Nutritional Determinants of Quality of Life in a Mediterranean Cohort: The SUN Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Dietary and Non-Dietary Variables
2.3. Main Outcome—Health Related Quality of Life (HRQoL) the SF-36 Questionnaire
2.4. Statistical Analysis
3. Results
3.1. Sample Characteristics and Overall HRQoL Scores
3.2. Dietary Analysis
3.2.1. Multivariate Linear Regression Models
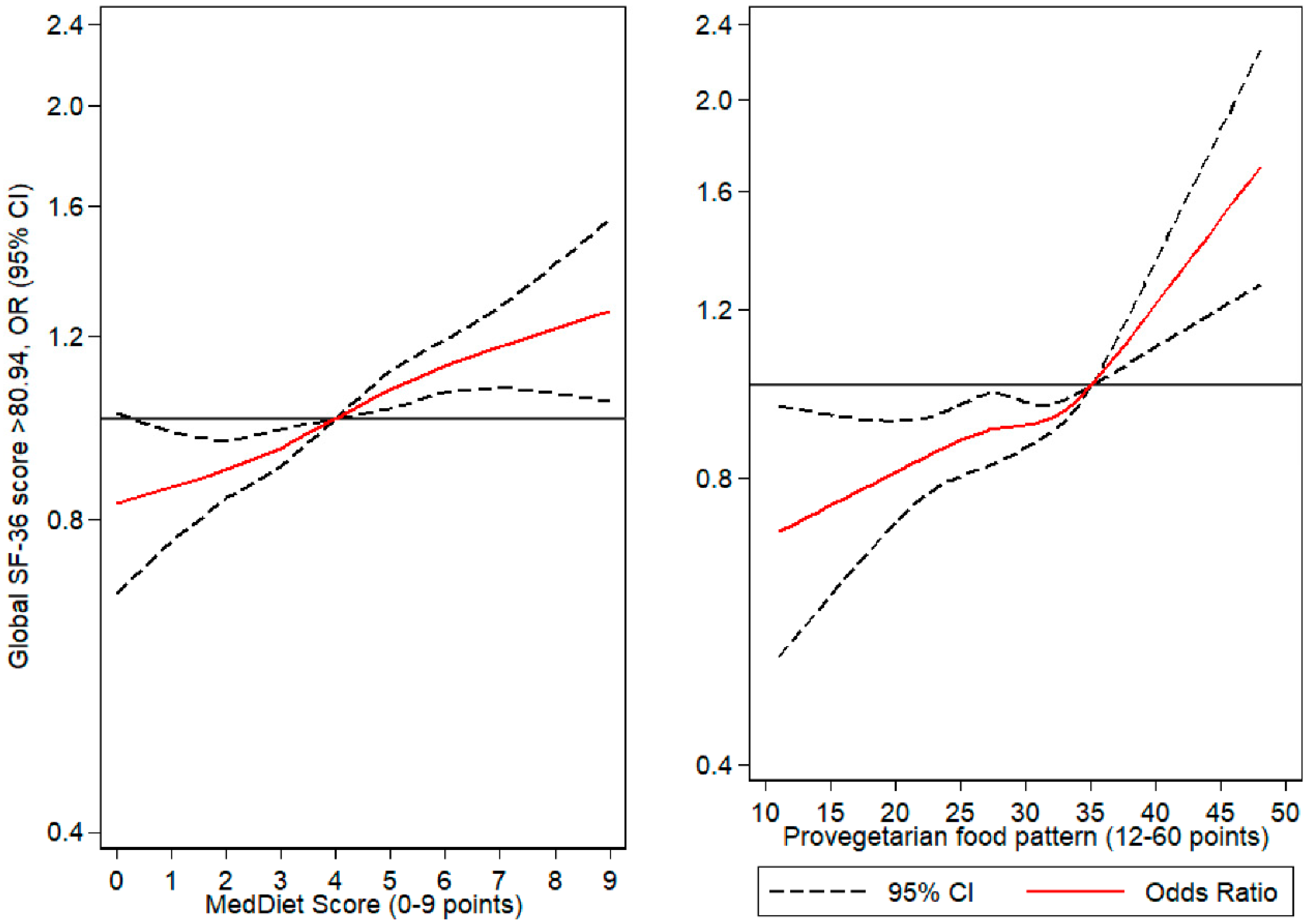
3.2.2. Flexible Regression Models (Cubic Splines)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization WHO | WHOQOL: Measuring Quality of Life. Available online: http://www.who.int/healthinfo/survey/whoqol-qualityoflife/en/ (accessed on 28 October 2019).
- Karimi, M.; Brazier, J. Health, Health-Related Quality of Life, and Quality of Life: What is the Difference? PharmacoEconomics 2016, 34, 645–649. [Google Scholar] [CrossRef]
- Barton, H.; Grant, M. A health map for the local human habitat. J. R. Soc. Promot Health 2006, 126, 252–253. [Google Scholar] [CrossRef]
- WHO The Determinants of Health. Health Impact Assessment. Available online: http://www.who.int/hia/evidence/doh/en/ (accessed on 31 July 2019).
