Low-Intensity Physical Exercise Improves Pain Catastrophizing and Other Psychological and Physical Aspects in Women with Fibromyalgia: A Randomized Controlled Trial
Abstract
:1. Introduction
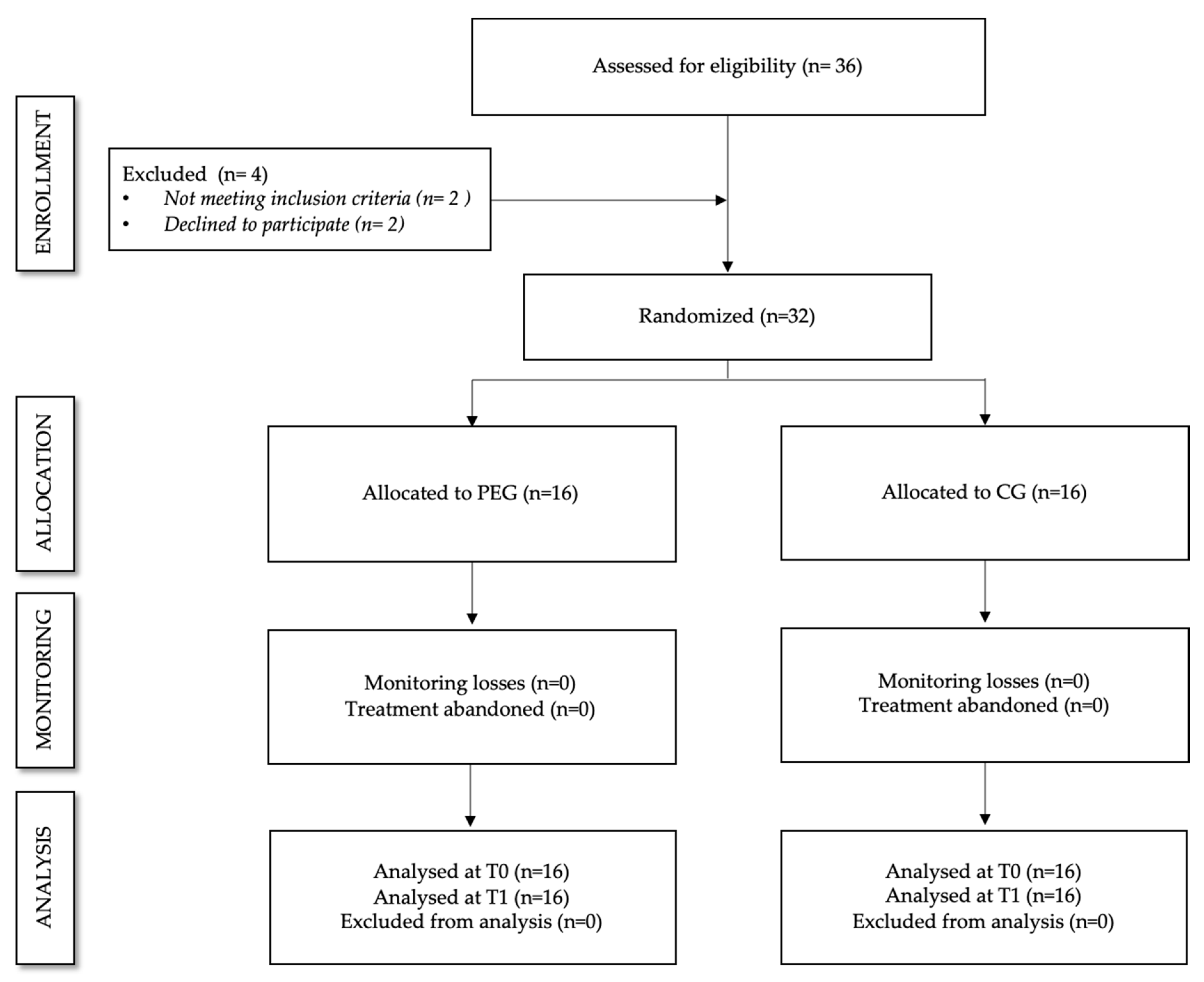
2. Materials and Methods
2.1. Participants
2.1.1. Study Design
2.1.2. Sample Size Calculation
2.1.3. Intervention Procedures
2.1.4. Low-Intensity Physical Exercise
2.1.5. Control Group
2.2. Assessments
2.2.1. Pain Catastrophizing
2.2.2. Anxiety
2.2.3. Depression
2.2.4. Stress
2.2.5. Perception of Pain
2.2.6. Quality of Life
2.2.7. Physical Conditioning
- Self-perceived functional capacity was assessed based on the “Physical Function” subscale of the FIQR (FIQR-PF). This subscale consists of nine items assessing the self-perceived abilities to perform daily living activities (e.g., walk for 20 min, climb one flight of stairs…). The maximal score is 30. The higher scores point to a poorer perception of physical function. It has shown a good reliability (ICC = 0.73) [57].
