Shell Growth of Large Benthic Foraminifera under Heavy Metals Pollution: Implications for Geochemical Monitoring of Coastal Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Selected Species
2.2. Experimental Culturing with HMs Additions
2.3. Culturing Conditions Monitoring
2.4. Statistical Analysis
3. Results
3.1. Foraminiferal Growth
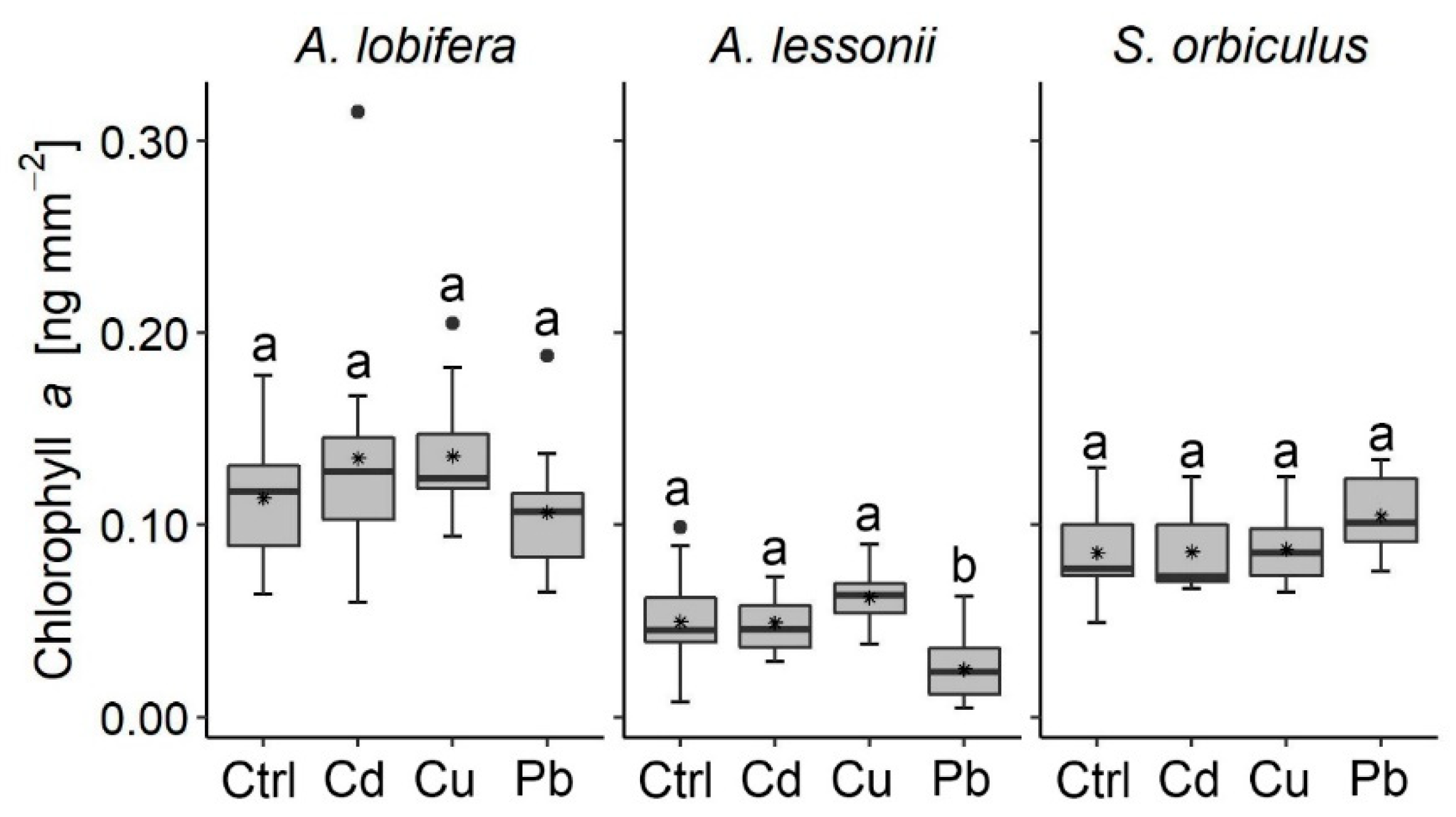
3.2. Algal Symbionts
3.3. Culturing Conditions Results
4. Discussion
4.1. LBF Shell Growth under HMs Extreme Pollution
4.2. Algal Symbionts Response to HMs Pollution
5. Conclusions
- All studied LBF species showed high tolerance to chronic exposure of 4–9 × CMC of Cu, Cd, and Pb.