- Ware, J.E.; Sherbourne, C.D. The MOS 36-ltem Short-Form Health Survey (SF-36). Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Hunt, S.M.; McKenna, S.P.; McEwen, J.; Williams, J.; Papp, E. The Nottingham health profile: Subjective health status and medical consultations. Soc. Sci. Med. Part A Med. Psychol. Med. Sociol. 1981, 15, 221–229. [Google Scholar] [CrossRef]
- Brooks, R. EuroQol: The current state of play. Health Policy 1996, 37, 53–72. [Google Scholar] [CrossRef]
- Makovski, T.T.; Schmitz, S.; Zeegers, M.P.; Stranges, S.; van den Akker, M. Multimorbidity and quality of life: Systematic literature review and meta-analysis. Ageing Res. Rev. 2019, 53. [Google Scholar] [CrossRef] [PubMed]
- The World Health Organization quality of life assessment (WHOQOL): Position paper from the World Health Organization. Soc. Sci. Med. 1995, 41, 1403–1409. [CrossRef]
- Lucas-Carrasco, R. The WHO quality of life (WHOQOL) questionnaire: Spanish development and validation studies. Qual. Life Res. 2012, 21, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Motamed, N.; Ayatollahi, A.R.; Zare, N.; Sadeghi-Hassanabadi, A. Validity and reliability of the Persian translation of the SF-36 version 2 questionnaire. East Mediterr. Health J. 2005, 11, 349–357. [Google Scholar]
- Alonso, J.; Prieto, L.; Antó, J.M. The Spanish version of the SF-36 Health Survey (the SF-36 health questionnaire): An instrument for measuring clinical results. Med. Clín. 1995, 104, 771–776. [Google Scholar]
- Ware, J.E. SF-36 Health Survey Update. Spine 2000, 25, 3130–3139. [Google Scholar] [CrossRef] [PubMed]
- Gigic, B.; Boeing, H.; Toth, R.; Böhm, J.; Habermann, N.; Scherer, D.; Schrotz-King, P.; Abbenhardt-Martin, C.; Skender, S.; Brenner, H.; et al. Associations Between Dietary Patterns and Longitudinal Quality of Life Changes in Colorectal Cancer Patients: The ColoCare Study. Nutr. Cancer 2018, 70, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, N.; Chen, Y.; Nie, X.; Li, Q.; Han, B.; Chen, Y.; Xia, F.; Cang, Z.; Lu, M.; et al. Health-related quality of life in type-2 diabetes patients: A cross-sectional study in East China. BMC Endocr. Disord. 2017, 17, 38. [Google Scholar] [CrossRef] [PubMed]
- Franquelo-Morales, P.; Gómez-Marcos, M.Á.; Martínez-Vizcaíno, V.; Notario-Pacheco, B.; Lahoz-García, N.; Miota-Ibarra, J.; Sánchez-López, M. Association Between Health-Related Quality of Life, Obesity, Fitness, and Sleep Quality in Young Adults: The Cuenca Adult Study. Behav. Sleep Med. 2016, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Q.; Pang, G.; Lin, L.; Origasa, H.; Wang, Y.; Di, J.; Shi, M.; Fan, C.; Shi, H. Association between Body Mass Index and Health-Related Quality of Life: The “Obesity Paradox” in 21,218 Adults of the Chinese General Population. PLoS ONE 2015, 10, e0130613. [Google Scholar] [CrossRef] [PubMed]
- Raymakers, A.J.N.; Gillespie, P.; O’Hara, M.C.; Griffin, M.D.; Dinneen, S.F. Factors influencing health-related quality of life in patients with Type 1 diabetes. Health Qual. Life Outcomes 2018, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Han, K.-T.; Park, E.-C.; Kim, J.-H.; Kim, S.J.; Park, S. Is marital status associated with quality of life? Health Qual. Life Outcomes 2014, 12, 109. [Google Scholar] [CrossRef]
- Zimbudzi, E.; Lo, C.; Ranasinha, S.; Gallagher, M.; Fulcher, G.; Kerr, P.G.; Russell, G.; Teede, H.; Usherwood, T.; Walker, R.; et al. Predictors of Health-Related Quality of Life in Patients with Co-Morbid Diabetes and Chronic Kidney Disease. PLoS ONE 2016, 11, e0168491. [Google Scholar] [CrossRef]