- Endurance and functional capacity were assessed by the six-minute walk test (6MWT). Participants walked down a 15-m long hallway for a total of six minutes. Any contra-indications were checked before the test started, so heart rate, oxygen level, and Borg Rate of Perceived fatigue were recorded besides the main variable, i.e., the walked distance. Patients were allowed to take as many standing rests as necessary, but the timer kept going. The instructions given to the patients were: “Walk to the turnaround point at each end. I am going to use this counter to keep track of the laps you complete. You may stand and rest, but you should walk as fast as you are able. Remember that the aim is to walk as far as possible, but do not run.” This test has shown an excellent reliability (ICC = 0.91) [58].
- Power was evaluated by the five-repetition sit-to-stand test (5STST) consisting of sitting down and standing up from an armless chair (43 cm high) five times as quickly as possible. Participants with arms crossed over their chest were instructed to stand up completely and make firm contact when sitting. Timing began at the command “ready-steady-go” and stopped when they sat after the fifth stand-up [59]. This test has shown an excellent reliability in adult women (ICC = 0.92) [60].Velocity was assessed by the Four-Meter Gait Speed Test (4mGST). The 4mGST consisted of walking a distance of 4 m at the usual pace. This test in addition to assessing the walking speed allows us to estimate the risk of disability for a given individual [61]. Both the test-retest and the inter-rater reliability have been shown to be excellent (ICC = 0.89 − 0.99 and ICC = 0.97, respectively) [62].
2.3. Statistics
3. Results
3.1. Participants
3.2. Intervenction Effects
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bidonde, J.; Busch, A.J.; Schachter, C.L.; Webber, S.-C.; Musselman, K.E.; Overend, T.J.; Boden, C. Mixed exercise training for adults with fibromyalgia. Cochrane Database Syst. Rev. 2019, 5, CD013340. [Google Scholar] [CrossRef] [PubMed]
- Schütze, R.; Rees, C.; Smith, A.; Slater, H.; O’Sullivan, P. Metacognition, perseverative thinking, and pain catastrophizing: A moderated-mediation analysis. Eur. J. Pain (United Kingdom) 2020, 24, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Protsenko, E.; Lazaridou, A.; Franceschelli, O.; Ellingsen, D.M.; Mawla, I.; Wasan, A.D. Encoding of Self-Referential Pain Catastrophizing in the Posterior Cingulate Cortex in Fibromyalgia. Arthritis Rheumatol. 2018, 70, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.R.; Calahan, C.; Mensing, G.; Smith, M.; Haythornthwaite, J.A. Pain, catastrophizing, and depression in the rheumatic diseases. Nat. Rev. Rheumatol. 2011, 7, 216–224. [Google Scholar] [CrossRef] [PubMed]
- McCracken, L.M.; Eccleston, C.; Bell, L. Clinical assessment of behavioral coping responses: Preliminary results from a brief inventory. Eur. J. Pain 2005, 9, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Lami, M.J.; Martínez, M.P.; Miró, E.; Sánchez, A.I.; Guzmán, M.A. Catastrophizing, Acceptance, and Coping as Mediators Between Pain and Emotional Distress and Disability in Fibromyalgia. J. Clin. Psychol. Med. Sett. 2018, 25, 80–92. [Google Scholar] [CrossRef]
- McCracken, L.M.; Vowles, K.E.; Eccleston, C. Accepctance of chronic pain: Component analysis and a revised assessment method. Pain 2004, 107, 159–166. [Google Scholar] [CrossRef]
