Visceral Fat Is a Negative Determinant of Bone Health in Obese Postmenopausal Women
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Participant Recruitment
2.2. BMD and Body Composition Analyses
2.3. Biochemical Analyses
2.4. Sample Size and Statistical Analyses
3. Results
3.1. Participant Characteristics
3.2. Spearman Correlations between Bone Markers, Body Fat and Metabolic Variables
3.3. Effect VAT on CTX-1, Osteocalcin and Bone ALP Levels
3.4. Effect of VAT on total BMD
3.5. Effect of VAT on Vitamin D Metabolites
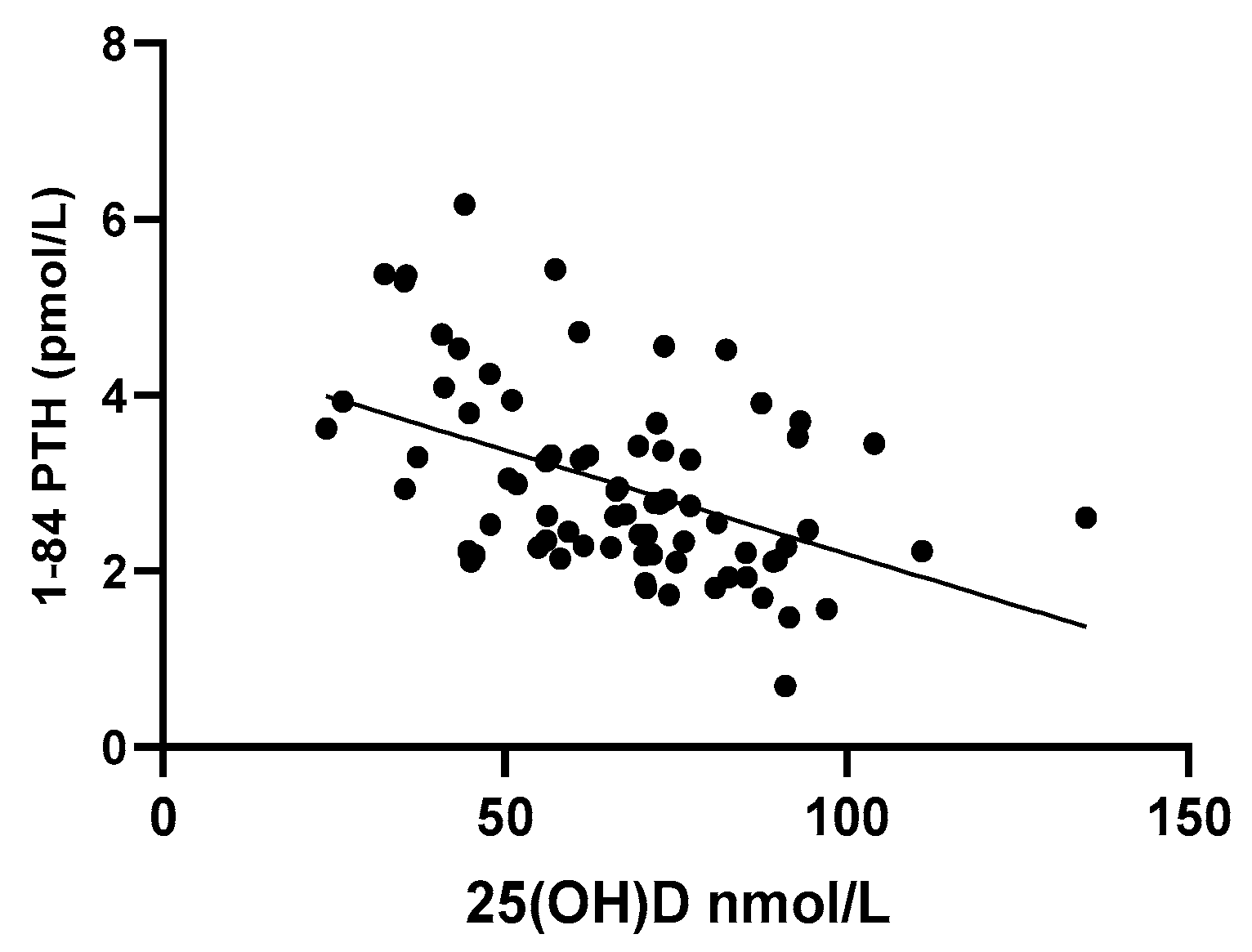
3.6. Effect of VAT on PTH Levels
3.7. Effect of VAT on Calcium, ALP and Phosphorus
4. Discussion
5. Conclusions
6. Study Highlights
- Postmenopausal women with higher visceral fat have reduced bone resorption (CTX-1 levels) despite elevated PTH levels.