- Peleias, M.; Tempski, P.; Paro, H.B.; Perotta, B.; Mayer, F.B.; Enns, S.C.; Gannam, S.; Pereira, M.A.D.; Silveira, P.S.; Santos, I.S.; et al. Leisure time physical activity and quality of life in medical students: Results from a multicentre study. BMJ Open Sport Exerc. Med. 2017, 3, e000213. [Google Scholar] [CrossRef]
- Laxy, M.; Teuner, C.; Holle, R.; Kurz, C. The association between BMI and health-related quality of life in the US population: Sex, age and ethnicity matters. Int. J. Obes. 2018, 42, 318–326. [Google Scholar] [CrossRef]
- Carlos, S.; de La Fuente-Arrillaga, C.; Bes-Rastrollo, M.; Razquin, C.; Rico-Campà, A.; Martínez-González, M.; Ruiz-Canela, M. Mediterranean Diet and Health Outcomes in the SUN Cohort. Nutrients 2018, 10, 439. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Beunza, J.J.; Delgado-Rodríguez, M.; Martínez-González, M.A. Validation of self reported diagnosis of hypertension in a cohort of university graduates in Spain. BMC Public Health 2005, 5, 94. [Google Scholar] [CrossRef] [PubMed]
- Guitérrez-Bedmar, M.; Seguí-Gómez, M.; Gómez-Gracia, E.; Bes-Rastrollo, M.; Martínez-González, M. Smoking Status, Changes in Smoking Status and Health-Related Quality of Life: Findings from the SUN (“Seguimiento Universidad de Navarra”) Cohort. Int. J. Environ Res. Public Health 2009, 6, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Bes-Rastrollo, M.; Pérez Valdivieso, J.R.; Sánchez-Villegas, A.; Alonso, Á.; Martínez-González, M.Á. Validación del peso e índice de masa corporal auto-declarados de los participantes de una cohorte de graduados universitarios. Rev. Esp. Obes. 2005, 3, 352–358. [Google Scholar]
- Chasan-Taber, S.; Rimm, E.B.; Stampfer, M.J.; Spiegelman, D.; Colditz, G.A.; Giovannucci, E.; Ascherio, A.; Willett, W.C. Reproducibility and Validity of a Self-Administered Physical Activity Questionnaire for Male Health Professionals. Epidemiology 1996, 7, 81–86. [Google Scholar] [CrossRef]
- Wolf, A.M.; Hunter, D.J.; Colditz, G.A.; Manson, J.E.; Stampfer, M.J.; Corsano, K.A.; Rosner, B.; Kriska, A.; Willet, W.C. Reproducibility and Validity of a Self-Administered Physical Activity Questionnaire. Int. J. Epidemiol. 1994, 23, 991–999. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; López-Fontana, C.; Varo, J.J.; Sánchez-Villegas, A.; Martinez, J.A. Validation of the Spanish version of the physical activity questionnaire used in the Nurses’ Health Study and the Health Professionals’ Follow-up Study. Public Health Nutr. 2005, 8, 920–927. [Google Scholar] [CrossRef]
- Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A.; Kushida, C.; et al. Recommended Amount of Sleep for a Healthy Adult: A Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep 2015, 38, 843–844. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Grandner, M.A.; Brown, D.; Conroy, M.B.; Jean-Louis, G.; Coons, M.; Bhatt, D.L. Sleep Duration and Quality: Impact on Lifestyle Behaviors and Cardiometabolic Health: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e367–e386. [Google Scholar] [CrossRef]
- Dalmases, M.; Benítez, I.D.; Mas, A.; Garcia-Codina, O.; Medina-Bustos, A.; Escarrabill, J.; Saltó, E.; Buysse, D.J.; Roure, N.; Sánchez-de-la-Torre, M.; et al. Assessing sleep health in a European population: Results of the catalan health survey 2015. PLoS ONE 2018, 13, e0194495. [Google Scholar] [CrossRef]