- Bucourt, E.; Martaillé, V.; Goupille, P.; Joncker-Vannier, I.; Huttenberger, B.; Réveillère, C.; Mulleman, D. A Comparative Study of Fibromyalgia, Rheumatoid Arthritis, Spondyloarthritis, and Sjögren’s Syndrome; Impact of the Disease on Quality of Life, Psychological Adjustment, and Use of Coping Strategies. Pain Med. 2019. [Google Scholar] [CrossRef]
- Bernik, M.; Sampaio, T.P.A.; Gandarela, L. Fibromyalgia comorbid with anxiety disorders and depression: Combined medical and psychological treatment. Curr. Pain Headache Rep. 2013, 17, 358. [Google Scholar] [CrossRef]
- Dell’Osso, L.; Bazzichi, L.; Consoli, G.; Carmassi, C.; Carlini, M.; Massimetti, E.; Ciapparelli, A. Manic spectrum symptoms are correlated to the severity of pain and the health-related quality of life in patients with fibromyalgia. Clin. Exp. Rheumatol. 2009, 27, S57–S61. [Google Scholar]
- Giesecke, T.; Williams, D.A.; Harris, R.E.; Cupps, T.R.; Tian, X.; Tian, T.X.; Clauw, D.J. Subgrouping of Fibromyalgia Patients on the Basis of Pressure-Pain Thresholds and Psychological Factors. Arthritis Rheum. 2003, 48, 2916–2922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larivière, C.; Bilodeau, M.; Forget, R.; Vadeboncoeur, R.; Mecheri, H. Poor back muscle endurance is related to pain catastrophizing in patients with chronic low back pain. Spine (Phila Pa 1976) 2010, 35, E1178–E1186. [Google Scholar] [CrossRef]
- Campbell, C.M.; Edwards, R.R. Mind–body interactions in pain: the neurophysiology of anxious and catastrophic pain-related thoughts. Bone 2009, 153, 97–101. [Google Scholar] [CrossRef] [Green Version]
- Estévez-López, F.; Álvarez-Gallardo, I.C.; Segura-Jiménez, V.; Soriano-Maldonado, A.; Borges-Cosic, M.; Pulido-Martos, M.; Geenen, R. The discordance between subjectively and objectively measured physical function in women with fibromyalgia: Association with catastrophizing and self-efficacy cognitions. The al-Ándalus project. Disabil. Rehabil. 2018, 40, 329–337. [Google Scholar] [CrossRef]
- Sempere-Rubio, N.; Aguilar-Rodríguez, M.; Inglés, M.; Izquierdo-Alventosa, R.; Serra-Añó, P. Physical Condition Factors that Predict a Better Quality of Life in Women with Fibromyalgia. Int. J. Environ. Res. Public Health 2019, 16, 3173. [Google Scholar] [CrossRef] [Green Version]
- Mannerkorpi, K.; Gard, G. Hinders for continued work among persons with fibromyalgia. BMC Musculoskelet Disord. 2012, 13, 96. [Google Scholar] [CrossRef] [Green Version]
- Rivera, J.; Rejas, J.; Esteve-Vives, J.; Vallejo, M.A.; ICAF, G. Resource utilisation and health care costs in patients diagnosed with fibromyalgia in Spain. Clin. Exp. Rheumatol. 2009, 27, S39–S45. [Google Scholar]
- Taylor, S.J.; Steer, M.; Ashe, S.C.; Furness, P.J.; Haywood-Small, S.; Lawson, K. Patients’ perspective of the effectiveness and acceptability of pharmacological and non-pharmacological treatments of fibromyalgia. Scand. J. Pain 2019, 19, 167–181. [Google Scholar] [CrossRef] [Green Version]
- Kayo, A.H.; Peccin, M.S.; Sanches, C.M.; Trevisani, V.F.M. Effectiveness of physical activity in reducing pain in patients with fibromyalgia: A blinded randomized clinical trial. Rheumatol. Int. 2012, 32, 2285–2292. [Google Scholar] [CrossRef]
- Sañudo, B.; Galiano, D.; Carrasco, L.; Blagojevic, M.; Hoyo, M.; Saxton, J. Aerobic Exercise Versus Combined Exercise Therapy in Women With Fibromyalgia Syndrome: A Randomized. Arch. Phys. Med. Rehabil. 2010, 91, 1838–1843. [Google Scholar] [CrossRef]
- Gowans, S.E.; DeHueck, A.; Voss, S.; Silaj, A.; Abbey, S.E.; Reynolds, W.J. Effect of a randomized, controlled trial of exercise on mood and physical function in individuals with fibromyalgia. Arthritis Rheum. 2001, 45, 519–529. [Google Scholar] [CrossRef]