- The studied LBF species showed higher shell growth rates than most smaller benthic foraminifera.
- A minor but statistically significant decrease in shell growth was found in S. orbiculus, indicating moderate stress but continuous calcification.
- Algal symbionts exhibited a general non-fatal response. The dinoflagellates symbionts within S. orbiculus and the diatoms symbionts within A. lobifera showed tolerance to the exposure of Cd, Cu, and Pb with no negative response detected, while the diatoms within A. lessonii negatively responded to the Pb and Cu treatments.
- Pb was found to negatively affect the algal symbionts more than the foraminifera host and Cu was found to negatively affect both the foraminifera as a host and the algae symbionts, affecting the organism as a holobiont. Cu was found to negatively affect the growth more than Cd and Pb.
- The continuous formation of the shell (new chambers) during exposure to extreme levels of HMs concentrations supports the applicability of LBF shells as living geochemical loggers of coastal pollution, a method currently not used in the regulatory sectors.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Phillips, D.J. The use of biological indicator organisms to monitor trace metal pollution in marine and estuarine environments—A review. Environ. Pollut. 1977, 13, 281–317. [Google Scholar] [CrossRef]
- Steinhardt, J.; Butler, P.G.; Carroll, M.L.; Hartley, J. The Application of Long-Lived Bivalve Sclerochronology in Environmental Baseline Monitoring. Front. Mar. Sci. 2016, 3, 27. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Zhang, J.; Fu, J.; Shi, J.; Jiang, G. Biomonitoring: An appealing tool for assessment of metal pollution in the aquatic ecosystem. Anal. Chim. Acta 2008, 606, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Titelboim, D.; Sadekov, A.; Hyams-Kaphzan, O.; Almogi-Labin, A.; Herut, B.; Kučera, M.; Abramovich, S. Foraminiferal single chamber analyses of heavy metals as a tool for monitoring permanent and short term anthropogenic footprints. Mar. Pollut. Bull. 2018, 128, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.E.; Cramer, B.S.; Franzese, A.; Hönisch, B.; Miller, K.; Rosenthal, Y.; Wright, J.D. Traditional and emerging geochemical proxies in foraminifera. J. Foraminifer. Res. 2010, 40, 165–192. [Google Scholar] [CrossRef]
- Gupta, B.K.S. Modern Foraminifera. Mod. Foraminifera 2003, 36, 384. [Google Scholar]
- Schönfeld, J.; Martins, M.V.A.; Geslin, E.; Jorissen, F.; Korsun, S.; Spezzaferri, S. The FOBIMO (FOraminiferal BIo-MOnitoring) initiative—Towards a standardised protocol for soft-bottom benthic foraminiferal monitoring studies. Mar. Micropaleontol. 2012, 94, 1–13. [Google Scholar] [CrossRef]
- Alve, E. Benthic foraminiferal responses to estuarine pollution: A review. J. Foraminifer. Res. 1995, 25, 190–203. [Google Scholar] [CrossRef]
- Prazeres, M.; Martínez-Colón, M.; Hallock, P. Foraminifera as bioindicators of water quality: The FoRAM Index revisited. Environ. Pollut. 2019, 257, 113612. [Google Scholar] [CrossRef]
- Alve, E.; Korsun, S.; Schönfeld, J.; Dijkstra, N.; Golikova, E.; Hess, S.; Husum, K.; Panieri, G. Foram-AMBI: A sensitivity index based on benthic foraminiferal faunas from North-East Atlantic and Arctic fjords, continental shelves and slopes. Mar. Micropaleontol. 2016, 122, 1–12. [Google Scholar] [CrossRef]
- Dimiza, M.; Triantaphyllou, M.V.; Koukousioura, O.; Hallock, P.; Simboura, N.; Karageorgis, A.P.; Papathanasiou, E. The Foram Stress Index: A new tool for environmental assessment of soft-bottom environments using benthic foraminifera. A case study from the Saronikos Gulf, Greece, Eastern Mediterranean. Ecol. Indic. 2016, 60, 611–621. [Google Scholar] [CrossRef]
- Jorissen, F.; De Stigter, H.C.; Widmark, J.G. A conceptual model explaining benthic foraminiferal microhabitats. Mar. Micropaleontol. 1995, 26, 3–15. [Google Scholar] [CrossRef]
- Schafer, C.T. Monitoring Nearshore Marine Environments Using Benthic Foraminifera: Some Protocols and Pitfalls. Micropaleontology 2000, 46, 161–169. [Google Scholar] [CrossRef]
- Murray, J.W.; Alve, E. Benthic foraminifera as indicators of environmental change: Estuaries, shelf and upper slope. In Environmental Quaternary Micropalaeontology; Haslett, S.R., Ed.; Hodder Arnold: London, UK, 2002; pp. 59–90. Available online: http://eprints.soton.ac.uk/id/eprint/54850 (accessed on 1 December 2019).