- Visceral fat is an independent negative predictor of total bone density, CTX-1 and osteocalcin levels.
- The clinical implications of low bone turnover with higher VAT levels are discussed briefly.
Author Contributions
Funding
Conflicts of Interest
References
- Evans, A.L.; Paggiosi, M.A.; Eastell, R.; Walsh, J.S. Bone density, microstructure and strength in obese and normal weight men and women in younger and older adulthood. J. Bone Miner. Res. 2015, 30, 920–928. [Google Scholar] [CrossRef]
- Compston, J.E.; Watts, N.B.; Chapurlat, R.; Cooper, C.; Boonen, S.; Greenspan, S.; Pfeilschifter, J.; Silverman, S.; Diez-Perez, A.; Lindsay, R.; et al. Obesity is not protective against fracture in postmenopausal women: GLOW. Am. J. Med. 2011, 124, 1043–1050. [Google Scholar] [CrossRef] [Green Version]
- Prieto-Alhambra, D.; Premaor, M.O.; Fina Avilés, F.; Hermosilla, E.; Martinez-Laguna, D.; Carbonell-Abella, C.; Nogués, X.; Compston, J.E.; Díez-Pérez, A. The association between fracture and obesity is site-dependent: A population-based study in postmenopausal women. J. Bone Miner. Res. 2012, 27, 294–300. [Google Scholar] [CrossRef]
- Johansson, H.; Kanis, J.A.; Oden, A.; McCloskey, E.; Chapurlat, R.D.; Christiansen, C.; Cummings, S.R.; Diez-Perez, A.; Eisman, J.A.; Fujiwara, S.; et al. A meta-analysis of the association of fracture risk and body mass index in women. J. Bone Miner. Res. 2014, 29, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Beck, T.J.; Petit, M.A.; Wu, G.; LeBoff, M.S.; Cauley, J.A.; Chen, Z. Does Obesity Really Make the Femur Stronger? BMD, Geometry, and Fracture Incidence in the Women’s Health Initiative-Observational Study. J. Bone Miner. Res. 2009, 24, 1369–1379. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Gong, X.; Jiang, W. Abdominal obesity and risk of hip fracture: A meta-analysis of prospective studies. Osteoporos Int. 2017, 28, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Dempster, D.W.; Recker, R.R.; Lappe, J.M.; Zhou, H.; Zwahlen, A.; Müller, R.; Zhao, B.; Guo, X.; Lang, T.; et al. Abdominal Fat Is Associated With Lower Bone Formation and Inferior Bone Quality in Healthy Premenopausal Women: A Transiliac Bone Biopsy Study. J. C. Endocrinol. Metab. 2013, 98, 2562–2572. [Google Scholar] [CrossRef]
- Gilsanz, V.; Chalfant, J.; Mo, A.O.; Lee, D.C.; Dorey, F.J.; Mittelman, S.D. Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J. Clin. Endocrinol. Metab. 2009, 94, 3387–3393. [Google Scholar] [CrossRef] [Green Version]
- Bredella, M.A.; Lin, E.; Gerweck, A.V.; Landa, M.G.; Thomas, B.J.; Torriani, M.; Bouxsein, M.L.; Miller, K.K. Determinants of bone microarchitecture and mechanical properties in obese men. J. Clin. Endocrinol. Metab. 2012, 97, 4115–4122. [Google Scholar] [CrossRef] [PubMed]
