Abstract
We aim to evaluate the risk of dry eye disease (DED) occurrence in patients with surgery-indicated chronic rhinosinusitis (CRS) via the national health insurance research database in Taiwan. After exclusion, patients with a diagnostic code of CRS and had received functional endoscopic sinus surgery (FESS) were regarded as having surgery-indicated CRS and enrolled in the study group, then each patient in the study group was age- and gender-matched to four non-CRS patients that served as the control group. The outcome was considered as the development of DED and Cox proportional hazard regression was used for the statistical analysis, which involved multiple potential risk factors of DED. A total of 6076 patients with surgery-indicated CRS that received FESS and another 24,304 non-CRS individuals were enrolled after exclusion. There were 317 and 770 DED events in the study group and the control group during the 16-year follow-up interval, and the study group demonstrated a significantly higher adjusted hazard ratio (1490, 95% confidence intervals (CI): 1.303-1.702) of DED development compared to the control group in the multivariable analysis. In addition, the cumulative probability analysis illustrated a positive correlation of DED occurrence and the disease period of surgery-indicated CRS (p < 0.0001). In the subgroup analysis, both genders revealed a higher but not significant incidence of developing DED in the study group. In conclusion, the existence of surgery-indicated CRS will increase the risk of developing DED, which correlated to the disease interval.
1. Introduction
Chronic rhinosinusitis (CRS) refers to inflammation in the paranasal sinuses that persists for at least three months [1], and affects approximately 6% of the population [2]. The clinical presentations of CRS include nasal stiffness, nasal discharge, facial pain, reduction of smell, headache and shortness of breath [1,3]. Except for the symptoms mentioned above, CRS may also be associated with other inflammatory disorders like allergic rhinitis and asthma [4]. In the severe form, the infection and inflammation of the paranasal sinus in CRS may even lead to the occurrence of fatal intracranial infection including brain abscesses [5].
Both medical and surgical approaches have been utilized to treat CRS [6]. The local corticosteroid therapy, systemic corticosteroid usage and antibiotic treatment have been used to treat CRS with favorable outcomes [3,6]. In addition, functional endoscopic sinus surgery (FESS) is a well-established procedure for severe CRS that shows poor response to medical management and can yield high anatomical success rate after the surgery [7,8,9]. Still, the recovery of maxillary sinus mucosa in patient with CRS is incomplete one year after the FESS performance [3]. Moreover, patients with certain risk factors, like higher Lund-Mackay CT scores and those with fungal-induced CRS, may still experience a poor quality of life or persistent nasal polyp formation even after successful FESS intervention [7,8]. The above lines of evidence suggest that the local effect of severe CRS would endure despite the FESS management.
Concerning the ocular complications related to the development of CRS, orbital cellulitis, preseptal cellulitis and subperiosteal abscess have been reported in a previous cross-sectional study [9]. In another report, inflammatory conjunctivitis and scleritis were observed in a patient with right maxillary sinusitis [10]. Symptoms of dry eye disease (DED) can include a sensation of dryness, irritation resulting from evaporative excess, aqueous deficiency, ocular surface damage, or all of these symptoms [11]. Recently, the major pathophysiology of DED is speculated as a multi-factorial vicious cycle that leads to inflammatory processes [12]. Since the etiology of CRS is usually related to inflammation [13], a certain association may exist between these two disorders, which has rarely been reported elsewhere.
Herein, we aim to investigate the relationship between surgery-indicated CRS and the development of DED via the National Health Insurance Research Database (NHIRD) in Taiwan. Additionally, we also conducted a multivariate model to estimate several risk factors of DED.
2. Materials and Methods
2.1. Ethics Declaration and Data Resource
This retrospective, population-based cohort study adhered to the Declaration of Helsinki in 1964 and its late amendment. In addition, the current study was approved by both the National Health Insurance Administration and the Institutional Review Board of Chung Shan Medical University, Taichung, Taiwan. In addition, the need for informed consent was waived by the above two institutions. The claims data used in the current study were obtained from the Longitudinal Health Insurance Database 2005 version (LHID), which derived the data from the NHIRD that contains data of insurance claims from more than 99% of Taiwan’s population. The data of LHID was randomly sampled from the NHIRD registry for the year 2005 by the database of the National Health Insurance Administration. The information/resource available from the LHID included the demographic data of the subjects, their socioeconomic conditions, the residence of the subjects, the International Classification of Diseases-Ninth Revision (ICD-9), the International Classification of Diseases-Tenth Revision (ICD-10), and the medications used for the study subjects. The time interval of LHID ranges from 1 January 2000 to 31 December 2016, with a total study interval of about 16 years.