- Hallock, P.; Lidz, B.H.; Cockey-Burkhard, E.M.; Donnelly, K.B. Foraminifera as Bioindicators in Coral Reef Assessment and Monitoring: The FORAM Index. Environ. Monit. Assess. 2003, 81, 221–238. [Google Scholar] [CrossRef]
- Hyams-Kaphzan, O.; Almogi-Labin, A.; Sivan, D.; Benjamini, C. Benthic foraminifera assemblage change along the southeastern Mediterranean inner shelf due to fall-off of Nile-derived siliciclastics. Neues Jahrbuch Geol. Paläontol. Abh. 2008, 248, 315–344. [Google Scholar] [CrossRef]
- Hyams-Kaphzan, O.; Almogi-Labin, A.; Benjamini, C.; Herut, B. Natural oligotrophy vs. pollution-induced eutrophy on the SE Mediterranean shallow shelf (Israel): Environmental parameters and benthic foraminifera. Mar. Pollut. Bull. 2009, 58, 1888–1902. [Google Scholar] [CrossRef]
- Frontalini, F.; Buosi, C.; Da Pelo, S.; Coccioni, R.; Cherchi, A.; Bucci, C. Benthic foraminifera as bio-indicators of trace element pollution in the heavily contaminated Santa Gilla lagoon (Cagliari, Italy). Mar. Pollut. Bull. 2009, 58, 858–877. [Google Scholar] [CrossRef]
- Frontalini, F.; Coccioni, R. Benthic foraminifera as bioindicators of pollution: A review of Italian research over the last three decades. Revue de Micropaléontologie 2011, 54, 115–127. [Google Scholar] [CrossRef]
- Saraswat, R.; Kurtarkar, S.R.; Mazumder, A.; Nigam, R. Foraminifers as indicators of marine pollution: A culture experiment with Rosalina leei. Mar. Pollut. Bull. 2004, 48, 91–96. [Google Scholar] [CrossRef]
- Munsel, D.; Kramar, U.; Dissard, D.; Nehrke, G.; Berner, Z.A.; Bijma, J.; Reichart, G.; Neumann, T. Heavy metal incorporation in foraminiferal calcite: Results from multi-element enrichment culture experiments with Ammonia tepida. Biogeosciences 2010, 7, 2339–2350. [Google Scholar] [CrossRef] [Green Version]
- Denoyelle, M.; Geslin, E.; Jorissen, F.; Cazes, L.; Galgani, F. Innovative use of foraminifera in ecotoxicology: A marine chronic bioassay for testing potential toxicity of drilling muds. Ecol. Indic. 2012, 12, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Fabrizio, F. The Response of Benthic Foraminiferal Assemblages to Copper Exposure: A Pilot Mesocosm Investigation. J. Environ. Prot. 2012, 3, 342–352. [Google Scholar] [CrossRef]
- Nardelli, M.P.; Sabbatini, A.; Negri, A. Experimental chronic exposure of the foraminifer Pseudotriloculina rotunda to zinc. Acta Protozool 2013, 52, 193–202. [Google Scholar] [CrossRef]
- Frontalini, F.; Greco, M.; Di Bella, L.; Lejzerowicz, F.; Reo, E.; Caruso, A.; Cosentino, C.; Maccotta, A.; Scopelliti, G.; Nardelli, M.P.; et al. Assessing the effect of mercury pollution on cultured benthic foraminifera community using morphological and eDNA metabarcoding approaches. Mar. Pollut. Bull. 2017, 129, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Titelboim, D.; Sadekov, A.; Almogi-Labin, A.; Herut, B.; Kučera, M.; Schmidt, C.; Hyams-Kaphzan, O.; Abramovich, S. Geochemical signatures of benthic foraminiferal shells from a heat-polluted shallow marine environment provide field evidence for growth and calcification under extreme warmth. Glob. Chang. Boil. 2017, 23, 4346–4353. [Google Scholar] [CrossRef]
- Price, E.B.; Kabengi, N.; Goldstein, S.T. Effects of heavy-metal contaminants (Cd, Pb, Zn) on benthic foraminiferal assemblages grown from propagules, Sapelo Island, Georgia (USA). Mar. Micropaleontol. 2019, 147, 1–11. [Google Scholar] [CrossRef]
- Frontalini, F.; Semprucci, F.; Di Bella, L.; Caruso, A.; Cosentino, C.; Maccotta, A.; Scopelliti, G.; Sbrocca, C.; Bucci, C.; Balsamo, M.; et al. The response of cultured meiofaunal and benthic foraminiferal communities to lead exposure: Results from mesocosm experiments. Environ. Toxicol. Chem. 2018, 37, 2439–2447. [Google Scholar] [CrossRef]