- Bircan, Ç.; Karasel, S.A.; Akgün, B.; El, Ö.; Alper, S. Effects of muscle strengthening versus aerobic exercise program in fibromyalgia. Rheumatol. Int. 2008, 28, 527–532. [Google Scholar] [CrossRef]
- Wang, C.; Schmid, C.H.; Fielding, R.A.; Harvey, W.F.; Reid, K.F.; Price, L.L.; McAlindon, T. Effect of tai chi versus aerobic exercise for fibromyalgia: Comparative effectiveness randomized controlled trial. BMJ 2018, 360, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Assumpçao, A.; Matsutani, L.A.; Yuan, S.L.; Santo, A.S.; Sauer, J.; Mango, P.; Marques, A.P. Muscle stretching exercises and resistance training in fibromyalgia: which is better? A three-arm randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2017, 54, 663–670. [Google Scholar] [CrossRef]
- Larsson, A.; Palstam, A.; Löfgren, M.; Ernberg, M.; Bjersing, J.; Bileviciute-ljungar, I.; Mannerkorpi, K. Resistance exercise improves muscle strength, health status and pain intensity in fibromyalgia—A randomized controlled trial. Arthritis Res. Ther. 2015, 17, 161. [Google Scholar] [CrossRef] [Green Version]
- Gavi, M.B.R.O.; Vassalo, D.V.; Amaral, F.T.; Macedo, D.C.F.; Gava, P.L.; Dantas, E.M.; Valim, V. Strengthening exercises improve symptoms and quality of life but do not change autonomic modulation in fibromyalgia: A randomized clinical trial. PLoS ONE 2014, 9, e90767. [Google Scholar] [CrossRef]
- Häkkinen, A.; Häkkinen, K.; Hannonen, P.; Alen, M. Strength training induced adaptations in neuromuscular function of premenopausal women with fibromyalgia: Comparison with healthy women. Ann. Rheum. Dis. 2001, 60, 21–26. [Google Scholar] [CrossRef]
- Jones, K.D.; Burckhardt, C.S.; Clark, S.R.; Bennett, R.M.; Potempa, K.M. A randomized controlled trial of muscle strengthening versus flexibility training in fibromyalgia. J. Rheumatol. 2002, 29, 1041–1048. [Google Scholar]
- Espí-lópez, G.V.; Inglés, M.; Ruescas-Nicolau, M.-A.; Moreno-Segura, N. Effect of low-impact aerobic exercise combined with music therapy on patients with fibromyalgia. A pilot study. Complement. Ther. Med. 2016, 28, 1–7. [Google Scholar] [CrossRef]
- Giannotti, E.; Koutsikos, K.; Pigatto, M.; Rampudda, M.E.; Doria, A.; Masiero, S. Medium-/Long-Term Effects of a Specific Exercise Protocol Combined with Patient Education on Spine Mobility, Chronic Fatigue, Pain, Aerobic Fitness and Level of Disability in Fibromyalgia. BioMed Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Latorre, P.A.; Santos, M.A.; Heredia-Jiménez, J.M.; Delgado-Fernández, M.; Soto, V.M.; Mañas, A.; Carbonell-Baeza, A. Effect of a 24-week physical training programme (in water and on land) on pain, functional capacity, body composition and quality of life in women with fibromyalgia. Clin. Exp. Rheumatol. 2013, 31, 72–80. [Google Scholar]
- Carbonell-Baeza, A.; Aparicio, V.A.; Ortega, F.B.; Cuevas, A.M.; Alvarez, I.C.; Ruiz, J.R.; Delgado-Fernandez, M. Does a 3-month multidisciplinary intervention improve pain, body composition and physical fi tness in women with fibromyalgia? Br. J. Sports Med. 2011, 45, 1189–1195. [Google Scholar] [CrossRef] [Green Version]
- García-Martínez, A.M.; De Paz, J.A.; Márquez, S. Effects of an exercise programme on self-esteem, self-concept and quality of life in women with fibromyalgia: A randomized controlled trial. Rheumatol. Int. 2012, 32, 1869–1876. [Google Scholar] [CrossRef]
- Tomas-Carus, P.; Gusi, N.; Häkkinen, A.; Häkkinen, K.; Leal, A.; Ortega-Alonso, A. Eight months of physical training in warm water improves physical and mental health in women with fibromyalgia: A randomized controlled trial. J. Rehabil. Med. 2008, 40, 248–252. [Google Scholar] [CrossRef] [Green Version]