2.2. Patient Selection
Patients were set as having surgery-indicated CRS if the following criteria was accomplished (1) in receipt of the diagnosis of CRS, (2) the arrangement of FESS within two year after the diagnosis of CRS, (3) the use of corticosteroid or antibiotics for at least two years from the diagnosis of CRS and (4) receipt of the CRS diagnosis by an otorhinolaryngologist. The index date was defined as the date of two years after the diagnosis of surgery-indicated CRS. In addition, the following exclusion criteria were applied to exclude certain impaired ocular conditions: (1) receipt of a diagnosis of legal blindness at any time; (2) receipt a diagnosis of ophthalmic tumors at any time; (3) receipt of a diagnosis of severe ocular trauma at any time; (4) receipt of eyeball removal surgery before the index date; and (5) receipt a diagnosis of DED (diagnostic codes are listed in the next paragraph) before the index date. In addition, each individual in the study group was age and gender-matched to four non-CRS subjects, as discussed in the following sections, which served the control group. Still, individuals with surgery-indicated CRS who could not be matched with four non-CRS patients were excluded from the current study.
2.3. Main Outcome Measurement
The development of DED was defined as the main outcome in the current study, which was based on the DED-related diagnostic codes after the index date. In clinical practice, ICD-9/ICD-10 codes for “unspecific corneal disorder” may also be used for some forms of DED, but these ICD-9/ICD-10 codes were eliminated to prevent overestimation and confusion of the primary outcome. Moreover, only those DED patients diagnosed by an ophthalmologist were considered as having achieved the primary outcome and included in the current study.
2.4. Demographic Variables and Co-Morbidities
To standardize the health condition of participants, we also considered the effects of demographic conditions including, age, gender, and income level and the following co-morbidities in the analysis: hypertension, diabetes mellitus, ischemic heart diseases, hyperlipidemia, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease and asthma, rheumatic disease, peptic ulcer disease, liver disease, and hemiplegia/paraplegia. To make the ocular condition of the study population more homogenous, we also included the effect of keratopathy, uveitis, glaucoma, and age-related macular degeneration (AMD) in the multivariable model. We would then longitudinally follow the patients’ condition from the index date until the date of any type of DED diagnosis, or until the last date of data collection from the LHID, which means 31 December 2016.
2.5. Statistical Analysis
SAS version 9.4 (SAS Institute Inc., NC, USA) was employed for all the statistical analyses in the current study. After the age and gender-matching of the study group and the control group with a 1:4 ratios, the incidence rate ratio, crude relative risk and corresponding 95% confidence intervals (CI) were calculated by the Poisson regression. Then, the Cox proportional hazard regression was adopted to yield adjusted hazard ratios (aHR) of DED by incorporating the above demographic data, ocular diseases and systemic comorbidities in the multivariable analysis. In addition, the Kaplan–Meier curves were plotted to show the cumulative probability of DED between the study and control groups, and we applied the log-rank test to evaluate the significant difference between the two survival curves. For the subgroup analysis, the sensitivity analysis with aHR of DED that stratified by the gender and age subgroups were conducted. Because most individuals in the LHID/NHIRD are Han Taiwanese, race was not considered a covariate. Statistical significance was set at p < 0.05.