- Ros, M.L.; Al-Enezi, E.; Cesarini, E.; Canonico, B.; Bucci, C.; Martins, M.V.A.; Papa, S.; Frontalini, F. Assessing the Cadmium Effects on the Benthic Foraminifer Ammonia cf. parkinsoniana: An Acute Toxicity Test. Water 2020, 12, 1018. [Google Scholar] [CrossRef] [Green Version]
- Prazeres, M.D.F.; Martins, S.E.; Bianchini, A. Biomarkers response to zinc exposure in the symbiont-bearing foraminifer Amphistegina lessonii (Amphisteginidae, Foraminifera). J. Exp. Mar. Biol. Ecol. 2011, 407, 116–121. [Google Scholar] [CrossRef]
- Frontalini, F.; Nardelli, M.P.; Curzi, D.; Martín-González, A.; Sabbatini, A.; Negri, A.; Losada, M.; Gobbi, P.; Coccioni, R.; Bernhard, J. Benthic foraminiferal ultrastructural alteration induced by heavy metals. Mar. Micropaleontol. 2018, 138, 83–89. [Google Scholar] [CrossRef]
- Le Cadre, V.; Debenay, J.-P. Morphological and cytological responses of Ammonia (foraminifera) to copper contamination: Implication for the use of foraminifera as bioindicators of pollution. Environ. Pollut. 2006, 143, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Nigam, R.; Linshy, V.N.; Kurtarkar, S.; Saraswat, R. Effects of sudden stress due to heavy metal mercury on benthic foraminifer Rosalina leei: Laboratory culture experiment. Mar. Pollut. Bull. 2009, 59, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Linshy, V.N.; Saraswat, R.; Kurtarkar, S.R.; Nigam, R. Experiment to decipher the effect of heavy metal cadmium on coastal benthic foraminifer Pararotalia nipponica (ASANO). J. Palaeontol. Soc. India 2013, 58, 205–211. [Google Scholar]
- Frontalini, F.; Curzi, D.; Giordano, F.; Bernhard, J.; Falcieri, E.; Coccioni, R. Effects of Lead Pollution on Ammonia Parkinsoniana (Foraminifera): Ultrastructural and Microanalytical Approaches. Eur. J. Histochem. 2015, 59, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frontalini, F.; Curzi, D.; Cesarini, E.; Canonico, B.; Giordano, F.M.; De Matteis, R.; Bernhard, J.M.; Pieretti, N.; Gu, B.; Eskelsen, J.; et al. Mercury-Pollution Induction of Intracellular Lipid Accumulation and Lysosomal Compartment Amplification in the Benthic Foraminifer Ammonia parkinsoniana. PLoS ONE 2016, 11, e0162401. [Google Scholar] [CrossRef]
- De Nooijer, L.J.; Reichart, G.; Dueñas-Bohórquez, A.; Wolthers, M.; Ernst, S.R.; Mason, P.R.D.; Van Der Zwaan, G.J. Copper incorporation in foraminiferal calcite: Results from culturing experiments. Biogeosci. Discuss. 2007, 4, 961–991. [Google Scholar] [CrossRef] [Green Version]
- Nardelli, M.P.; Malferrari, D.; Ferretti, A.; Bartolini, A.; Sabbatini, A.; Negri, A. Zinc incorporation in the miliolid foraminifer Pseudotriloculina rotunda under laboratory conditions. Mar. Micropaleontol. 2016, 126, 42–49. [Google Scholar] [CrossRef]
- Herut, B.; Segal, Y.; Gertner, Y. The National Monitoring Program of Israel’s Mediterranean Waters—Scientific Report on Marine Pollution for 2017, Israel Oceanographic and Limnological Research; IOLR Report H50/2018; IOLR (Israel Oceanographic and Limnological Research): Haifa, Israel, 2018. [Google Scholar]
- Herut, B.; Halicz, L. Preliminary Screening for Organic and Metal Pollutants in the Northern Gulf of Eilat; IOLR Report H11/2004; IOLR (Israel Oceanographic and Limnological Research): Haifa, Israel, 2018. [Google Scholar]
- Buchman, M.F. Screening Quick Reference Tables (SQuiRTs); Office of Response and Restoration Division, National Oceanic and Atmospheric Administration: Seattle, WA, USA, 2008. Available online: https://repository.library.noaa.gov/view/noaa/9327 (accessed on 1 December 2019).
