Effects of Particulate Matter Education on Self-Care Knowledge Regarding Air Pollution, Symptom Changes, and Indoor Air Quality among Patients with Chronic Obstructive Pulmonary Disease
Abstract
1. Introduction
1.1. Influence of PM on Respiratory Diseases
1.2. Relation between PM, Symptom Changes, and Self-Care Behaviors in Patients with COPD
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Measures
2.4. Environment Assessment
2.5. COPD Symptoms
2.6. Self-Care Knowledge of PM
2.7. Age-Adjusted Charlson Comorbidity Index (ACCI)
2.8. Lung Function Test
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dominici, F.; Peng, R.D.; Bell, M.L.; Pham, L.; McDermott, A.; Zeger, S.L.; Samet, J.M. Fine Particulate Air Pollution and Hospital Admission for Cardiovascular and Respiratory Diseases. JAMA 2006, 295, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Halonen, J.I.; Lanki, T.; Yli-Tuomi, T.; Tittanen, P.; Kulmala, M.; Pekkanen, J. Particulate air pollution and acute cardiorespiratory hospital admissions and mortality among the elderly. Epidemiology 2009, 20, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Janssen, N.A.; De Hartog, J.J.; Hoek, G.; Brunekreef, B.; Lanki, T.; Timonen, K.L.; Pekkanen, J. Personal exposure to fine particulate matter in elderly subjects: Relation between personal, indoor, and outdoor concentrations. J. Air Waste Manag. Assoc. 2000, 50, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.Y.; Guo, Y.; Markevych, I.; Qian, Z.; Bloom, M.S.; Heinrich, J.; Dharmage, S.C.; Rolling, C.A.; Jordan, S.S.; Komppula, M.; et al. Association of Long-term Exposure to Ambient Air Pollutants With Risk Factors for Cardiovascular Disease in China. JAMA Netw. Open 2019, 2, e190318. [Google Scholar] [CrossRef] [PubMed]
- Taiwan Society of Pulmonary and Critical Care Medicine. 2014 Chronic Obstructive Pulmonary Guidelines; Taiwan Society of Pulmonary and Critical Care Medicine: Taipei City, Taiwan, 2014. [Google Scholar]
- Guarascio, A.J.; Ray, S.M.; Finch, C.K.; Self, T.H. The clinical and economic burden of chronic obstructive pulmonary disease in the USA. Clin. Outcomes Res. 2013, 5, 235–245. [Google Scholar]
- 2018 National Health Insurance Statistical Report. Available online: https://www.mohw.gov.tw/dl-58386-626f953f-85c5-4d14-b86f-434d98a06ace.html (accessed on 29 March 2020).
- Wang, Y.P. The Association of Air Pollution and Health Effects Using GIS Model in Central Taiwan. Master’s Thesis, Institute of Environmnetal Health, China Medical University, Taichung City, Taiwan, 1998. [Google Scholar]
- Mariani, E.; Bonati, E.; Veronesi, L. Respiratory function in subjects with Chronic Obstructive Pulmonary Disease (COPD) and atmospheric pollution in the city of Parma. Preliminary analysis. Acta Biomed. 2010, 81, 109–114. [Google Scholar]
- Paulin, L.; Hansel, N. Particulate air pollution and impaired lung function. F1000Research 2016, 5. [Google Scholar] [CrossRef]
- Detels, R.; Tashkin, D.P.; Sayre, J.W.; Rokaw, S.N.; Coulson, A.H.; Massey, F.J., Jr.; Wegman, D.H. The UCLA population studies of chronic obstructive respiratory disease. Lung function changes associated with chronic exposure to photochemical oxidants; a cohort study among never-smokers. Chest 1987, 92, 594–603. [Google Scholar] [CrossRef]
- Rajak, R.; Chattopadhyay, A. Short and long-term exposure to ambient air pollution and impact on health in India: A systematic review. Int. J. Environ. Health Res. 2019, 1–25. [Google Scholar] [CrossRef]
- Wang, Y.C. Prevalence and Risks for Chronic Pulmonary Diseases in Taiwan. Master’s Thesis, Institute of Environmental Health, National Taiwan University, Taipei City, Taiwan, 2007. [Google Scholar]
- Ding, P.H. Effect of Urban Air Pollution and Climate on Patients with Chronic Obstructive Pulmonary Disease in Taipei. Master’s Thesis, Institute of Environmental Health, National Taiwan University, Taipei City, Taiwan, 2017. [Google Scholar]