3. Results
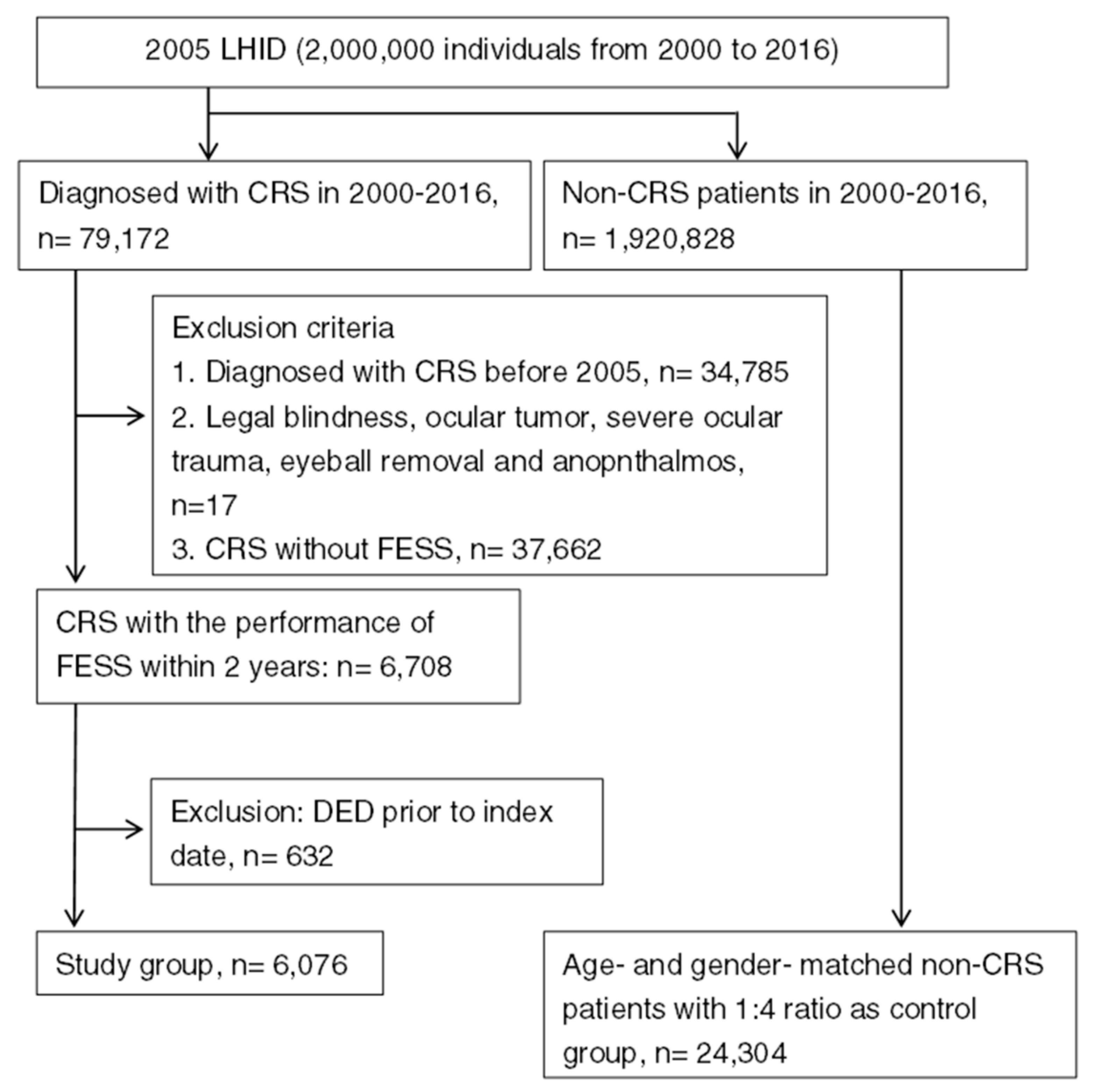
A total numbers of 6076 patients with surgery-indicated CRS were enrolled in the study group, while another 24,304 individuals were enrolled into the control group after exclusion, and the flowchart of patient selection is shown in Figure 1. The age and gender ratio are identical due to the matching process, while the different characteristics of co-morbidities between the study and control group are listed in Table 1.
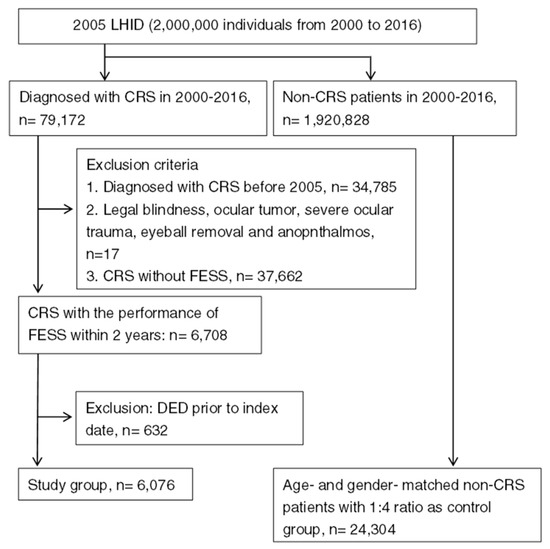
Figure 1.
The flowchart of patient selection. LHID 2005: Longitudinal Health Insurance Database 2005 version, CRS: chronic rhinosinusitis, FESS: functional endoscopic sinus surgery, DED: dry eye disease.

Table 1.