- Enviromental Protection Agency. Recommended Aquatic Life Ambient Water Quality Criteria for Cadmium—2016; Environmental Protection Agency: Washington, DC, USA, 2016.
- ANZECC, ARMCANZ. Australian and New Zealand Guidelines for Fresh and Marine Water Quality. 2000. Available online: https://www.waterquality.gov.au/media/61 (accessed on 1 April 2020).
- Canadian Council of Ministers of the Environment. Canadian Water Quality Guidelines for the Protection of Aquatic Life: Cadmium. 2014. Available online: http://ceqg-rcqe.ccme.ca/download/en/148/ (accessed on 1 April 2020).
- Bernhard, J.M.; Blanks, J.K.; Hintz, C.J.; Chandler, T.G. Use of the fluorescent calcite marker calcein to label foraminifera tests. J. Foraminifer. Res. 2004, 34, 96–101. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; Dezonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Arar, E.J.; Collin, S.G. Method 445.0 In Vitro Determination of Chlorophyll a and Pheophytin ain Marine and Freshwater Algae by Fluorescence; U.S. Environmental Protection Agency: Washington, DC, USA, 1997. Available online: https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NERL&dirEntryId=309417 (accessed on 1 February 2018).
- Oron, S.; Abramovich, S.; Almogi-Labin, A.; Woeger, J.; Erez, J. Depth related adaptations in symbiont bearing benthic foraminifera: New insights from a field experiment on Operculina ammonoides. Sci. Rep. 2018, 8, 9560. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Jones, R. Photoinhibition and photoprotection in symbiotic dinoflagellates from reef-building corals. Mar. Ecol. Prog. Ser. 1999, 183, 73–86. [Google Scholar] [CrossRef] [Green Version]
- Joshi, M.; Mohanty, P. Chlorophyll a Fluorescence as a Probe of Heavy Metal Ion Toxicity in Plants. In Functional Organization of the Plant Nucleus; Springer Science and Business Media LLC: Berlin, Germany, 2007; Volume 19, pp. 637–661. [Google Scholar]
- Ralph, P.; Smith, R.A.; Macinnis-Ng, C.; Seery, C.R. Use of fluorescence-based ecotoxicological bioassays in monitoring toxicants and pollution in aquatic systems: Review. Toxicol. Environ. Chem. 2007, 89, 589–607. [Google Scholar] [CrossRef]
- Friedrichs, A.; Busch, J.A.; Van Der Woerd, H.J.; Zielinski, O. SmartFluo: A Method and Affordable Adapter to Measure Chlorophyll a Fluorescence with Smartphones. Sensors 2017, 17, 678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prazeres, M.; Uthicke, S.; Pandolfi, J.M. Ocean acidification induces biochemical and morphological changes in the calcification process of large benthic foraminifera. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142782. [Google Scholar] [CrossRef] [Green Version]
- Broadshaw, J.S. Laboratory Studies on the Rate of Growth of the Foraminifer, Streblus beccarii (Linné) var. tepida (Cushman). JSTOR 1957, 31, 1138–1147. Available online: https://www.jstor.org/stable/1300499 (accessed on 1 May 2020).