- Lazaridou, A.; Koulouris, A.; Devine, J.K.; Haack, M.; Jamison, R.N.; Edwards, R.R.; Schreiber, K.L. Impact of daily yoga-based exercise on pain, catastrophizing, and sleep amongst individuals with fibromyalgia. J. Pain Res. 2019, 12, 2915–2923. [Google Scholar] [CrossRef] [Green Version]
- Jiao, J.; Russell, I.J.; Wang, W.; Wang, J.; Zhao, Y.Y.; Jiang, Q. Ba-Duan-Jin alleviates pain and fibromyalgia-related symptoms in patients with fibromyalgia: results of a randomised controlled trial. Clin. Exp. Rheumatol. 2019, 37, 953–962. [Google Scholar]
- Jones, K.D.; Adams, D.; Winters-Stone, K.; Burckhardt, C.S. A comprehensive review of 46 exercise treatment studies in fibromyalgia (1988-2005). Health Qual Life Outcomes 2006, 4, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Pescatello, L. ACSM’s Guidelines for Exercise Testing and Prescription, 9th ed.; Pescatello, L.S., Arena, R., Riebe, D., Thompson, P.D., Eds.; The Journal of the Canadian Chiropractic Association; ASCM Group Publisher: Baltimor, MD, USA; Philadelphia, PA, USA, 2014; Volume 58, p. 328. [Google Scholar]
- Walitt, B.; Nahin, R.L.; Katz, R.S.; Bergman, M.J.; Wolfe, F. The prevalence and characteristics of fibromyalgia in the 2012 national health interview survey. PLoS ONE 2015, 10, e0138024. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.A.; Goldenberg, D.L.; Häuser, W.; Katz, R.L.; Walitt, B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin. Arthritis Rheum. 2016, 46, 319–329. [Google Scholar] [CrossRef]
- Saghaei, M. Random allocation software for parallel group randomized trials. BMC Med. Res. Methodol. 2004, 4, 26. [Google Scholar] [CrossRef] [Green Version]
- Koele, R.; Volker, G.; van Vree, F.; van Gestel, M.; Köke, A.; Vliet Vlieland, T. Multidisciplinary Rehabilitation for Chronic Widespread Musculoskeletal Pain: Results from Daily Practice. Musculoskeletal Care 2014, 12, 210–220. [Google Scholar] [CrossRef] [Green Version]
- Boonstra, A.M.; Preuper, H.R.S.; Balk, G.A.; Stewart, R.E. Cut-off points for mild, moderate, and severe pain on the visual analogue scale for pain in patients with chronic musculoskeletal pain. Pain 2014, 155, 2545–2550. [Google Scholar] [CrossRef]
- Borg, G. Borg’s Perceived Exertion and Pain Scales, 1st ed.; Kinetics Human: Champaign, IL, USA, 1998; 120p. [Google Scholar]
- Soriano-Maldonado, A.; Ruiz, J.R.; Álvarez-Gallardo, I.C.; Segura-Jiménez, V.; Santalla, A.; Munguía-Izquierdo, D. Validity and reliability of rating perceived exertion in women with fibromyalgia: exertion-pain discrimination. J. Sports Sci. 2015, 33, 1515–1522. [Google Scholar] [CrossRef]
- García Campayo, J.; Rodero, B.; Alda, M.; Sobradiel, N.; Montero, J.; Moreno, S. Validación de la versión española de la escala de la catastrofización ante el dolor (Pain Catastrophizing Scale) en la fibromialgia. Med. Clin. (Barc) 2008, 131, 487–492. [Google Scholar] [CrossRef]
- Ryde-Brandt, B. Anxiety and depression in mothers of children with psychotic disorders and mental retardation. Br. J. Psychiatry 1990, 156, 118–121. [Google Scholar] [CrossRef]
- Herrero, M.J.; Blanch, J.; Peri, J.M.; De Pablo, J.; Pintor, L.; Bulbena, A. A validation study of the hospital anxiety and depression scale (HADS) in a Spanish population. Gen. Hosp. Psychiatry 2003, 25, 277–283. [Google Scholar] [CrossRef]