Different characteristics of co-morbidities between the study and control group.
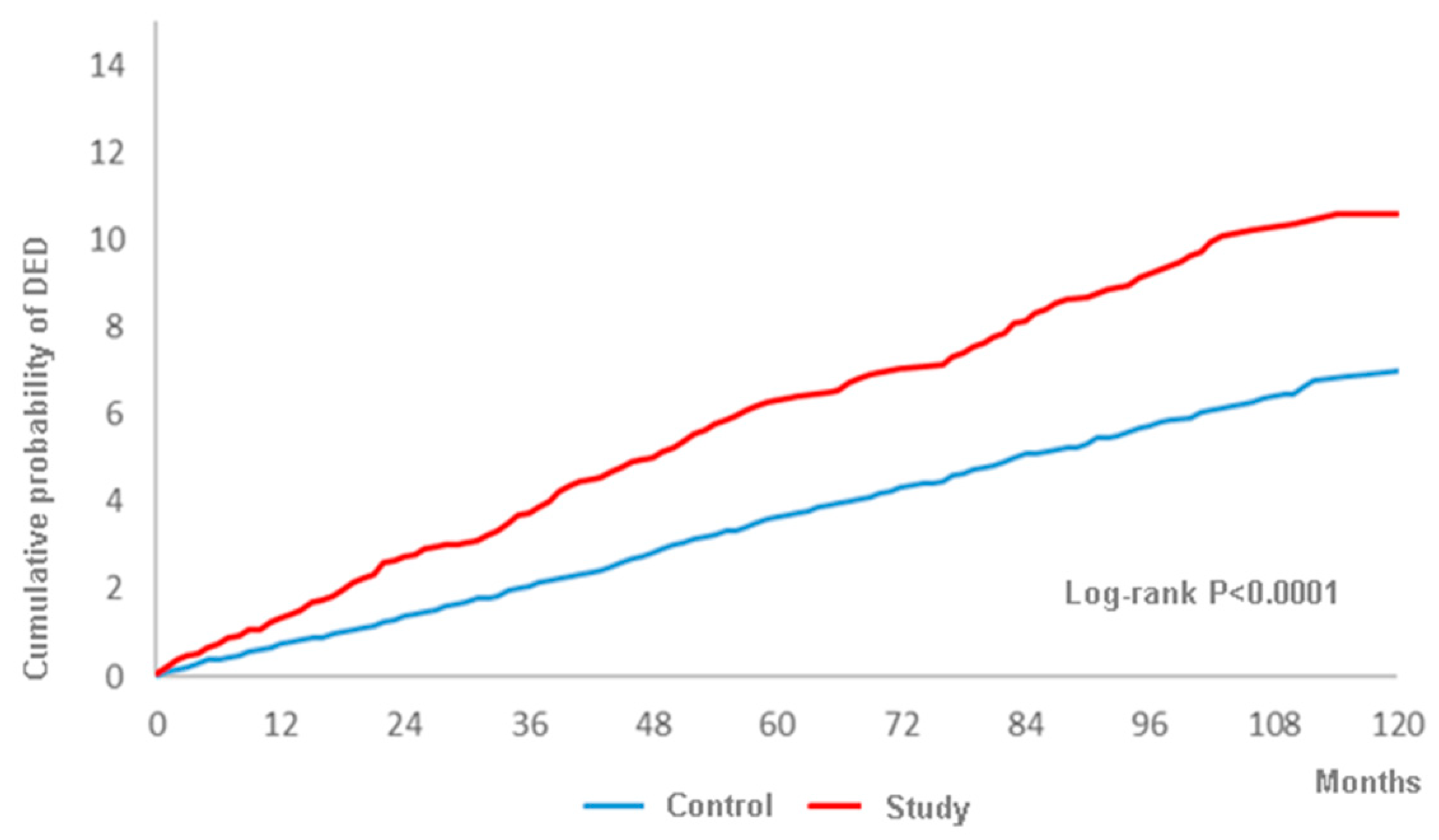
A total of 317 and 770 DED events were observed in the study group and the control group during the whole follow-up period up to 16 years, and the study group showed a higher crude relative risk (1685, 95% CI: 1.479-1.921), as shown in Table 2. Moreover, the study group revealed a significantly higher aHR (1490, 95% CI: 1.303-1.702) compared to the control group after adjusting for multiple potential risk factors including demographic data, systemic diseases and ocular diseases, as shown in Table 3. Besides, the cumulative probability of DED was also significantly higher in the study group according to the result of the log-rank test (p < 0.0001) (Figure 2). In addition to surgery-indicated CRS, peripheral vascular disease, chronic pulmonary diseases, peptic ulcer disease, liver disease, keratopathy, uveitis, glaucoma and AMD were prominently related to the higher rate of DED development (Table 3). About the subgroup analysis, both the male and female population in the surgery-indicated CRS yielded a significant higher aHR of developing DED and the aHR of DED increased with older age (Table 4).