- McIntyre-Wressnig, A.; Bernhard, J.; McCorkle, D.; Hallock, P. Non-lethal effects of ocean acidification on the symbiont-bearing benthic foraminifer Amphistegina gibbosa. Mar. Ecol. Prog. Ser. 2013, 472, 45–60. [Google Scholar] [CrossRef]
- Reymond, C.; Lloyd, A.; Kline, D.; Dove, S.G.; Pandolfi, J.M. Decline in growth of foraminifer Marginopora rossi under eutrophication and ocean acidification scenarios. Glob. Chang. Biol. 2012, 19, 291–302. [Google Scholar] [CrossRef]
- Stuhr, M.; Meyer, A.; Reymond, C.; Narayan, G.R.; Rieder, V.; Rahnenführer, J.; Kucera, M.; Westphal, H.; Muhando, C.A.; Hallock, P. Variable thermal stress tolerance of the reef-associated symbiont-bearing foraminifera Amphistegina linked to differences in symbiont type. Coral Reefs 2018, 37, 811–824. [Google Scholar] [CrossRef]
- Titelboim, D.; Almogi-Labin, A.; Herut, B.; Kučera, M.; Asckenazi-Polivoda, S.; Abramovich, S. Thermal tolerance and range expansion of invasive foraminifera under climate changes. Sci. Rep. 2019, 9, 4198. [Google Scholar] [CrossRef]
- Nobes, K.; Uthicke, S.; Henderson, R. Is light the limiting factor for the distribution of benthic symbiont bearing foraminifera on the Great Barrier Reef? J. Exp. Mar. Biol. Ecol. 2008, 363, 48–57. [Google Scholar] [CrossRef]
- Hallock, P. Light dependence in Amphistegina. J. Foraminifer. Res. 1981, 11, 40–46. [Google Scholar] [CrossRef]
- Ter Kuile, B.; Erez, J. In situ growth rate experiments on the symbiont-bearing foraminifera Amphistegina lobifera and Amphisorus hemprichii. J. Foraminifer. Res. 1984, 14, 262–276. [Google Scholar] [CrossRef]
- Nigam, R.; Saraswat, R.; Kurtarkar, S.R. Laboratory experiment to study the effect of salinity variations on benthic foraminiferal species—Pararotalia nipponica (Asano). J. Geol. Soc. India 2006, 67, 41–46. [Google Scholar]
- Schmidt, C.; Morard, R.; Almogi-Labin, A.; Weinmann, A.E.; Titelboim, D.; Abramovich, S.; Kučera, M. Recent Invasion of the Symbiont-Bearing Foraminifera Pararotalia into the Eastern Mediterranean Facilitated by the Ongoing Warming Trend. PLoS ONE 2015, 10, e0132917. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Morard, R.; Prazeres, M.; Barak, H.; Kučera, M. Retention of high thermal tolerance in the invasive foraminifera Amphistegina lobifera from the Eastern Mediterranean and the Gulf of Aqaba. Mar. Biol. 2016, 163, 228. [Google Scholar] [CrossRef]
- Sharifi, A.R.; Croudace, I.W.; Austin, R.L. Benthic foraminiferids as pollution indicators in Southampton Water, southern England, U.K. J. Micropalaeontol. 1991, 10, 109–113. [Google Scholar] [CrossRef] [Green Version]