- Sanz, J.; García-Vera, M.P.; Espinosa, R.; Fortún, M.; Vázquez, C. Adaptación española del Inventario para la Depresión de Beck-II (BDI-II): 3. Propiedades psicométricas en pacientes con trastornos psicológicos. Clínica y salud 2005, 16, 121–142. [Google Scholar]
- Olaya-Contreras, P.; Persson, T.; Styf, J. Comparison between the Beck Depression inventory and psychiatric evaluation of distress in patients on long-term sick leave due to chronic musculoskeletal pain. J. Multidiscip. Healthc. 2010, 3, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.; Mayer, T.G.; Williams, M.J.; Gatchel, R.J. What is the best screening test for depression in chronic spinal pain patients? Spine J. 2014, 14, 1175–1182. [Google Scholar] [CrossRef]
- Wiebe, J.S.; Penley, J.A. A psychometric comparison of the Beck Depression Inventory - II in English and Spanish. Psychol. Assess. 2005, 17, 481–485. [Google Scholar] [CrossRef]
- Trujillo, H.M.; González-Cabrera, J.M. Psychometric properties of the Spanish version of the Perceived Stress Scale (PSS). Psicol. Conductual. 2006, 9, 86–93. [Google Scholar]
- González, A.; Fernández, P.; Torres, I. Aceptación del dolor crónico en pacientes con fibromialgia: adaptación del Chronic Pain Acceptance Questionnaire (CPAQ). Psicothema 2010, 22, 997–1003. [Google Scholar]
- Harden, R.N.; Revivo, G.; Song, S.; Nampiaparampil, D.; Golden, G.; Kirincic, M.; Houle, T.T. A critical analysis of the tender points in fibromyalgia. Pain Med. 2007, 8, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Farasyn, A.; Meeusen, R. Pressure pain thresholds in healthy subjects: Influence of physical activity, history of lower back pain factors and the use of endermology as a placebo-like treatment. J. Bodyw. Mov. Ther. 2003, 7, 53–61. [Google Scholar] [CrossRef]
- Salgueiro, M.; García-Leiva, J.M.; Ballesteros, J.; Hidalgo, J.; Molina, R.; Calandre, E.P. Validation of a Spanish version of the Revised Fibromyalgia Impact Questionnaire (FIQR). Health Qual Life Outcomes 2013, 11, 132. [Google Scholar] [CrossRef] [Green Version]
- Pankoff, B.A.; Overend, T.J.; Lucy, S.D.; White, K.P. Reliability of the six-minute walk test in people with fibromyalgia. Arthritis Rheum. 2000, 13, 291–295. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Bubela, D.J.; Magasi, S.R.; Wang, Y.C.; Gershon, R.C. Sit-to-stand test: Performance and determinants across the age-span. Isokinet. Exerc. Sci. 2010, 18, 235–240. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, A.; Chavis, M.; Watkins, J.; Wilson, T. The five-times-sit-to-stand test: Validity, reliability and detectable change in older females. Aging Clin. Exp. Res. 2012, 24, 339–344. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Ferrucci, L.; Pieper, C.F.; Leveille, S.G.; Markides, K.S.; Ostir, G.V.; Wallace, R.B. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2000, 55, 221–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, K.S.; Friedman, L.A.; Dinglas, V.D.; Hough, C.L.; Morris, P.E.; Mendez-Tellez, P.A.; Needham, D.M. Evaluating Physical Outcomes in ARDS Survivors: Validity, Responsiveness & Minimal Important Difference of 4-Meter Gait Speed Test. Crit. Care Med. 2016, 44, 859–868. [Google Scholar] [CrossRef]
- Johnson, M.H. How Does Distraction Work in the Management of Pain? Curr. Sci. Inc. 2005, 9, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Puterman, E.; O’Donovan, A.; Adler, N.E.; Tomiyama, A.J.; Kemeny, M.; Wolkowitz, O.M.; Epel, E. Physical activity moderates stressor-induced rumination on cortisol reactivity. Psychosom. Med. 2011, 73, 604–611. [Google Scholar] [CrossRef] [Green Version]