Table 2.
Incidence rate of dry eye disease in the study group.

Table 3.
Multiple Cox proportional hazard regression for estimation of adjusted hazard ratios on dry eye disease.
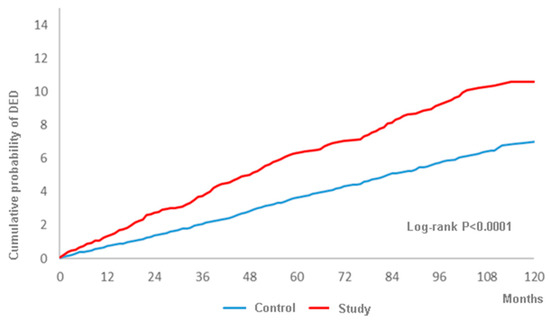
Figure 2.
The cumulative probability of dry eye disease between the study and control groups.

Table 4.
The sensitivity analysis for the adjusted hazard ratio stratified by follow-up time of gender and age groups.
4. Discussion
In the current study, the occurrence of recent-onset DED is significantly higher in those with surgery-indicated CRS compared to non-CRS individuals after adjusting for multiple potential risk factors in the multivariate analysis. On the other hand, the chance of developing DED is also significantly raised in those individuals diagnosed with peripheral vascular disease, chronic pulmonary diseases, peptic ulcer disease, liver disease, keratopathy, uveitis, glaucoma and AMD.
About the possible pathophysiology between CRS and DED, several mechanisms may lead to such a relationship. Recently, CRS has been found to be an inflammatory disorder and tissue-deforming process rather than an infectious lesion in the majority of cases [4,14,15]. The interleukin is elevated in patients with CRS [16], with raised matrix metalloproteinases detected [17]. In addition, tissue from patients with CRS demonstrated inflammatory cell infiltration, thus suggesting an inflammatory nature of CRS [18]. On the other hand, the vicious cycle in developing DED includes components such as meibomian gland dysfunction (MGD), aqueous deficiency, exposure-related damage and goblet cell deficiency [12]. Among them, both the MGD and goblet cell deficiency are related to the increment inflammatory reaction [12,19], in which MGD will lead to the evaporative excess subtype of DED and affects about 50 percent of patients with DED [20]. Besides, several inflammatory mediators—like interleukin and matrix metalloproteinases—are elevated in those individuals with DED [12,21]. As a consequence, the persistent inflammatory process in prolonged CRS may activate the inflammatory reaction in the DED cycle. In addition, both CRS and DED may manifest vasculitis concurrently [22,23], and thus presence of CRS and underlying vasculitis may be related to the occurrence of DED. Moreover, severe CRS may lead to poor sleep quality [24], which is also a significant risk factor for the formation of DED [25]. Accordingly, persistent and severe CRS may contribute to the development of DED, as demonstrated in the current study.
Concerning the relationship between CRS and DED, one study found that DED would exist in patients with CRS [26]. In the current study, patients with surgery-indicated CRS showed a higher aHR of developing DED (1.490) compared to those without CRS after adjusting several potential risk factors in a multivariate analysis. To our knowledge, this is a preliminary experience to reveal the association between these two diseases. Additionally, we excluded those with preceding DED before the index date (i.e., two years after the diagnosis of CRS), thus the chance of enrolling previous DED cases and mistaking them as the effect of CRS is minimal. Moreover, the log-rank test illustrated a positive and significantly cumulative probability of DED occurring, which was correlated to the disease interval of surgery-indicated CRS. This finding further demonstrates that the effect of surgery-indicated CRS to induce DED is a long-term influence rather than an acute stress result from the FESS. As a consequence, we speculate a causal relationship between surgery-indicated CRS and the development of DED.