- Samir, A.; El-Din, A. Benthic foraminiferal assemblages and morphological abnormalities as pollution proxies in two Egyptian bays. Mar. Micropaleontol. 2001, 41, 193–227. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Smith, G. The effect of sudden changes in temperature, light and salinity on the population density and export of zooxanthellae from the reef corals Stylophora pistillata Esper and Seriatopora hystrix Dana. J. Exp. Mar. Biol. Ecol. 1989, 129, 279–303. [Google Scholar] [CrossRef]
- Gleason, D.F.; Wellington, G.M. Ultraviolet radiation and coral bleaching. Nature 1993, 365, 836–838. [Google Scholar] [CrossRef]
- Glynn, P.W. Coral reef bleaching: Ecological perspectives. Coral Reefs 1993, 12, 1–17. [Google Scholar] [CrossRef]
- Brown, B.E. Coral bleaching: Causes and consequences. Coral Reefs 1997, 16, S129–S138. [Google Scholar] [CrossRef]
- Rowan, R. Thermal adaptation in reef coral symbionts. Nature 2004, 430, 742. [Google Scholar] [CrossRef]
- Schmidt, C.; Heinz, P.; Kučera, M.; Uthicke, S. Temperature-induced stress leads to bleaching in larger benthic foraminifera hosting endosymbiotic diatoms. Limnol. Oceanogr. 2011, 56, 1587–1602. [Google Scholar] [CrossRef]
- Dettmering, C.; Röttger, R.; Hohenegger, J.; Schmaljohann, R. The trimorphic life cycle in foraminifera: Observations from cultures allow new evaluation. Eur. J. Protistol. 1998, 34, 363–368. [Google Scholar] [CrossRef]
- Harney, J.N.; Hallock, P.; Talge, H.K. Observations on a trimorphic life cycle in Aamphistegina Gibbosa populations from the Florida Keys. J. Foraminifer. Res. 1998, 28, 141–147. [Google Scholar] [CrossRef]
- Bradshaw, A.D.; Hardwick, K. Evolution and stress-genotypic and phenotypic components. Biol. J. Linn. Soc. 1989, 37, 137–155. [Google Scholar] [CrossRef]
- Lee, J.J.; Morales, J.; Symons, A.; Hallock, P. Diatom symbionts in larger foraminifera from Caribbean hosts. Mar. Micropaleontol. 1995, 26, 99–105. [Google Scholar] [CrossRef]
- Pochon, X.; Garcia-Cuetos, L.; Baker, A.C.; Castellà, E.; Pawlowski, J. One-year survey of a single Micronesian reef reveals extraordinarily rich diversity of Symbiodinium types in soritid foraminifera. Coral Reefs 2007, 26, 867–882. [Google Scholar] [CrossRef] [Green Version]
- Thomas, W.; Hollibaugh, J.; Seibert, D.; Wallace, G. Toxicity of a Mixture of Ten Metals to Phytoplankton. Mar. Ecol. Prog. Ser. 1980, 2, 213–220. [Google Scholar] [CrossRef]
- Bilgrami, K.; Kumar, S. Effects of copper, lead and zinc on phytoplankton growth. Biol. Plant. 1997, 39, 315–317. [Google Scholar] [CrossRef]
- Talge, H.K.; Hallock, P. Ultrastructural responses in field-bleached and experimentally stressed Amphistegina gibbosa (Class Foraminifera). J. Eukaryot. Microbiol. 2003, 50, 324–333. [Google Scholar] [CrossRef] [PubMed]