- Bredella, M.A.; Torriani, M.; Ghomi, R.H.; Thomas, B.J.; Brick, D.J.; Gerweck, A.V.; Harrington, L.M.; Breggia, A.; Rosen, C.J.; Miller, K.K. Determinants of bone mineral density in obese premenopausal women. Bone 2011, 48, 748–754. [Google Scholar] [CrossRef] [Green Version]
- Hind, K.; Pearce, M.; Birrell, F. Total and Visceral Adiposity Are Associated With Prevalent Vertebral Fracture in Women but Not Men at Age 62 Years: The Newcastle Thousand Families Study. J. Bone Miner. Res. 2017, 32, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.T.; Broe, K.E.; Zhou, Y.; Boyd, S.K.; Cupples, L.A.; Hannan, M.T.; Lim, E.; McLean, R.R.; Samelson, E.J.; Bouxsein, M.L.; et al. Visceral Adipose Tissue Is Associated With Bone Microarchitecture in the Framingham Osteoporosis Study. J. Bone Miner. Res. 2017, 32, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Lee, J.Y.; Sung, J. Obesity and Bone Health Revisited: A Mendelian Randomization Study for Koreans. J. Bone Miner. Res. 2019, 34, 1058–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, S.E.; ElShishtawy, H.F.; Helmy, A.; Galal, Z.A.; Abdel Salam, M.H. Serum leptin concentration, bone mineral density and bone biochemical markers in a sample of Egyptian women: A possible relationship. Egypt. Rheumatol. 2011, 33, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Kaul, S.; Rothney, M.P.; Peters, D.M.; Wacker, W.K.; Davis, C.E.; Shapiro, M.D.; Ergun, D.L. Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity 2012, 20, 1313–1318. [Google Scholar] [CrossRef] [Green Version]
- Rothney, M.P.; Xia, Y.; Wacker, W.K.; Martin, F.P.; Beaumont, M.; Rezzi, S.; Giusti, V.; Ergun, D.L. Precision of a new tool to measure visceral adipose tissue (VAT) using dual-energy X-Ray absorptiometry (DXA). Obesity 2013, 21, E134–E136. [Google Scholar] [CrossRef]
- Morris, H.A.; Eastell, R.; Jorgensen, N.R.; Cavalier, E.; Vasikaran, S.; Chubb, S.A.P.; Kanis, J.A.; Cooper, C.; Makris, K. Clinical usefulness of bone turnover marker concentrations in osteoporosis. Clin. Chim. Acta 2017, 467, 34–41. [Google Scholar] [CrossRef]
- Vasikaran, S.; Eastell, R.; Bruyère, O.; Foldes, A.J.; Garnero, P.; Griesmacher, A.; McClung, M.; Morris, H.A.; Silverman, S.; Trenti, T.; et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: A need for international reference standards. Osteoporos. Int. 2011, 22, 391–420. [Google Scholar] [CrossRef]
- Waist circumference and Waist-Hip Ratio, Report of a WHO Expert Consultation GENEVA, 8–11 December 2008. Available online: https://www.who.int/nutrition/publications/obesity/WHO_report_waistcircumference_and_waisthip_ratio/en/ (accessed on 5 July 2019).