In the field of epidemiology, prevalence of DED ranges from 5 to 50 percent [20]. In the current study, the occurrence rate is 14.15% in the study group, which is significantly higher than the occurrence rate in the control group and numerically higher than another epidemiologic study operated in the same region [27]. The finding implies that surgery-indicated CRS not only leads to a higher rate of DED, but also influences a majority of the population with surgery-indicated CRS. Furthermore, CRS is also a prominent disease that affects up to 5 percent of the population, thus decreasing their quality of life [4]. Since both CRS and DED may influence a large number of patients, a referral to the ophthalmic department for to be screened for possible DED may be suggested in patients with long-lasting CRS. On the other hand, the patients with DED and nasal discomfort may also be referred to the otorhinolaryngologic department for a preliminary survey of CRS.
Other diseases that correlated to the occurrence of DED, including keratopathy, uveitis, glaucoma and AMD, showed a significant correlation to DED in the current study. Because the persistent DED may cause damage in the ocular surface including the cornea [12], it is reasonable for the correlation between the two diseases in the current study. In addition, the preservatives in anti-glaucomatous medications may cause further damage of ocular surface and lead to inflammation of the ocular surface and DED [28]. Thus an association between glaucoma and DED is expectable. The uveitis is also an inflammatory disease like DED [29], and age is risk factor for both AMD and DED, which could explain such a correlation [20,30]. The systemic diseases related to DED include peripheral vascular disease, chronic pulmonary diseases, peptic ulcer disease and liver disease, while the exact etiology needs further investigation. Both genders in the study group showed a higher probability to develop DED, while the female gender revealed a numerically higher aHR than the males, which corresponded to the previous epidemiological study [27]. Although older age is a risk factor of DED [20], the bias may be minimized due to our age-matched manipulation.
There are some limitations in the current study. First, a group with patients diagnosed with CRS but without the arrangement of FESS should be included to make the statistical analysis more complete, but this could not be conducted due to the overestimation rate of CRS, which may lead to some confusion [31,32,33,34]. In addition, the retrospective and observational nature of study design might reduce the homogeneity of the patient population even after multivariate analysis. Notwithstanding, we used claimed data rather than real medical documents, thus missing some important information like the laterality of DED and the postoperative condition of CRS after a FESS procedure. In addition, seasonal allergies, in which the DED could be a surrogate for allergic disease in CRS subjects, cannot be included as a potential confounder for the same reason that real medical documents were not available in the current study. Moreover, the usage of cyclosporine emulsion (not reimbursed by the health insurance until the end of 2013) could not be accessed in the majority of the study population, thus it was hard to investigate the percentage of severe DED. Last but not least, only the DED diagnosed by an ophthalmologist was enrolled in the current study to gain the accuracy of DED diagnosis, while some general physicians may also handle a number of DED individuals; thus, the DED population in the current study may be underestimated.
5. Conclusions
In conclusion, the presence of surgery-indicated CRS contributes to the development of DED after adjusting for multiple potential risk factors. Furthermore, the occurrence of DED is correlated to the disease interval of surgery-indicated CRS. Further large-scale prospective study to evaluate the effect of CRS on the severity of DED is warranted.
Author Contributions