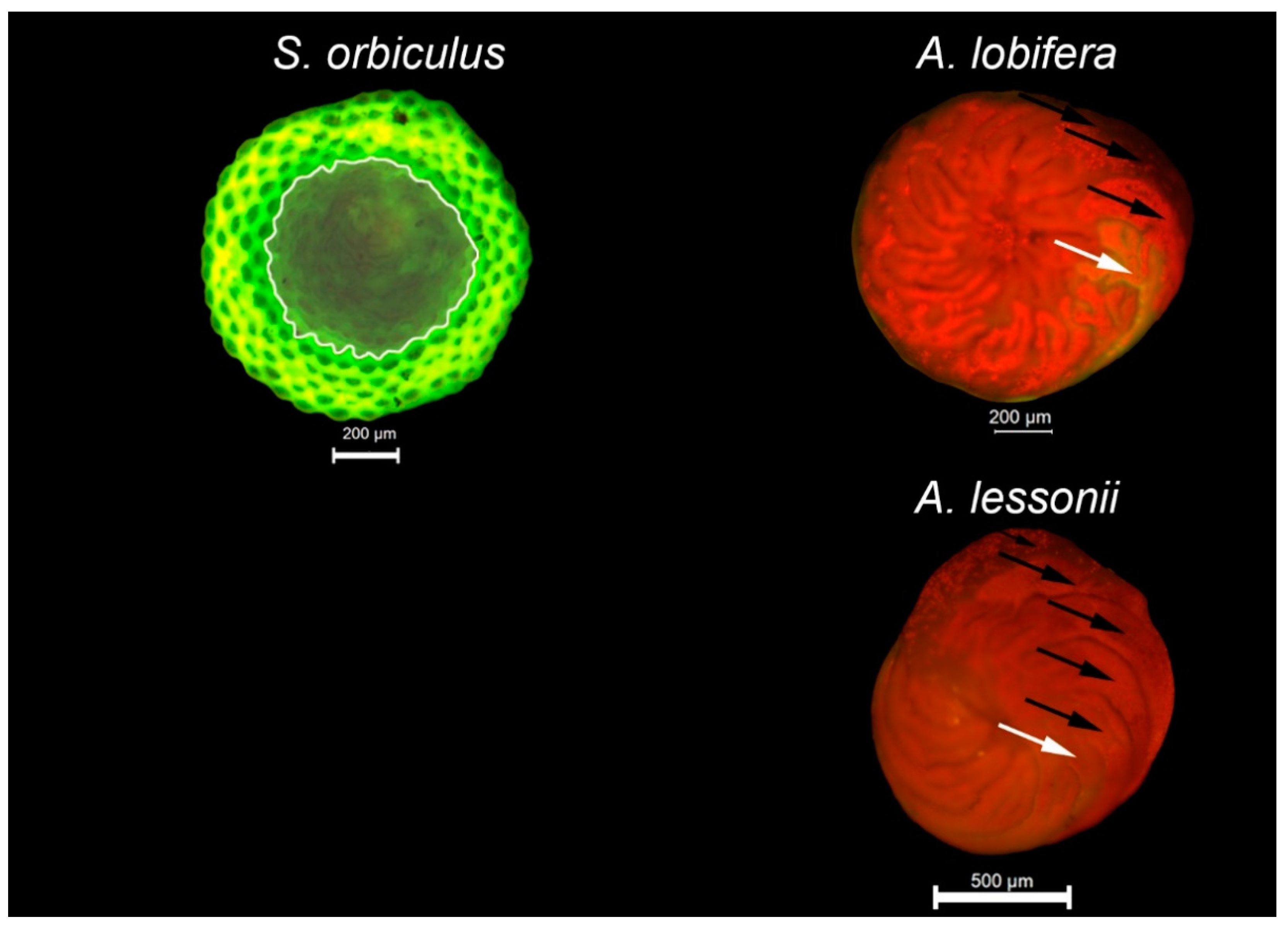



| Heavy Metal | CMC | This Study | |
|---|---|---|---|
| A. Lobifera, S. Orbiculus | A. Lessonii | ||
| Cadmium | 33 | 166 ± 3 (4 × CMC) | 165 ± 3 (4 × CMC) |
| Copper | 4.8 | 43 ± 1 (9 × CMC) | 33 ± 1 (7 × CMC) |
| Lead | 210 | 1001 ± 41 (5 × CMC) | 1206 ± 3 (6 × CMC) |
| Species | HMs | Concentration Relative to CMC | Modified Growth Rates [Chamber Week−1] | Time [Day] | Source |
|---|---|---|---|---|---|
| Ammonia tepida | Cu + Mn + Ni | Control | 0.10 | 82 | Modified after [21] |
| 5 fold 13 × CMC 3 µg L−1 0.1 × CMC | 0.07 | ||||
| 10 fold 22 × CMC 6 µg L−1 0.3 × CMC | 0.05 | ||||
| 20 fold 44 × CMC 11 µg L−1 0.5 × CMC | 0.03 | ||||
| Amminia tepida | Cd | Control 25 × CMC 63 × CMC 125 × CMC 250 × CMC 500 × CMC | 0.20 ± 0.02 0.23 ± 0.01 0.20 ± 0.02 0.16 ± 0.02 0.10 ± 0.02 0 ± 0 | 30 | Modified after [22] |
| Pseudotriloculina rotunda | Zn | Control 0.1 × CMC 1 × CMC 11 × CMC 111 × CMC 1111 × CMC | 0.17 ± 0.02 0.16 ± 0.04 0.08 ± 0.01 0.04 ± 0.04 0.07 ± 0.02 0 ± 0 | 70 | Modified after [24] |
| Rosalina leei | Hg | Control 0.01 × CMC 0.02 × CMC 0.03 × CMC 0.04 × CMC 0.06 × CMC 0.07 × CMC 0.08 × CMC 0.09 × CMC 0.10 × CMC | [µm day−1] 2.3 1.4 0.8 1.2 1.0 0.5 0.3 0.5 0.7 0.3 | 66 | Modified after [20] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben-Eliahu, N.; Herut, B.; Rahav, E.; Abramovich, S. Shell Growth of Large Benthic Foraminifera under Heavy Metals Pollution: Implications for Geochemical Monitoring of Coastal Environments. Int. J. Environ. Res. Public Health 2020, 17, 3741. https://doi.org/10.3390/ijerph17103741
Ben-Eliahu N, Herut B, Rahav E, Abramovich S. Shell Growth of Large Benthic Foraminifera under Heavy Metals Pollution: Implications for Geochemical Monitoring of Coastal Environments. International Journal of Environmental Research and Public Health. 2020; 17(10):3741. https://doi.org/10.3390/ijerph17103741
Chicago/Turabian StyleBen-Eliahu, Nir, Barak Herut, Eyal Rahav, and Sigal Abramovich. 2020. "Shell Growth of Large Benthic Foraminifera under Heavy Metals Pollution: Implications for Geochemical Monitoring of Coastal Environments" International Journal of Environmental Research and Public Health 17, no. 10: 3741. https://doi.org/10.3390/ijerph17103741
APA StyleBen-Eliahu, N., Herut, B., Rahav, E., & Abramovich, S. (2020). Shell Growth of Large Benthic Foraminifera under Heavy Metals Pollution: Implications for Geochemical Monitoring of Coastal Environments. International Journal of Environmental Research and Public Health, 17(10), 3741. https://doi.org/10.3390/ijerph17103741