- Sayón-Orea, C.; Bes-Rastrollo, M.; Carlos, S.; Beunza, J.J.; Basterra-Gortari, F.J.; Martínez-González, M.A. Association between sleeping hours and siesta and the risk of obesity: The SUN Mediterranean cohort. Obes. Fact. 2013, 6, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ballart, J.D.; Piñol, J.L.; Zazpe, I.; Corella, D.; Carrasco, P.; Toledo, E.; Perez-Bauer, M.; Martínez-González, M.Á.; Salas-Salvadó, J.; Martín-Moreno, J.M. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br. J. Nutr. 2010, 103, 1808–1816. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente-Arrillaga, C.; Vázquez Ruiz, Z.; Bes-Rastrollo, M.; Sampson, L.; Martinez-González, M.A. Reproducibility of an FFQ validated in Spain. Public Health Nutr. 2010, 13, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- Martin-Moreno, J.M.; Boyle, P.; Gorgojo, L.; Maisonnueve, P.; Fernandez-Rodriguez, J.C.; Salvini, S.; Willett, W.C. Development and Validation of a Food Frequency Questionnaire in Spain. Int. J. Epidemiol. 1993, 22, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Aranceta-Bartrina, J.; Partearroyo, T.; López-Sobaler, A.M.; Ortega, R.M.; Varela-Moreiras, G.; Serra-Majem, L.; Pérez-Rodrigo, C. Updating the Food-Based Dietary Guidelines for the Spanish Population: The Spanish Society of Community Nutrition (SENC) Proposal. Nutrients 2019, 11, 2675. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Kouris-Blazos, A.; Wahlqvist, M.L.; Gnardellis, C.; Lagiou, P.; Polychronopoulos, E.; Vassilakou, T.; Lipworth, L.; Trichopoulos, D. Diet and overall survival in elderly people. BMJ 1995, 311, 1457–1460. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Sánchez-Tainta, A.; Corella, D.; Salas-Salvadó, J.; Ros, E.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; Lamuela-Raventós, R.M.; Schröder, H.; et al. A provegetarian food pattern and reduction in total mortality in the Prevención con Dieta Mediterránea (PREDIMED) study. Am. J. Clin. Nutr. 2014, 100, 320–328. [Google Scholar] [CrossRef]
- Gómez-Donoso, C.; Martínez-González, M.A.; Martínez, J.A.; Gea, A.; Sanz-Serrano, J.; Perez-Cueto, F.J.A.; Bes-Rastrollo, M. A Provegetarian Food Pattern Emphasizing Preference for Healthy Plant-Derived Foods Reduces the Risk of Overweight/Obesity in the SUN Cohort. Nutrients 2019, 11, 1553. [Google Scholar] [CrossRef]
- Vilagut, G.; Ferrer, M.; Rajmil, L.; Rebollo, P.; Permanyer-Miralda, G.; Quintana, J.M.; Santed, R.; Valderas, J.M.; Ribera, A.; Domingo-Salvany, A.; et al. El Cuestionario de Salud SF-36 español: Una década de experiencia y nuevos desarrollos. Gac. Sanit. 2005, 19, 135–150. [Google Scholar] [CrossRef]
- Amiri, P.; Jalali-Farahani, S.; Rezaei, M.; Cheraghi, L.; Hosseinpanah, F.; Azizi, F. Which obesity phenotypes predict poor health-related quality of life in adult men and women? Tehran Lipid and Glucose Study. PLoS ONE 2018, 13, e0203028. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, A.F.; Graco, M.; Rasekaba, T.M.; Parikh, S.; Berlowitz, D.J.; Lim, W.K. Relationship between health-related quality of life, comorbidities and acute health care utilisation, in adults with chronic conditions. Health Qual. Life Outcomes 2015, 13, 69. [Google Scholar] [CrossRef] [PubMed]