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis; Pearson Education: Boston, MA, USA, 2014. [Google Scholar]
- Akinwande, O.; Dikko, H.G.; Agboola, S. Variance Inflation Factor: As a Condition for the Inclusion of Suppressor Variable(s) in Regression Analysis. Open J. Stat. 2015, 05, 754–767. [Google Scholar] [CrossRef] [Green Version]
- Garnero, P.; Sornay-Rendu, E.; Chapuy, M.-C.; Delmas, P.D. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J. Bone Miner. Res. 1996, 11, 337–349. [Google Scholar] [CrossRef]
- Choi, H.S.; Kim, K.J.; Kim, K.M.; Hur, N.W.; Rhee, Y.; Han, D.S.; Lee, E.J.; Lim, S.K. Relationship between visceral adiposity and bone mineral density in Korean adults. Calcif. Tissue Int. 2010, 87, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Pouliot, M.C.; Despres, J.P.; Lemieux, S.; Moorjani, S.; Bouchard, C.; Tremblay, A.; Nadeau, A.; Lupien, P.J. Waist circumference and abdominal sagittal diameter: Best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am. J. Cardiol. 1994, 73, 460–468. [Google Scholar] [CrossRef]
- Walsh, J.S.; Evans, A.L.; Bowles, S.; Naylor, K.E.; Jones, K.S.; Schoenmakers, I.; Jacques, R.M.; Eastell, R. Free 25-hydroxyvitamin D is low in obesity, but there are no adverse associations with bone health. Am. J. Clin. Nutr. 2016, 103, 1465–1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, F.; Traber, L.; Roth, H.J.; Heckel, V.; Schmidt-Gayk, H. Markers of bone resorption--measurement in serum, plasma or urine? Clin. Lab. 2003, 49, 203–207. [Google Scholar]
- Cavalier, E.; Eastell, R.; Jørgensen, N.R.; Makris, K.; Vasikaran, S.; Morris, H.A. Bone Turnover Markers. In Encyclopedia of Endocrine Diseases, 2nd ed.; Huhtaniemi, I., Martini, L., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 116–127. [Google Scholar] [CrossRef]
- Garcia-Martin, A.; Cortes-Berdonces, M.; Luque-Fernandez, I.; Rozas-Moreno, P.; Quesada-Charneco, M.; Munoz-Torres, M. Osteocalcin as a marker of metabolic risk in healthy postmenopausal women. Menopause 2011, 18, 537–541. [Google Scholar] [CrossRef]
- Lee, S.W.; Jo, H.H.; Kim, M.R.; You, Y.O.; Kim, J.H. Association between obesity, metabolic risks and serum osteocalcin level in postmenopausal women. Gynecol. Endocrinol. 2012, 28, 472–477. [Google Scholar] [CrossRef]
- Tonks, K.T.; White, C.P.; Center, J.R.; Samocha-Bonet, D.; Greenfield, J.R. Bone Turnover Is Suppressed in Insulin Resistance, Independent of Adiposity. J. Clin. Endocrinol. Metab. 2017, 102, 1112–1121. [Google Scholar] [CrossRef] [Green Version]
- Viljakainen, H.; Ivaska, K.K.; Paldanius, P.; Lipsanen-Nyman, M.; Saukkonen, T.; Pietilainen, K.H.; Andersson, S.; Laitinen, K.; Makitie, O. Suppressed bone turnover in obesity: A link to energy metabolism? A case-control study. J. Clin. Endocrinol. Metab. 2014, 99, 2155–2163. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.K.; Sowa, H.; Hinoi, E.; Ferron, M.; Ahn, J.D.; Confavreux, C.; Dacquin, R.; Mee, P.J.; McKee, M.D.; Jung, D.Y.; et al. Endocrine regulation of energy metabolism by the skeleton. Cell 2007, 130, 456–469. [Google Scholar] [CrossRef] [Green Version]