- Anderson, H.R.; Atkinson, R.W.; Peacock, J.L.; Sweeting, M.J.; Marston, L. Ambient Particulate Matter and Health Effects: Publication Bias in Studies of Short-Term Associations. Epidemiology 2005, 16, 155–163. [Google Scholar] [CrossRef]
- Ostro, B.; Broadwin, R.; Green, S.; Feng, W.Y.; Lipsett, M. Fine particulate air pollution and mortality in nine California counties: Results from CALFINE. Environ. Health Perspect. 2006, 114, 29–33. [Google Scholar] [CrossRef]
- Hwang, S.L.; Guo, S.E.; Chi, M.C.; Chou, C.T.; Lin, Y.C.; Lin, C.M.; Chou, Y.L. Association between Atmospheric Fine Particulate Matter and Hospital Admissions for Chronic Obstructive Pulmonary Disease in Southwestern Taiwan: A Population-Based Study. Int. J. Environ. Res. Public Health 2016, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- Chi, M.C.; Guo, S.E.; Hwang, S.L.; Chou, C.T.; Lin, C.M.; Lin, Y.C. Exposure to Indoor Particulate Matter Worsens the Symptoms and Acute Exacerbations in Chronic Obstructive Pulmonary Disease Patients of Southwestern Taiwan: A Pilot Study. Int. J. Environ. Res. Public Health 2017, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Seemungal, T.A.R.; Donaldson, G.C.; Paul, E.A.; Bestall, J.C.; Jeffries, D.J.; Wedzicha, J.A. Effect of Exacerbation on Quality of Life in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 1998, 157, 1418–1422. [Google Scholar] [CrossRef] [PubMed]
- Langsetmo, L.; Platt, R.W.; Ernst, P.; Bourbeau, J. Underreporting Exacerbation of Chronic Obstructive Pulmonary Disease in a Longitudinal Cohort. Am. J. Respir. Crit. Care Med. 2008, 177, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Collet, J.P.; Shapiro, S.; Lin, Y.; Yang, T.; Wang, C.; Bourbeau, J. Negative impacts of unreported COPD exacerbations on health-related quality of life at 1 year. Eur. Respir. J. 2010, 35, 1022–1030. [Google Scholar] [CrossRef]
- Chuang, K.C. The Effect of Nursing Intervention for the Satisfaction of Home Self Care Needs Level of Patients with Chronic Obstructive Pulmonary Disease. Master’s Thesis, School of Nursing, Taipei Medical University, Taipei City, Taiwan, 2008. [Google Scholar]
- Yao, B.L. Factors Associated with Self-Care Behavior of Patients with Chronic Obstructive Pulmonary Disease. Master’s Thesis, School of Nursing, National Taiwan University, Taipei City, Taiwan, 2010. [Google Scholar]
- Chen, K.H. Development and Construction of a Self-Management Scale and Model for Patients with Chronic Obstructive Pulmonary Disease. Master’s Thesis, School of Nursing, Chung Shan Medical University, Taichung City, Taiwan, 2010. [Google Scholar]
- Gillissen, A.; Wirtz, H.; Juergens, U. Patient and physician factors contributing to poor outcomes in patients with asthma and COPD. Dis. Manag. Health Outcomes 2007, 15, 355–376. [Google Scholar] [CrossRef]
- Lo, C.T.; Weng, H.C. Building Explanatory Model of asthma-specific Quality of Life an Analysis of Patients Enrolled in an Asthma Disease Management Program. Taiwan J. Public Health 2006, 25, 125–134. [Google Scholar]
- Clari, M.; Matarese, M.; Ivziku, D.; De Marinis, M.G. Self-care of people with chronic obstructive pulmonary disease: A meta-synthesis. Patient 2017, 10, 407–427. [Google Scholar] [CrossRef]
- Bucknall, C.E.; Miller, G.; Lloyd, S.M.; Cleland, J.; McCluskey, S.; Cotton, M.; Stevenson, R.D.; Cotton, P.; McConnachie, A. Glasgow supported self-management trial (GSuST) for patients with moderate to severe COPD: Randomised controlled trial. BMJ 2012, 344, e1060. [Google Scholar] [CrossRef]
- Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pilmonary Disease 2019 Report. 2019. Available online: https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf (accessed on 3 April 2020).