- Ambak, R.; Mohamad Nor, N.S.; Puteh, N.; Mohd Tamil, A.; Omar, M.A.; Shahar, S.; Ahmad, N.A.; Aris, T. The effect of weight loss intervention programme on health-related quality of life among low income overweight and obese housewives in the MyBFF@home study. BMC Women Health 2018, 18, 111. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.C.; Lash, J.P.; Xie, D.; Pan, Q.; DeLuca, J.; Kanthety, R.; Kusek, J.W.; Lora, C.M.; Nessel, L.; Ricardo, A.C.; et al. Predictors and Outcomes of Health-Related Quality of Life in Adults with CKD. Clin. J. Am. Soc. Nephrol. 2016, 11, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Fanning, J.; Walkup, M.P.; Ambrosius, W.T.; Brawley, L.R.; Ip, E.H.; Marsh, A.P.; Rejeski, W.J. Change in health-related quality of life and social cognitive outcomes in obese, older adults in a randomized controlled weight loss trial: Does physical activity behavior matter? J. Behav. Med. 2018, 41, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Napoli, N.; Shah, K.; Waters, D.L.; Sinacore, D.R.; Qualls, C.; Villareal, D.T. Effect of weight loss, exercise, or both on cognition and quality of life in obese older adults. Am. J. Clin. Nutr. 2014, 100, 189–198. [Google Scholar] [CrossRef]
- van Gemert, W.A.M.; van der Palen, J.; Monninkhof, E.M.; Rozeboom, A.; Peters, R.; Wittink, H.; Schuit, A.J.; Peeters, P.H. Quality of Life after Diet or Exercise-Induced Weight Loss in Overweight to Obese Postmenopausal Women: The SHAPE-2 Randomised Controlled Trial. PLoS ONE 2015, 10, e0127520. [Google Scholar] [CrossRef]
- Ruano, C.; Henriquez, P.; Martínez-González, M.Á.; Bes-Rastrollo, M.; Ruiz-Canela, M.; Sá Nchez-Villegas, A. Empirically Derived Dietary Patterns and Health-Related Quality of Life in the SUN Project. PLoS ONE 2013, 8, e61490. [Google Scholar] [CrossRef]
- de Cuevillas García, B.; Álvarez Álvarez, I.; Cuervo Zapatel, M.; Fernández Montero, A.; Navas Carretero, S.; Martínez Hernández, J.A. Definition of nutritionally qualitative categorizing (proto)nutritypes and a pilot quantitative nutrimeter for mirroring nutritional well-being based on a quality of life health related questionnaire. Nutr. Hosp. 2019, 36, 862–874. [Google Scholar]
- Oyewole, O.O.; Odusan, O.; Oritogun, K.S.; Idowu, A.O. Predictability of physical activity and bodyweight on health-related quality of life amongst Nigerian type 2 diabetes mellitus. Int. J. Diabetes Dev. C 2015, 35, 194–200. [Google Scholar] [CrossRef]
- Cameron, A.J.; Magliano, D.J.; Dunstan, D.W.; Zimmet, P.Z.; Hesketh, K.; Peeters, A.; Shaw, J.E. A bi-directional relationship between obesity and health-related quality of life: Evidence from the longitudinal AusDiab study. Int. J. Obes. 2012, 36, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Galilea-Zabalza, I.; Buil-Cosiales, P.; Salas-Salvadó, J.; Toledo, E.; Ortega-Azorín, C.; Díez-Espino, J.; Vázquez-Ruiz, Z.; Zomeño, M.D.; Vioque, J.; Martínez, J.A.; et al. Mediterranean diet and quality of life: Baseline cross-sectional analysis of the PREDIMED-PLUS trial. PLoS ONE 2018, 13, e0198974. [Google Scholar] [CrossRef] [PubMed]
- Kosinski, M.; Zhao, S.Z.; Dedhiya, S.; Osterhaus, J.T.; Ware, J.E. Determining minimally important changes in generic and disease-specific health-related quality of life questionnaires in clinical trials of rheumatoid arthritis. Arthritis Rheum. 2000, 43, 1478–1487. [Google Scholar] [CrossRef]
- Paulis, W.D.; Palmer, M.; Chondros, P.; Kauer, S.; van Middelkoop, M.; Sanci, L.A. Health profiles of overweight and obese youth attending general practice. Arch. Dis. Child. 2017, 102, 434–439. [Google Scholar] [CrossRef]
- Li, C.-L.; Chang, H.-Y.; Hsu, C.-C.; Lu, J.R.; Fang, H.-L. Joint predictability of health-related quality of life and leisure time physical activity on mortality risk in people with diabetes. BMC Public Health 2013, 13, 67. [Google Scholar] [CrossRef]
- Sayón-Orea, C.; Santiago, S.; Bes-Rastrollo, M.; Martínez-González, M.; Pastor, M.; Moreno-Aliaga, M.; Tur, J.; Garcia, A.; Martínez, J. Determinants of Self-Rated Health Perception in a Sample of a Physically Active Population: PLENUFAR VI Study. Int. J. Environ. Res. Public Health 2018, 15, 2104. [Google Scholar] [CrossRef]