- Casey, M.; Cotter, N.; Kelly, C.; Mc Elchar, L.; Dunne, C.; Neary, R.; Doody, C. Exercise and Acceptance and Commitment Therapy for Chronic Pain: A Case Series with One-Year Follow-Up. Musculoskeletal. Care 2020, 18, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.J.E.M.; Vlaeyen, J.W.S.; Kester, A.D.M.; Knottnerus, J.A. Reduction of Pain Catastrophizing Mediates the Outcome of Both Physical and Cognitive-Behavioral Treatment in Chronic Low Back Pain. J. Pain 2006, 7, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Baeza, A.; Aparicio, V.A.; Sjöström, M.; Ruiz, J.R.; Delgado-Fernández, M. Pain and Functional Capacity in Female Fibromyalgia Patients. Pain Med. 2011, 12, 1667–1675. [Google Scholar] [CrossRef] [Green Version]
- Soriano-Maldonado, A.; Ortega, F.B.; Munguía-Izquierdo, D. Association of cardiorespiratory fitness with pressure pain sensitivity and clinical pain in women with fibromyalgia. Rheumatol. Int. 2015, 35, 899–904. [Google Scholar] [CrossRef]
- LaChapelle, D.L.; Lavoie, S.; Boudreau, A. The meaning and process of pain acceptance. Perceptions of women living with arthritis and fibromyalgia. Pain Res. Manag. 2008, 13, 201–210. [Google Scholar] [CrossRef]
- Lago, T.R.; Hsiung, A.; Leitner, B.P.; Duckworth, C.J.; Chen, K.Y.; Ernst, M.; Grillon, C. Exercise decreases defensive responses to unpredictable, but not predictable, threat. Depress. Anxiety 2018, 35, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Cary, M.A.; Gyurcsik, N.C.; Brawley, L.R. Prediction of adaptive self-regulatory responses to arthritis pain anxiety in exercising adults: Does pain acceptance matter? Pain Res. Manag. 2015, 20, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Miller, D.L.; Roecklein, K.A. The Aging Hippocampus: Interactions between Exercise, Depression, and BDNF. Neuroscientist 2012, 18, 82–97. [Google Scholar] [CrossRef]
- Bote, M.E.; Garcia, J.J.; Hinchado, M.D.; Ortega, E. Fibromyalgia: Anti-Inflammatory and Stress Responses after Acute Moderate Exercise. PLoS ONE 2013, 8, e74524. [Google Scholar] [CrossRef]
- O’Keefe, E.L.; O’Keefe, J.H.; Lavie, C.J. Exercise Counteracts the Cardiotoxicity of Psychosocial Stress. Mayo Clin. Proc. 2019, 94, 1852–1864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, A.; de Azevedo Klumb Steffens, R.; Sieczkowska, S.M.; Peyré, L.A.; Torres Vilarino, G. A systematic review of the effects of strength training in patients with fibromyalgia: clinical outcomes and design considerations. Adv. Rheumatol. (London England) 2018, 58, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunsky, A. The Effect of Balance and Coordination Exercises on Quality of Life in Older Adults: A Mini-Review. Front Aging Neurosci. 2019, 11, 318. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.; Campos, M.A.; Párraga-Montilla, J.A.; Aragón-Vela, J.; Latorre-Román, P.A. Effects of a functional training program in patients with fibromyalgia: A 9-year prospective longitudinal cohort study. Scand. J. Med. Sci. Sport 2020, 30, 904–913. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Health-Related Physical Fitness Assessment Manual; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Topinková, E. Sarcopenia: European consensus on definition and diagnosis. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Gallardo, I.C.; Carbonell-Baeza, A.; Segura-Jiménez, V.; Soriano-Maldonado, A.; Intemann, T.; Aparicio, V.A.; Delgado-Fernández, M. Physical fitness reference standards in fibromyalgia: The al-Ándalus project. Scand. J. Med. Sci. Sport 2016, 27, 1477–1488. [Google Scholar] [CrossRef]