- Starup-Linde, J.; Lykkeboe, S.; Gregersen, S.; Hauge, E.M.; Langdahl, B.L.; Handberg, A.; Vestergaard, P. Bone Structure and Predictors of Fracture in Type 1 and Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 928–936. [Google Scholar] [CrossRef] [Green Version]
- Coluzzi, F.; Pergolizzi, J.; Raffa, R.B.; Mattia, C. The unsolved case of “bone-impairing analgesics”: The endocrine effects of opioids on bone metabolism. Ther. Clin. Risk Manag. 2015, 11, 515–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idris, A.I.; Ralston, S.H. Cannabinoids and Bone: Friend or Foe? Calcif. Tissue Int. 2010, 87, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, D.; Sessa, G.; Salvatore, T.; Sasso, F.C.; Giugliano, D.; Lefebvre, P.J.; Torella, R. The involvement of the opioid system in human obesity: A study in normal weight relatives of obese people. J. Clin. Endocrinol. Metab. 1996, 81, 713–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, F.; Punzo, F.; Umano, G.R.; Argenziano, M.; Miraglia Del Giudice, E. Role of Cannabinoids in Obesity. Int. J. Mol. Sci. 2018, 19, 2690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arunabh, S.; Pollack, S.; Yeh, J.; Aloia, J.F. Body fat content and 25-hydroxyvitamin D levels in healthy women. J. Clin. Endocrinol. Metab. 2003, 88, 157–161. [Google Scholar] [CrossRef]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef]
- Saarnio, E.; Pekkinen, M.; Itkonen, S.T.; Kemi, V.; Karp, H.; Ivaska, K.K.; Risteli, J.; Koivula, M.K.; Karkkainen, M.; Makitie, O.; et al. Low free 25-hydroxyvitamin D and high vitamin D binding protein and parathyroid hormone in obese Caucasians. A complex association with bone? PLoS ONE 2018, 13, e0192596. [Google Scholar] [CrossRef] [Green Version]
- Snijder, M.B.; van Dam, R.M.; Visser, M.; Deeg, D.J.; Dekker, J.M.; Bouter, L.M.; Seidell, J.C.; Lips, P. Adiposity in relation to vitamin D status and parathyroid hormone levels: A population-based study in older men and women. J. Clin. Endocrinol. Metab. 2005, 90, 4119–4123. [Google Scholar] [CrossRef] [Green Version]
- Need, A.G.; Morris, H.A.; Horowitz, M.; Nordin, C. Effects of skin thickness, age, body fat, and sunlight on serum 25-hydroxyvitamin D. Am. J. Clin. Nutr. 1993, 58, 882–885. [Google Scholar] [CrossRef]
- Drincic, A.T.; Armas, L.A.; Van Diest, E.E.; Heaney, R.P. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity 2012, 20, 1444–1448. [Google Scholar] [CrossRef]
- Drincic, A.; Fuller, E.; Heaney, R.P.; Armas, L.A. 25-Hydroxyvitamin D response to graded vitamin D(3) supplementation among obese adults. J. Clin. Endocrinol. Metab. 2013, 98, 4845–4851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallagher, J.C.; Yalamanchili, V.; Smith, L.M. The effect of vitamin D supplementation on serum 25(OH)D in thin and obese women. J. Steroid Biochem. Mol. Biol. 2013, 136, 195–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lips, P. Which circulating level of 25-hydroxyvitamin D is appropriate? J. Steroid Biochem. Mol. Biol. 2004, 89, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Vanderschueren, D.; Pye, S.R.; O’Neill, T.W.; Lee, D.M.; Jans, I.; Billen, J.; Gielen, E.; Laurent, M.; Claessens, F.; Adams, J.E.; et al. Active vitamin D (1,25-dihydroxyvitamin D) and bone health in middle-aged and elderly men: The European Male Aging Study (EMAS). J. Clin. Endocrinol. Metab. 2013, 98, 995–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lips, P. Vitamin D physiology. Prog. Biophys. Mol. Biol. 2006, 92, 4–8. [Google Scholar] [CrossRef]