- Pawełczyk, T.; Piątkowska-Janko, E.; Bogorodzki, P.; Gębski, P.; Grancow-Grabka, M.; Trafalska, E.; Żurner, N.; Pawełczyk, A. Omega-3 fatty acid supplementation may prevent loss of gray matter thickness in the left parieto-occipital cortex in first episode schizophrenia: A secondary outcome analysis of the OFFER randomized controlled study. Schizophr. Res. 2018, 195, 168–175. [Google Scholar] [CrossRef]
- Mazza, E.; Fava, A.; Ferro, Y.; Rotundo, S.; Romeo, S.; Bosco, D.; Pujia, A.; Montalcini, T. Effect of the replacement of dietary vegetable oils with a low dose of extravirgin olive oil in the Mediterranean Diet on cognitive functions in the elderly. J. Trans. Med. 2018, 16, 10. [Google Scholar] [CrossRef]
- Godos, J.; Castellano, S.; Marranzano, M. Adherence to a Mediterranean Dietary Pattern Is Associated with Higher Quality of Life in a Cohort of Italian Adults. Nutrients 2019, 11, 981. [Google Scholar] [CrossRef]
- Alvarez-Alvarez, I.; Toledo, E.; Lecea, O.; Salas-Salvadó, J.; Corella, D.; Buil-Cosiales, P.; Zomeño, M.D.; Vioque, J.; Martinez, J.A.; Konieczna, J.; et al. Adherence to a priori dietary indexes and baseline prevalence of cardiovascular risk factors in the PREDIMED-Plus randomised trial. Eur. J. Nutr. 2020, 59, 1219–1232. [Google Scholar] [CrossRef]
- Boldt, P.; Knechtle, B.; Nikolaidis, P.; Lechleitner, C.; Wirnitzer, G.; Leitzmann, C.; Rosemann, T.; Wirnitzer, K. Quality of life of female and male vegetarian and vegan endurance runners compared to omnivores—Results from the NURMI study (step 2). J. Int. Soc. Sports Nutr. 2018, 15, 33. [Google Scholar] [CrossRef] [PubMed]

| Individual Characteristics | Total (n = 15,674) | MedDiet Score | p-Value | ||
|---|---|---|---|---|---|
| Low (0–2 pts) | Moderate (3–6 pts) | High (7–9 pts) | |||
| Sex (%, men) | 40.2 | 39.4 | 40.0 | 43.1 | * |
| Age (years) | 38.5 (12.1) | 34.5 (10.3) | 38.8 (12.1) | 43.7 (12.7) | * |
| Marital status: (%) | |||||
| Single | 43.0 | 52.7 | 42.0 | 33.2 | ** |
| Married | 52.2 | 44.5 | 52.9 | 60.0 | |
| Widow | 0.9 | 0.4 | 0.9 | 1.8 | |
| Separated | 2.5 | 1.5 | 2.6 | 3.1 | |
| Other | 1.5 | 0.9 | 1.6 | 1.9 | |
| Diabetes (%) | 1.9 | 0.8 | 2.0 | 2.8 | ** |
| Hypertension (%) | 11.0 | 7.5 | 11.2 | 15.1 | ** |
| Hypercholesterolemia (%) | 17.9 | 12.1 | 18.0 | 27.4 | ** |
| Family history of chronic disease (%) | 63.8 | 58.7 | 64.2 | 69.8 | ** |
| Sleeping hours (h/day) | 7.3 (0.8) | 7.4 (0.8) | 7.3 (0.8) | 7.2 (0.8) | * |
| Siesta (min/day) | 16.8 (43.2) | 16.0 (44.9) | 16.9 (43.0) | 17.7 (41.6) | - |
| Body Mass Index (BMI) (kg/m2) | 23.6 (3.5) | 23.1 (3.5) | 23.6 (3.5) | 23.9 (3.6) | ** |
| Total energy intake (kcal/day) | 2344 (615) | 2202 (593) | 2349 (622) | 2549 (543) | * |
| Mediterranean Diet Score (0–9 points) | 4.2 (1.8) | 1.6 (0.6) | 4.4 (1.1) | 7.3 (0.5) | * |
| Provegetarian Pattern (12–60 points) | 30.0 (5.0) | 25.7 (4.0) | 30.3 (4.4) | 35.4 (4.0) | * |
| Physical Activity (METs-h/week) | 21.6 (22.6) | 17.6 (20.2) | 21.9 (22.4) | 26.9 (25.8) | * |
| Individual Characteristics | Global | Physical | Mental | Transition |
|---|---|---|---|---|
| Sex: | ||||
| Female | 77.2 (0.1) * | 83.7 (0.1) * | 70.7 (0.1) * | 50.9 (0.2) * |
| Male (ref.) | 79.9 (0.1) | 86.8 (0.2) | 73.0 (0.1) | 51.9 (0.2) |
| Age: | ||||
| <36 years (ref.) | 78.9 (0.1) | 86.6 (0.2) | 71.1 (0.1) | 52.6 (0.2) |
| >36 years | 77.8 (0.1) * | 83.4 (0.2) * | 72.1 (0.1) * | 50.1 (0.2) * |