| 1. Preacher curl while standing, palms facing forward |
| 2. Leg extension while seated by lifting a sandbell |
| 3. Bilateral dumbbell front raise while standing |
| 4. Standing hip abduction with a soft elastic band |
| 5. Chest lateral pull-ups while standing |
| 6. Dumbbell shoulder external and internal rotation while standing |
| 7. Sitting down and standing up from a chair without using arms |
| 8. Throwing a ball above the head and catching it |
| 9. Standing calf raise |
| 10. Low Step-ups |
| Physical Exercise Group | Control Group | |||||
|---|---|---|---|---|---|---|
| Pre-Treatment | Post-Treatment | Effect Size (d) | Pre-Treatment | Post-Treatment | Effect Size (d) | |
| Pain catastrophizing | 27.31 (11.55) | 20.00 (10.86) * | 0.65 | 28.25 (13.32) | 27.06 (10.91) | - |
| Anxiety | 11.81 (3.54) | 9.94 (3.57) * | 0.53 | 12.19 (4.07) | 11.19 (3.69) | - |
| Depression | 31.13 (9.06) | 23.81 (7.93) * | 0.86 | 29.31 (11.55) | 27.94 (11.14) | - |
| Stress | 25.31 (7.18) | 22.88 (7.51) * | 0.33 | 24.50(6.34) | 24.75 (7.22) | - |
| Pain acceptance | 38.00 (14.33) | 42.94 (7.96) * | 0.43 | 39.38 (14.67) | 40.81 (13.54) | - |
| Pressure pain threshold (kg/cm2) | 1.75 (0.98) | 2.07 (1.03) * | 0.32 | 1.76 (0.42) | 1.50 (0.59) * | 0.51 |
| Quality of life | 71.47 (14.21) | 61.49 (17.65) * | 0.62 | 62.44 (17.33) | 67.07 (15.87) | - |
| Physical Exercise Group | Control Group | |||||
|---|---|---|---|---|---|---|
| Pre-Treatment | Post-Treatment | Effect Size (d) | Pre-Treatment | Post-Treatment | Effect Size (d) | |
| Self-perceived functional capacity | 20.06 (6.23) | 17.46 (5.16) * | 0.46 | 17.56 (7.28) | 19.42 (6.03) | - |
| Endurance and functional capacity (m) | 481.00 (71.23) | 513.00 (64.84) * | 0.47 | 493.19 (68.48) | 497.31 (76.29) | - |
| Power (s) | 18.18 (11.71) | 11.33 (2.35) * | 0.81 | 11.66 (3.06) | 12.21 (3.01) | - |
| Velocity (s) | 2.79 (0.39) | 2.39 (0.27) * | 1.19 | 2.47 (0.42) | 2.36 (0.47) | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izquierdo-Alventosa, R.; Inglés, M.; Cortés-Amador, S.; Gimeno-Mallench, L.; Chirivella-Garrido, J.; Kropotov, J.; Serra-Añó, P. Low-Intensity Physical Exercise Improves Pain Catastrophizing and Other Psychological and Physical Aspects in Women with Fibromyalgia: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 3634. https://doi.org/10.3390/ijerph17103634
Izquierdo-Alventosa R, Inglés M, Cortés-Amador S, Gimeno-Mallench L, Chirivella-Garrido J, Kropotov J, Serra-Añó P. Low-Intensity Physical Exercise Improves Pain Catastrophizing and Other Psychological and Physical Aspects in Women with Fibromyalgia: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2020; 17(10):3634. https://doi.org/10.3390/ijerph17103634
Chicago/Turabian StyleIzquierdo-Alventosa, Ruth, Marta Inglés, Sara Cortés-Amador, Lucia Gimeno-Mallench, Javier Chirivella-Garrido, Juri Kropotov, and Pilar Serra-Añó. 2020. "Low-Intensity Physical Exercise Improves Pain Catastrophizing and Other Psychological and Physical Aspects in Women with Fibromyalgia: A Randomized Controlled Trial" International Journal of Environmental Research and Public Health 17, no. 10: 3634. https://doi.org/10.3390/ijerph17103634
APA StyleIzquierdo-Alventosa, R., Inglés, M., Cortés-Amador, S., Gimeno-Mallench, L., Chirivella-Garrido, J., Kropotov, J., & Serra-Añó, P. (2020). Low-Intensity Physical Exercise Improves Pain Catastrophizing and Other Psychological and Physical Aspects in Women with Fibromyalgia: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 17(10), 3634. https://doi.org/10.3390/ijerph17103634








