers of phytoplankton biomass in eutrophic Lake Taihu. Sci. Total Environ. 2018, 645, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Herbst, D.B.; Bradley, T.J. Salinity and nutrient limitations on growth of benthic algae from two alkaline salt lakes of the western great basin (USA). J. Phycol. 1989, 25, 673–678. [Google Scholar] [CrossRef]
- Mohapatra, P.K.; Mohanty, R.C. Differential effect of dimethoate toxicity to Anabaena doliolum with change in nutrient status. Bull. Environ. Contam. Toxicol. 1992, 48, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, P.K.; Dash, R.C.; Panda, S.S.; Mishra, R.K.; Mohanty, R.C. Effects of nutrients at different salinities on growth of the freshwater green alga Scenedesmus bijugatus in water of Narendra Pond, Puri, Orissa. Internat. Rev. Hvdrobiol. 1998, 83, 297–304. [Google Scholar] [CrossRef]
- Larson, C.A.; Belovsky, G.E. Salinity and nutrients influence species richness and evenness of phytoplankton communities in microcosm experiments from Great Salt Lake, Utah, USA. J. Plankton Res. 2013, 35, 1154–1166. [Google Scholar] [CrossRef]
- Dash, R.C.; Mohapatra, P.K.; Mohanty, R.C. Salt induced changes in the growth of Chlorococcum humicolo and Scenedesmus bijugatus under nutrient limited cultures. Bull. Environ. Contam. Toxicol. 1995, 54, 695–702. [Google Scholar] [CrossRef]
- Chia, A.M.; Musa, I.R. Effect of indigo dye effluent on the growth, biomass production and phenotypic plasticity of Scenedesmus quadricauda (Chlorococcales). Anais Acad. Bras. Ciênc. 2014, 86, 419–428. [Google Scholar] [CrossRef] [Green Version]
- Choi, C.H.; Jung, S.W.; Yun, S.M.; Kim, S.H.; Park, J.G. Changes in phytoplankton communities and environmental factors in Saemangeum artificial lake, South Korea between 2006 and 2009. Korean J. Environ. Biol. 2013, 31, 213–224. [Google Scholar] [CrossRef]
- An, S.S.; Friedl, T.; Hegewald, E. Phylogenetic relationships of Scenedesmus and Scenedesmus-like coccoid green algae as inferred from ITS-2 rDNA sequence comparisons. Plant Biol. 1999, 1, 418–428. [Google Scholar] [CrossRef]
- Panigrahi, S.; Wikner, J.; Panigrahy, R.C.; Satapathy, K.K.; Acharya, B.C. Variability of nutrients and phytoplankton biomass in a shallow brackish water ecosystem (Chilika Lagoon, India). Limnology 2009, 10, 73–85. [Google Scholar] [CrossRef]
- Lee, J.K.; Ahn, T.W.; Oh, J.M. A study on the influence of water quality on the phosphorus fraction properties from reservoir sediments. J. Korean Soc. Environ. Eng. 2010, 32, 840–850. [Google Scholar]
- Andersen, R.A.; Kawachi, M. Traditional microalgae isolation techniques. In Algal Culturing Techniques; Andersen, R., Ed.; Phycological Society of America; Elsevier Academic Press: San Diego, CA, USA, 2005; Chapter 6; pp. 83–100. [Google Scholar]
- Allen, M.M. Simple conditions for growth of unicellular blue-green algae on plates. J. Phycol. 1968, 4, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Krzemińska, I.; Pawlik-Skowrońska, B.; Trzcińska, M.; Tys, J. Influence of photoperiods on the growth rate and biomass productivity of green microalgae. Bioprocess Biosyst. Eng. 2014, 37, 735–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [Green Version]
- Kessler, E. Mass culture of Chlorella strains under conditions of high salinity, acidity and temperature. Arch. Hydrobiol. Suppl. 1980, 60, 80–86. [Google Scholar]
- Fedina, I.S.; Benderliev, K.M. Response of Scenedesmus incrassatulus to salt stress as affected by methyl jasmonate. Biol. Plant 2000, 43, 625–627. [Google Scholar] [CrossRef]
- Meena, M.; Divyanshu, K.; Kumar, S.; Swapnil, P.; Zehra, A.; Shukla, V.; Yadav, M.; Upadhyay, R.S. Regulation of L-proline biosynthesis, signal transduction, transport, accumulation and its vital role in plants during variable environmental conditions. Heliyon 2019, 5, e02951. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, A.M.M.; De Figueiredo, D.; Pereira, M.J. The effects of different salinity concentrations on growth of three freshwater green algae. Fresenius Environ. Bull. 2006, 15, 1382–1386. [Google Scholar]