| Marital status: | ||||
| Single (ref.) | 78.1 (0.1) | 84.6 (0.2) | 71.5 (0.2) | 51.5 (0.2) |
| Married | 78.6 (0.1) * | 85.4 (0.2) * | 71.9 (0.1) | 51.2 (0.2) |
| Widow | 78.1 (0.9) | 85.9 (1.2) | 70.3 (1.0) | 52.0 (1.3) |
| Separated | 77.1 (0.5) | 83.8 (0.7) | 70.5 (0.6) | 52.3 (0.8) |
| Other | 76.6 (0.7) * | 82.8 (0.9) * | 70.3 (0.8) | 52.9 (1.0) |
| Diabetes: | ||||
| No (ref.) | 78.4 (0.1) | 85.1 (0.1) | 71.7 (0.1) | 51.3 (0.1) |
| Yes | 75.4 (0.6) * | 80.3 (0.8) * | 70.5 (0.7) | 51.3 (0.9) |
| Hypertension: | ||||
| No (ref.) | 78.6 (0.1) | 85.4 (0.1) | 71.8 (0.1) | 51.3 (0.1) |
| Yes | 76.4 (0.3) * | 81.9 (0.3) * | 70.2 (0.3) * | 51.5 (0.4) |
| Hypercholesterolemia: | ||||
| No (ref.) | 78.5 (0.1) | 85.3 (0.1) | 71.8 (0.1) | 51.2 (0.1) |
| Yes | 77.2 (0.2) * | 83.5 (0.3) * | 70.9 (0.2) * | 52.0 (0.3) * |
| Family history of diseases | ||||
| None (ref.) | 78.8 (0.1) | 85.7 (0.2) | 71.9 (0.2) | 51.3 (0.2) |
| At least one disease | 78.0 (0.1) * | 84.5 (0.1) * | 71.4 (0.1) * | 51.3 (0.2) |
| Sleeping hours (h/day): | ||||
| <7 | 77.2 (0.2) * | 84.2 (0.3) * | 70.3 (0.2) * | 50.8 (0.3) * |
| 7–8 (ref.) | 78.6 (0.1) | 85.3 (0.1) | 72.0 (0.1) | 51.5 (0.1) |
| >8 | 77.7 (0.3) * | 84.0 (0.3) * | 71.4 (0.3) | 51.2 (0.4) |
| Siesta (min/day) | ||||
| <30 (ref.) | 78.5 (0.1) | 85.3 (0.1) | 71.8 (0.1) | 51.3 (0.1) |
| >30 | 77.0 (0.2) * | 83.2 (0.3) * | 70.8 (0.2) * | 51.3 (0.3) |
| BMI categories: | ||||
| Underweight | 78.3 (0.4) | 85.2 (0.6) | 71.4 (0.5) | 51.6 (0.6) |
| Normal (ref.) | 78.7 (0.1) | 85.6 (0.1) | 71.8 (0.1) | 51.2 (0.2) |
| Overweight | 77.7 (0.2) * | 84.0 (0.2) * | 71.5 (0.2) | 51.5 (0.3) |
| Obesity | 75.6 (0.4) * | 80.7 (0.5) * | 70.5 (0.4) * | 52.4 (0.6) * |
| Smoking Status: | ||||
| Never (ref.) | 78.9 (0.1) | 85.6 (0.2) | 72.1 (0.1) | 51.1 (0.2) |
| Current | 77.4 (0.2) * | 84.3 (0.2) * | 70.5 (0.2) * | 51.4 (0.3) |
| Former | 78.0 (0.2) * | 84.3 (0.2) * | 71.7 (0.2) | 51.6 (0.2) |
| Personal Characteristics | Global | Physical | Mental | Transition |
|---|---|---|---|---|
| Female (male ref.) | −3.28 (−3.68, −2.89) * | −4.06 (−4.57, −3.55) * | −2.50 (−2.94, −2.07) * | −0.84 (−1.43, −0.25) * |
| Age (years) | −0.03 (−0.05, −0.02) * | −0.14 (−0.16, −0.11) * | 0.07 (0.05, 0.09) * | −0.16 (−0.19, −0.13) * |
| Marital Status | ||||
| Single (ref.) | 0 (Ref) | 0 (Ref) | 0 (Ref) | 0 (Ref) |
| Married | 0.70 (0.29, 1.10) * | 0.95 (0.43, 1.48) * | 0.44 (−0.01, 0.89) | −0.44 (−1.04, 0.17) |
| Widow | 0.32 (−1.44, 2.09) | 1.63 (−0.68, 3.93) | −0.98 (−2.94, 0.98) | 0.42 (−2.23, 3.07) |
| Separated | −0.74 (−1.83, 0.35) | −0.66 (−2.08, 0.76) | −0.82 (−2.03, 0.39) | 0.63 (−1.00, 2.26) |
| Other | −1.38 (−2.72, −0.02) * | −1.57 (−3.33, 0.19) | −1.16 (−2.66, 0.33) | 1.08 (−0.93, 3.09) |
| Pre-existing Diabetes | −2.27 (−3.48, −1.06) * | −3.75 (−5.33, −2.16) * | −0.79 (−2.14, 0.56) | −0.48 (−2.30, 1.33) |
| Pre-existing Hypertension | −1.79 (−2.36, −1.22) * | −2.39 (−3.13, −1.65) * | −1.20 (−1.83, 0.57) | 0.06 (−0.79, 0.90) |
| Pre-existing Hypercholesterolemia | −1.04 (−1.48, −0.59) * | −1.37 (−1.95, –0.79) * | −0.70 (−1.20, −0.21) * | 0.70 (−0.03, 1.36) |
| Family history of diseases Prev. | −0.65 (−1.00, −0.30) * | −0.91 (−1.36, −0.46) * | −0.40 (−0.79, −0.02) * | −0.15 (−0.66, 0.37) |
| Sleeping hours at night (h/day) | ||||
| <7 | −1.10 (−1.54, −0.67) * | −0.68 (−1.25, −0.11) * | −1.53 (−2.01, −1.05) * | −0.77 (−1.42, −0.12) * |
| 7–8 | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| >8 | −0.68 (−1.22, −0.14) * | −0.95 (−1.66, −0.25) * | −0.40 (−1.00, 0.20) | −0.16 (−0.96, 0.65) |