- Chen, Z.; Xie, X.; Cai, J.; Chen, D.; Gao, B.; He, B.; Cheng, N.; Xu, B. Understanding meteorological influences on PM2.5 concentrations across China: A temporal and spatial perspective. Atmos. Chem. Phys. 2018, 18, 5343–5358. [Google Scholar] [CrossRef]
- Hwang, S.L.; Chi, M.C.; Guo, S.E.; Lin, Y.C.; Chou, C.T.; Lin, C.M. Seasonal variation and source apportionment of PM2.5-bound trace elements at a coastal area in southwestern Taiwan. Environ. Sci. Pollut. Res. Int. 2018, 25, 9101–9113. [Google Scholar] [CrossRef] [PubMed]
- Environmental Protection Administration, Executive Yuan, Taiwn. Ari Quality Standards. Available online: https://airtw.epa.gov.tw/ENG/Information/Standard/Rules.aspx (accessed on 13 March 2020).
- Jones, P.W.; Harding, G.; Berry, P.; Wiklund, I.; Chen, W.H.; Leidy, N.K. Development and first validation of the COPD assessment test. Eur. Respir. J. 2009, 34, 648–654. [Google Scholar] [CrossRef]
- Gupta, N.; Pinto, L.M.; Morogan, A.; Bourbeau, J. The COPD assessment test: A systematic review. Eur. Respir. J. 2014, 44, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.J.; Liu, T.; Cai, B.Q. Evaluation of clinical significance of chronic obstructive pulmonary disease assessment test. Chin. J. Tuberc. Respir. Dis. 2011, 34, 256–258. [Google Scholar]
- Shi, J.; Mo, X.; Sun, Z. Content Validity Index in Scale Development. J. Cent. South Univ. Med Sci. 2012, 37, 152–155. [Google Scholar]
- Chuang, M.H.; Chuang, T.L.; Huang, K.Y.; Wang, Y.F. Age-adjusted Charlson Comorbidity Index scores predict major adverse cardiovascular events and all-cause mortality among systemic lupus erythematosus patients. Tzu-Chi Med. J. 2017, 29, 154–158. [Google Scholar]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Liang, K.Y.; Zeger, S.L. Longitudinal data analysis using generalized linear models. Biometrika 1986, 73, 13–22. [Google Scholar] [CrossRef]
- Gibson, P.G.; Powell, H.; Coughlan, J.; Wilson, A.J.; Hensley, M.J.; Abramson, M.; Bauman, A.; Walters, E.H. Limited (information only) patient education programs for adults with asthma. Cochrane Database Syst. Rev. 2002, CD001005. [Google Scholar] [CrossRef]
- Gallefoss, F.; Bakke, P.S.; Rsgaard, P.K. Quality of life assessment after patient education in a randomized controlled study on asthma and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care. Med. 1999, 159, 812–817. [Google Scholar] [CrossRef]
- Sangren, P.S. Dialectics of alienation: Individuals and collectivities in Chinese religion. Man 1991, 26, 67–86. [Google Scholar] [CrossRef]
- Chang, H. Intangible Cultural Heritage: The Concept of Incense-fire and the Ritual of Pilgrimage in Taiwanese Folk Religion. J. Cult. Herit. Conserv. 2011, 16, 37–46. [Google Scholar]
- Bootdee, S.; Chantara, S.; Prapamontol, T. Determination of PM2.5 and polycyclic aromatic hydrocarbons from incense burning emission at shrine for health risk assessment. Atmos. Pollut. Res. 2016, 7, 680–689. [Google Scholar] [CrossRef]
- Shen, H.; Tsai, C.M.; Yuan, C.S.; Jen, Y.H.; Ie, I.R. How incense and joss paper burning during the worship activities influences ambient mercury concentrations in indoor and outdoor environments of an Asian temple? Chemosphere 2017, 167, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.H.; Guo, A.M.; Zhang, X.J. Effects of self-management education on quality of life of patients with chronic obstructive pulmonary disease. Int. J. Nurs. Sci. 2014, 1, 53–57. [Google Scholar] [CrossRef]