Conceptualization, C.-Y.L. and K.-L.Y.; methodology, C.-Y.L., K.-L.Y. and H.-C.C. (Hung-Chih Chen); validation, J.-Y.H. and S.-F.Y.; formal analysis, J.-Y.H. and H.-C.C. (Hung-Chih Chen); investigation, C.-C.S.; writing—original draft preparation, C.-Y.L., K.-L.Y. and H.-C.C. (Hung-Chi Chen); writing—review and editing, C.-C.S., H.-C.C. (Hung-Chi Chen) and S.-F.Y.; supervision, S.-F.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siedek, V.; Stelter, K.; Betz, C.S.; Berghaus, A.; Leunig, A. Functional endoscopic sinus surgery—A retrospective analysis of 115 children and adolescents with chronic rhinosinusitis. Int. J. Pediatr. Otorhinolaryngol. 2009, 73, 741–745. [Google Scholar] [CrossRef]
- Gulati, S.P.; Chaudhry, D.; Kalra, V.; Wadhera, R.; Garg, A. The role of functional endoscopic sinus surgery (fess) in patients with asthma with chronic sinusitis. Indian J. Otolaryngol. Head Neck Surg.: Off. Publ. Assoc. Otolaryngol. India 2008, 60, 152–155. [Google Scholar] [CrossRef]
- Anselmo-Lima, W.T.; Ferreira, M.D.; Valera, F.C.; Rossato, M.; de Mello, V.R.; Demarco, R.C. Histological evaluation of maxillary sinus mucosa after functional endoscopic sinus surgery. Am. J. Rhinol. 2007, 21, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, A.R. Chronic rhinosinusitis. Am. Fam. Physician 2017, 96, 500–506. [Google Scholar] [PubMed]
- Constantin, F.; Niculescu, P.A.; Petre, O.; Balasa, D.; Tunas, A.; Rusu, I.; Lupascu, M.; Orodel, C. Orbital cellulitis and brain abscess—rare complications of maxillo-spheno-ethmoidal rhinosinusitis. Rom. J. Ophthalmol. 2017, 61, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Fokkens, W.J.; Lund, V.J.; Mullol, J.; Bachert, C.; Alobid, I.; Baroody, F.; Cohen, N.; Cervin, A.; Douglas, R.; Gevaert, P.; et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinology. 2012, 50, 1–12. [Google Scholar] [CrossRef]
- Tan, B.K.; Lane, A.P. Endoscopic sinus surgery in the management of nasal obstruction. Otolaryngol. Clin. N. Am. 2009, 42, 227–240. [Google Scholar] [CrossRef]
- Ramakrishnan, V.R.; Kennedy, D.W. Advances in the surgical management of chronic sinusitis and nasal polyps. Curr. Allergy Asthma Rep. 2011, 11, 220–229. [Google Scholar] [CrossRef]
- Welch, K.C.; Stankiewicz, J.A. A contemporary review of endoscopic sinus surgery: Techniques, tools, and outcomes. Laryngoscope 2009, 119, 2258–2268. [Google Scholar] [CrossRef]
- Barac, A.; Pekmezovic, M.; Spiric, V.T.; Trivic, A.; Marinkovic, J.; Pekic, S.; Arsenijevic, V.A. Chronic rhinosinusitis: Association of recalcitrant nasal polyposis and fungal finding in polyp’s single-cell suspension. Eur. Arch. Oto-Rhino-Laryngol. 2015, 272, 3727–3734. [Google Scholar] [CrossRef]
- Brooks, S.G.; Trope, M.; Blasetti, M.; Doghramji, L.; Parasher, A.; Glicksman, J.T.; Kennedy, D.W.; Thaler, E.R.; Cohen, N.A.; Palmer, J.N.; et al. Preoperative lund-mackay computed tomography score is associated with preoperative symptom severity and predicts quality-of-life outcome trajectories after sinus surgery. Int. Forum Allergy Rhinol. 2018, 8, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.S.; Chen, P.L.; Hung, J.H.; Chen, H.Y.; Lai, C.C.; Ou, C.Y.; Chang, C.M.; Wang, C.K.; Cheng, H.C.; Tseng, S.H. Orbital complications of paranasal sinusitis in taiwan, 1988 through 2015: Acute ophthalmological manifestations, diagnosis, and management. PLoS ONE 2017, 12, e0184477. [Google Scholar] [CrossRef] [PubMed]
- Nejabat, M.; Mahmoudi Nezhad, G.S.; Shenavandeh, S.; Ashraf, M.J.; Jalalpour, M.H. Conjunctivitis as a manifestation of wegener’s granulomatosis. J. Curr. Ophthalmol. 2018, 30, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K.; Yokoi, N.; Shimazaki, J.; Watanabe, H.; Dogru, M.; Yamada, M.; Kinoshita, S.; Kim, H.M.; Tchah, H.W.; Hyon, J.Y.; et al. New perspectives on dry eye definition and diagnosis: A consensus report by the asia dry eye society. Ocul. Surf. 2017, 15, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Milner, M.S.; Beckman, K.A.; Luchs, J.I.; Allen, Q.B.; Awdeh, R.M.; Berdahl, J.; Boland, T.S.; Buznego, C.; Gira, J.P.; Goldberg, D.F.; et al. Dysfunctional tear syndrome: Dry eye disease and associated tear film disorders—New strategies for diagnosis and treatment. Curr. Opin. Ophthalmol. 2017, 28 (Suppl. 1), 3–47. [Google Scholar] [CrossRef] [PubMed]