- Shetty, P.; Gitau, M.M.; Maróti, G. Salinity stress responses and adaptation mechanisms in eukaryotic green microalgae. Cells 2019, 8, 1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshiba, Y.; Kiyosue, T.; Katagiri, T.; Ueda, H.; Mizoguchi, T.; Yamaguchi-Shinozaki, K.; Wada, K.; Harada, Y.; Shinozaki, K. Correlation between the induction of a gene for delta 1-pyrroline-5-carboxylate synthetase and the accumulation of proline in Arabidopsis thaliana under osmotic stress. Plant J. 1995, 7, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Dubey, R.S. Effect of cadmium on proline accumulation and ribonuclease activity in rice seedlings: role of proline as a possible enzyme protectant. Biol. Plant 1998, 40, 121–130. [Google Scholar] [CrossRef]
- Rai, A.K.; Abraham, G. Relationship of combined nitrogen sources to salt tolerance in freshwater cyanobacterium Anabaena doliolum. J. Appl. Microbiol. 1995, 78, 501–506. [Google Scholar]
- Senthilkumar, T.; Jeyachandran, S. Effect of salinity stress on the marine cyanobacterium Oscillatoria acuminata Gomont with reference to proline accumulation. Seaweed Res. Utiln. 2006, 28, 99–104. [Google Scholar]
- Saygideger, S.; Deniz, F. Effect of 24-epibrassinolide on biomass, growth and free proline concentration in Spirulina platensis (Cyanophyta) under NaCl stress. Plant Growth Regul. 2008, 56, 219–223. [Google Scholar] [CrossRef]



| Experimental Condition | N:P Ratio | N (mg/L) | P (mg/L) |
|---|---|---|---|
| 1 | 2 | 1.0 | 0.5 |
| 5 | 0.2 | ||
| 10 | 0.1 | ||
| 20 | 0.05 | ||
| 2 | 2 | 0.1 | 0.05 |
| 5 | 0.25 | ||
| 10 | 0.5 | ||
| 20 | 1.0 | ||
| 3 | 2 | 1.0 | 0.5 |
| 5 | 2.5 | ||
| 10 | 5.0 | ||
| 20 | 10.0 |
| Class | Species | Source | Proline (pg/Cell) 1 | Salinity (psu) 2 | Major Treatment |
|---|---|---|---|---|---|
| Chlorophyceae | Scenedesmus bijugatus | [26] | 10.20–16.10 | 0.2–13.6 | Nutrient enrichment (NO3−, HPO42− and SiO32−) |
| Scenedesmus incrassatulus | [40] | 0.56–0.78 | 10.5 | Light and dark | |
| Scenedesmusquadricauda | This study (Exp. 1) | 0.18–1.79 | 0–12 | P addition | |
| Scenedesmusquadricauda | This study (Exp. 2) | 0.21–2.27 | 0–12 | Low N addition | |
| Scenedesmusquadricauda | This study (Exp. 3) | 0.06–0.73 | 0–12 | High N addition | |
| Cyanophyceae | Oscillatoria acuminata | [47] | 7.20–14.16 | 5–40 | Different salinities |
| Spirulina platensis | [48] | 3.44 –4.64 | 0–12 | 24-epiBL addition 3 | |
| Bacillariophyceae | Fragilariopsis cylindrus | [10] | 1.57–1.76 | 70 | Hyper-salinity |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.-H.; Park, C.-H.; Sim, Y.B.; Hwang, S.-J. Response of Scenedesmus quadricauda (Chlorophyceae) to Salt Stress Considering Nutrient Enrichment and Intracellular Proline Accumulation. Int. J. Environ. Res. Public Health 2020, 17, 3624. https://doi.org/10.3390/ijerph17103624
Park M-H, Park C-H, Sim YB, Hwang S-J. Response of Scenedesmus quadricauda (Chlorophyceae) to Salt Stress Considering Nutrient Enrichment and Intracellular Proline Accumulation. International Journal of Environmental Research and Public Health. 2020; 17(10):3624. https://doi.org/10.3390/ijerph17103624
Chicago/Turabian StylePark, Myung-Hwan, Chae-Hong Park, Yeon Bo Sim, and Soon-Jin Hwang. 2020. "Response of Scenedesmus quadricauda (Chlorophyceae) to Salt Stress Considering Nutrient Enrichment and Intracellular Proline Accumulation" International Journal of Environmental Research and Public Health 17, no. 10: 3624. https://doi.org/10.3390/ijerph17103624
APA StylePark, M.-H., Park, C.-H., Sim, Y. B., & Hwang, S.-J. (2020). Response of Scenedesmus quadricauda (Chlorophyceae) to Salt Stress Considering Nutrient Enrichment and Intracellular Proline Accumulation. International Journal of Environmental Research and Public Health, 17(10), 3624. https://doi.org/10.3390/ijerph17103624