- Lieben, L.; Carmeliet, G.; Masuyama, R. Calcemic actions of vitamin D: Effects on the intestine, kidney and bone. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 561–572. [Google Scholar] [CrossRef]
- Shapses, S.A.; Sukumar, D. The Hormonal Milieu in Obesity and Influences on the Trabecular, Cortical, and Geometric Properties of Bone. In Nutritional Influences on Bone Health: 8th International Symposium; Burckhardt, P., Dawson-Hughes, B., Weaver, M.C., Eds.; Springer: London, UK, 2013; pp. 43–60. [Google Scholar] [CrossRef]
| VAT cm3 | Quartile 1 231 (190–425) | Quartile 2 780 (690–874) | Quartile 3 1208 (1050–1302) | Quartile 4 1723 (1615–2226) | p-Value <0.0001 |
|---|---|---|---|---|---|
| Age, y | 65.7 (61.8–68.8) | 65.4 (61.4–68.1) | 66.3 (64.2–69) | 64.8 (61.2–67.3) | 0.4 (NS) |
| Weight, cm | 57.7 (48.2–63.8) | 68.5 (62.7–78) | 74.8 (72.1–81) | 93 (83.3–100.2) | <0.0001 |
| Height, cm | 159.4 (155.1–162.6) | 165.6 (156.8–169.5) | 163.3 (157.4–165.8) | 161.6 (158.2–164.5) | 0.15 (NS) |
| BMI, kg/m2 | 22.9 (20.2–24.0) | 27.3 (24.0–27.9) | 28.0 (27.2–29.5) | 35.5 (32.0–39.5) | <0.0001 |
| WC, cm | 75 (71–77.5) | 85.6 (81.5–94.7) | 93.5 (88–96) | 109.5 (102–118) | <0.0001 |
| Total fat mass, kg | 18.9 (13.0–22.3) | 28.1 (24.1–31.2) | 32.6 (29.1–36.5) | 44.9 (37.9–49.8) | <0.0001 |
| Total lean mass, kg | 3.5 (3.3–3.9) | 39.7 (34.5–43.4) | 38.6 (36.3–41.2) | 45.1 (39.7–48.6) | <0.0001 |
| Android fat mass, kg | 4.1 (3.4–4.4) | 5 (4.7–5.9) | 5.8 (5.3–6.1) | 7.6 (6.8–8.8) | <0.0001 |
| Android lean mass, kg | 2.7 (2.5–2.9) | 2.9 (2.6–3.2) | 2.7 (2.5–3.2) | 3.3 (2.9–3.9) | <0.0001 |
| Android fat mass, kg | 1.4 (0.8–1.5) | 2.3 (1.9–2.6) | 2.9 (2.6–3.2) | 4.2 (3.8–4.8) | <0.0001 |
| Android fat (%) | 33.7 (24.8–36.9) | 44.8 (42.2–49.9) | 52.1 (46.4–54.2) | 56.5 (54.7–58.8) | <0.0001 |
| Gynoid fat (%) | 39.5 (33.8–43.8) | 47.4 (42.6–48.8) | 47.8 (44.1–49.9) | 50.1 (48.2–53.1) | <0.0001 |
| SAT, cm3 | 1178 (759–1407) | 1714 (1321–2107) | 1817 (1605–2178) | 2510 (2138–3376) | <0.0001 |
| Android/Gynoid ratio | 0.88 (0.74–0.94) | 1.0 (0.9–1.04) | 1.1 (1.0–1.17) | 1.13 (1.08–1.17) | <0.0001 |
| BMD, g/cm2 | 1.04 (0.91–1.14) | 1.11 (0.99–1.14) | 1.12 (0.99–1.2) | 1.17 (1.10–1.23) | 0.016 |
| VAT cm3 | Quartile 1 231 (190–425) | Quartile 2 780 (690–874) | Quartile 3 1208 (1050–1302) | Quartile 4 1723 (1615–2226) | p-Value <0.0001 |
|---|---|---|---|---|---|
| Calcium mmol/L | 2.34 (2.28–2.41) | 2.35 (2.32–2.38) | 2.36 (2.26–2.41) | 2.33 (2.29–2.37) | 0.9 |
| Phosphorus mmol/L | 1.21 (1.09–1.23) | 1.18 (1.11–1.27) | 1.16 (1.14–1.23) | 1.08 (0.96–1.21) | 0.2 |
| ALP U/L | 56.6 (48.7–68.2) | 69.4 (57.6–74.8) | 60.9 (54.6–89.4) | 77.7 (60.8–91.6) | 0.007 |