| Siesta (min/day) | ||||
| <30 | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| >30 | −1.09 (−1.55, −0.64) * | −1.57 (−2.16, −0.97) * | −0.62 (−1.13, −0.11) * | 0.04 (−0.63, 0.72) |
| BMI (kg/m2) | −0.18 (−0.24, −0.12) * | −0.30 (−0.37, −0.23) * | −0.06 (−0.12, 0.003) | 0.09 (−0.01, 0.18) |
| Physical activity (METs-h/wk) | 0.03 (0.02, 0.03) * | 0.03 (0.02, 0.04) * | 0.03 (0.02, 0.03) * | 0.02 (0.01, 0.03) * |
| Smoking status | ||||
| Never | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| Current | −1.26 (−1.68, −0.83) * | −1.14 (−1.69, −0.58) * | −1.38 (−1.85, −0.91) * | 0.34 (−0.29, 1.00) |
| Former | −0.81 (−1.21, −0.41) * | −1.23 (−1.75, −0.71) * | −0.39 (−0.84, 0.05) | 0.32 (−0.27, 0.91) |
| Diet | Global | Physical | Mental | Transition |
|---|---|---|---|---|
| MedDiet | 0.32 (0.22, 0.42) * | 0.37 (0.25, 0.50) * | 0.27 (0.16, 0.37) * | 0.39 (0.25, 0.54) * |
| MUFA/SFA | 0.45 (0.11, 0.80) * | 0.28 (−0.17, 0.73) | 0.63 (0.25, 1.01) * | 0.11 (−0.41, 0.62) |
| Alcohol | 0.53 (0.16, 0.89) * | 0.81 (0.33, 1.29) * | 0.24 (−0.17, 0.65) | −0.37 (−0.92, 0.18) |
| Cereal | 0.16 (−0.20, 0.52) | 0.21 (−0.26, 0.67) | 0.11 (−0.28, 0.51) | −0.19 (−0.34, 0.72) |
| Vegetables | 0.35 (−0.003, 0.69) | 0.60 (0.15, 1.05) * | 0.09 (−0.30, 0.48) | 0.80 (0.28, 1.32) * |
| Fruits | 0.74 (0.39, 1.09) * | 0.68 (0.23, 1.14) * | 0.80 (0.41, 1.20) * | 0.81 (0.28, 1.33) * |
| Fish | 0.39 (0.05, 0.73) * | 0.27 (−0.18, 0.71) | 0.51 (0.14, 0.89) * | 0.31 (−0.20, 0.82) |
| Legumes | 0.40 (0.06, 0.73) * | 0.38 (−0.06, 0.82) | 0.41 (0.04, 0.79) * | 0.50 (−0.001, 1.01) |
| Dairy | −0.12 (−0.48, 0.24) | 0.18 (−0.29, 0.65) | −0.42 (−0.82, 0.02) | 0.44 (−0.10, 0.98) |
| Meat | −0.05 (−0.41, 0.30) | −0.01 (−0.46, 0.47) | −0.11 (−0.51, 0.28) | 0.37 (−0.16, 0.90) |
| Provegetarian FP | 0.09 (0.06, 0.12) * | 0.11 (0.07, 0.15) * | 0.07 (0.03, 0.11) * | 0.14 (0.09, 0.19) * |
| Very Low | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) | 0 (Ref.) |
| Low | 0.59 (0.21, 0.98) * | 0.66 (0.16, 1.16) * | 0.53 (0.11, 0.96) * | 0.40 (−0.17, 0.97) |
| Moderate | 0.72 (0.24, 1.20) * | 1.00 (0.38, 1.62) * | 0.44 (−0.09, 0.97) | 1.36 (0.65, 2.07) * |
| High | 1.38 (0.44, 2.31) * | 1.20 (−0.03, 2.42) | 1.55 (0.51, 2.60) * | 2.39 (0.99, 3.80) * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pano, O.; Sayón-Orea, C.; Gea, A.; Bes-Rastrollo, M.; Martínez-González, M.Á.; Martínez, J.A. Nutritional Determinants of Quality of Life in a Mediterranean Cohort: The SUN Study. Int. J. Environ. Res. Public Health 2020, 17, 3897. https://doi.org/10.3390/ijerph17113897
Pano O, Sayón-Orea C, Gea A, Bes-Rastrollo M, Martínez-González MÁ, Martínez JA. Nutritional Determinants of Quality of Life in a Mediterranean Cohort: The SUN Study. International Journal of Environmental Research and Public Health. 2020; 17(11):3897. https://doi.org/10.3390/ijerph17113897
Chicago/Turabian StylePano, Octavio, Carmen Sayón-Orea, Alfredo Gea, Maira Bes-Rastrollo, Miguel Ángel Martínez-González, and J. Alfredo Martínez. 2020. "Nutritional Determinants of Quality of Life in a Mediterranean Cohort: The SUN Study" International Journal of Environmental Research and Public Health 17, no. 11: 3897. https://doi.org/10.3390/ijerph17113897
APA StylePano, O., Sayón-Orea, C., Gea, A., Bes-Rastrollo, M., Martínez-González, M. Á., & Martínez, J. A. (2020). Nutritional Determinants of Quality of Life in a Mediterranean Cohort: The SUN Study. International Journal of Environmental Research and Public Health, 17(11), 3897. https://doi.org/10.3390/ijerph17113897