- Van Crombruggen, K.; Zhang, N.; Gevaert, P.; Tomassen, P.; Bachert, C. Pathogenesis of chronic rhinosinusitis: Inflammation. J. Allergy Clin. Immunol. 2011, 128, 728–732. [Google Scholar] [CrossRef]
- Gurrola, J., 2nd; Borish, L. Chronic rhinosinusitis: Endotypes, biomarkers, and treatment response. J. Allergy Clin. Immunol. 2017, 140, 1499–1508. [Google Scholar] [CrossRef]
- Stevens, W.W.; Lee, R.J.; Schleimer, R.P.; Cohen, N.A. Chronic rhinosinusitis pathogenesis. J. Allergy Clin. Immunol. 2015, 136, 1442–1453. [Google Scholar] [CrossRef]
- Olcott, C.M.; Han, J.K.; Cunningham, T.D.; Franzese, C.B. Interleukin-9 and interleukin-17c in chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2016, 6, 841–847. [Google Scholar] [CrossRef]
- Homma, T.; Kato, A.; Sakashita, M.; Takabayashi, T.; Norton, J.E.; Suh, L.A.; Carter, R.G.; Harris, K.E.; Peters, A.T.; Grammer, L.C.; et al. Potential involvement of the epidermal growth factor receptor ligand epiregulin and matrix metalloproteinase-1 in pathogenesis of chronic rhinosinusitis. Am. J. Respir. Cell Mol. Biol. 2017, 57, 334–345. [Google Scholar] [CrossRef]
- Chowdhury, N.I.; Chandra, R.K.; Li, P.; Ely, K.; Turner, J.H. Investigating the correlation between mucus cytokine levels, inflammatory cell counts, and baseline quality-of-life measures in chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2019, 9, 538–544. [Google Scholar] [CrossRef]
- Chhadva, P.; Goldhardt, R.; Galor, A. Meibomian gland disease: The role of gland dysfunction in dry eye disease. Ophthalmology 2017, 124, S20–s26. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. Tfos dews ii epidemiology report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- Liu, R.; Gao, C.; Chen, H.; Li, Y.; Jin, Y.; Qi, H. Analysis of th17-associated cytokines and clinical correlations in patients with dry eye disease. PLoS ONE 2017, 12, e0173301. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; Reh, D.D. Chapter 11: Granulomatous diseases and chronic sinusitis. Am. J. Rhinol. Allergy 2013, 27 (Suppl. 1), S39–S41. [Google Scholar] [CrossRef]
- Mathews, P.M.; Hahn, S.; Hessen, M.; Kim, J.; Grader-Beck, T.; Birnbaum, J.; Baer, A.N.; Akpek, E.K. Ocular complications of primary sjogren syndrome in men. Am. J. Ophthalmol. 2015, 160, 447–452.e441. [Google Scholar] [CrossRef] [PubMed]
- DeConde, A.S.; Soler, Z.M. Chronic rhinosinusitis: Epidemiology and burden of disease. Am. J. Rhinol. Allergy 2016, 30, 134–139. [Google Scholar] [CrossRef]
- Wu, M.; Liu, X.; Han, J.; Shao, T.; Wang, Y. Association between sleep quality, mood status, and ocular surface characteristics in patients with dry eye disease. Cornea 2019, 38, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Chang, Y.T.; Chen, S.F.; Lin, W.C.; Su, Y.Y.; Luo, S.D. The symptom burden of autonomic dysfunction is positively associated with chronic rhinosinusitis status. Rhinology 2018, 56, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Yen, J.C.; Hsu, C.A.; Li, Y.C.; Hsu, M.H. The prevalence of dry eye syndrome’s and the likelihood to develop sjogren’s syndrome in taiwan: A population-based study. Int. J. Environ. Res. Public Health 2015, 12, 7647–7655. [Google Scholar] [CrossRef]
- Anwar, Z.; Wellik, S.R.; Galor, A. Glaucoma therapy and ocular surface disease: Current literature and recommendations. Curr. Opin. Ophthalmol. 2013, 24, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.R.; Huang, J.C.; Tao, Y.; Kaburaki, T.; Lee, C.S.; Lin, T.C.; Hsu, C.C.; Chiou, S.H.; Hwang, D.K. Noninfectious uveitis in the asia-pacific region. Eye (Lond. Engl.) 2019, 33, 66–77. [Google Scholar] [CrossRef]
- Al-Zamil, W.M.; Yassin, S.A. Recent developments in age-related macular degeneration: A review. Clin. Interv. Aging 2017, 12, 1313–1330. [Google Scholar] [CrossRef] [PubMed]
- Dietz de Loos, D.; Lourijsen, E.S.; Wildeman, M.A.M.; Freling, N.J.M.; Wolvers, M.D.J.; Reitsma, S.; Fokkens, W.J. Prevalence of chronic rhinosinusitis in the general population based on sinus radiology and symptomatology. J. Allergy Clin. Immunol. 2019, 143, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).