| 25 (OH)D nmol/L | 72.2 (61.5–91.6) | 67.7 (47.8–77.1) | 71.5 (51–80.8) | 57.4 (41.1–73.2) | 0.029 |
| 1,25 (OH)2D pmol/L | 126 (107–157) | 126 (102–141) | 116 (98.5–154) | 117 (106–138) | 0.767 |
| 1–84 PTH pmol/L | 2.4 (2.2–2.6) | 2.5 (2.1–3.5) | 2.8 (2.2–3.5) | 3.4 (3.0–4.1) | 0.002 |
| Osteocalcin nmol/L | 3.8 (3.4–4.4) | 4.1 (3.5–4.5) | 3.6 (2.9–4.4) | 3.3 (2.9–3.5) | 0.025 |
| Bone ALP µg/L | 11.6 (9.4–13.1) | 13.1 (11.1–15.8) | 12.4 (9.2–17.3) | 14.1 (9.6–19.6) | 0.378 |
| CTX-1 ng/L | 454.2 (397.5–640.6) | 476.5 (346.2–701.2) | 402.4 (320.5–615.7) | 314 (248–418) | 0.041 |
| Variables | 25(OH)D nmol/l | 1–84 PTH pmol/l | Osteocalcin nmol/l | CTX-1 ng/l | Bone ALP µg/l | ALP U/L | Calcium mmol/l | Phosphorus mmol/l | |
|---|---|---|---|---|---|---|---|---|---|
| BMI | r | −0.30 | 0.39 | −0.31 | −0.32 | 0.16 | 0.32 | 0.018 | −0.28 |
| p | 0.008 | 0.001 | 0.007 | 0.005 | NS | 0.004 | NS | 0.014 | |
| WC (cm) | r | −0.22 | 0.33 | −0.30 | −0.29 | 0.086 | 0.24 | 0.07 | −0.28 |
| p | NS | 0.003 | 0.008 | 0.010 | NS | 0.038 | NS | 0.016 | |
| VAT (cm3) | r | −0.24 | 0.37 | −0.35 | −0.36 | 0.18 | 0.37 | 0.002 | −0.24 |
| p | 0.034 | 0.001 | 0.002 | 0.002 | NS | 0.001 | NS | 0.041 | |
| SAT (cm3) | r | −0.34 | 0.36 | −0.23 | −0.26 | 0.20 | 0.32 | −0.05 | −0.17 |
| p | 0.003 | 0.002 | 0.042 | 0.025 | NS | 0.004 | NS | NS | |
| Gynoid fat (%) | r | −0.24 | 0.3 | −0.16 | −0.20 | 0.13 | 0.22 | −0.05 | −0.28 |
| p | 0.035 | 0.008 | NS | 0.076 | NS | 0.06 | NS | 0.014 |
| Independent Predictors | Standardized Coefficient (β) | t | p |
|---|---|---|---|
| 1,25(OH)2D | 0.404 | 3.980 | <0.001 |
| VAT | −0.263 | −2.591 | 0.012 |
| Constant | 193 | 1.932 | 0.057 |
| Independent Predictors | Standardized Coefficient (β) | t | p |
|---|---|---|---|
| 1,25(OH)2D | 0.311 | 2.940 | 0.004 |
| VAT | −0.277 | −2.617 | 0.011 |
| Constant | 2.89 | 6.341 | <0.001 |
| Independent Predictors | Standardized Coefficient (β) | t | p |
|---|---|---|---|
| BMI | 0.871 | 5.833 | <0.001 |
| CTX-1 | −0.279 | −3.198 | 0.002 |
| VAT | −0.368 | −2.435 | 0.017 |
| Constant | 0.764 | 11.383 | <0.001 |
| Independent Predictors | Standardized Coefficient (β) | t | p |
|---|---|---|---|
| 25(OH)D | −0.361 | −3.455 | 0.001 |
| VAT | 0.274 | 2.620 | 0.011 |
| Constant | 3.727 | 8.501 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, D.K.; Anderson, P.H.; Morris, H.A.; Clifton, P.M. Visceral Fat Is a Negative Determinant of Bone Health in Obese Postmenopausal Women. Int. J. Environ. Res. Public Health 2020, 17, 3996. https://doi.org/10.3390/ijerph17113996
Sharma DK, Anderson PH, Morris HA, Clifton PM. Visceral Fat Is a Negative Determinant of Bone Health in Obese Postmenopausal Women. International Journal of Environmental Research and Public Health. 2020; 17(11):3996. https://doi.org/10.3390/ijerph17113996
Chicago/Turabian StyleSharma, Deepti K., Paul H. Anderson, Howard A. Morris, and Peter M. Clifton. 2020. "Visceral Fat Is a Negative Determinant of Bone Health in Obese Postmenopausal Women" International Journal of Environmental Research and Public Health 17, no. 11: 3996. https://doi.org/10.3390/ijerph17113996