- Kim, K.; Choi, J.S.; Choi, E.; Nieman, C.L.; Joo, J.H.; Lin, F.R.; Gitlin, L.N.; Han, H.R. Effects of community-based health worker interventions to improve chronic disease management and care among vulnerable populations: A systematic review. Am. J. Public Health 2016, 106, e3–e28. [Google Scholar] [CrossRef]
- Liu, J.; Meng, G.; Ma, Y.; Zhang, X.; Chen, D.; Chen, M. Influence of COPD Assessment Text (CAT) evaluation and rehabilitation education guidance on the respiratory and motor functions of COPD patients. Open Med. 2015, 10, 394–398. [Google Scholar] [CrossRef]
- Emery, C.F.; Schein, R.L.; Hauck, E.R.; MacIntyre, N.R. Psychological and cognitive outcomes of a randomized trial of exercise among patients with chronic obstructive pulmonary disease. Health Psychol. 1998, 17, 232–240. [Google Scholar] [CrossRef]
- Baraniak, A.; Sheffield, D. The efficacy of psychologically based interventions to improve anxiety, depression and quality of life in COPD: A systematic review and meta-analysis. Patient Educ. Couns. 2011, 83, 29–36. [Google Scholar] [CrossRef]
- Connolly, M.J.; Yohannes, A.M. The impact of depression in older patients with chronic obstructive pulmonary disease and asthma. Maturitas 2016, 92, 9–14. [Google Scholar] [CrossRef]
- Hanania, N.A.; O’Donnell, D.E. Activity-related dyspnea in chronic obstructive pulmonary disease: Physical and psychological consequences, unmet needs, and future directions. Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Brien, S.B.; Stuart, B.; Dickens, A.P.; Kendrick, T.; Jordan, R.E.; Adab, P.; Thomas, M. Independent determinants of disease-related quality of life in COPD–scope for nonpharmacologic interventions? Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 247. [Google Scholar] [CrossRef]
- Small, S.A. Age-related memory decline: Current concepts and future directions. Arch. Neurol. 2001, 58, 360–364. [Google Scholar] [CrossRef]
- Meusel, L.A.; Grady, C.L.; Ebert, P.E.; Anderson, N.D. Brain–behavior relationships in source memory: Effects of age and memory ability. Cortex 2017, 91, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Csíkszentmihály, M. Flow: The Psychology of Optimal Experience; Harper & Row: New York, NY, USA, 1990. [Google Scholar]
- Csíkszentmihály, M. Toward a psychology of optimal experience. In Flow and the Foundations of Positive Psychology: The Collected Works of Mihaly Csíkszentmihály; Csíkszentmihály, M., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 209–226. [Google Scholar]
- Nilsson, I.; Nygård, L. Geriatric Rehabilitation: Elderly Clients’ Experiences of a Pre-discharge Occupational Therapy Group Programme. Scand. J. Occup. Ther. 2003, 10, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Goossens, E.; Fieuws, S.; Van Deyk, K.; Luyckx, K.; Gewillig, M.; Budts, W.; Moons, P. Effectiveness of structured education on knowledge and health behaviors in patients with congenital heart disease. J. Pdiatr. 2015, 166, 1370–1376. [Google Scholar] [CrossRef]
- Blackstock, F.; Webster, K.E. Disease-specific health education for COPD: A systematic review of changes in health outcomes. Health Educ. Res. 2007, 22, 703–717. [Google Scholar] [CrossRef][Green Version]
- Wang, T.; Tan, J.Y.; Xiao, L.D.; Deng, R. Effectiveness of disease-specific self-management education on health outcomes in patients with chronic obstructive pulmonary disease: An updated systematic review and meta-analysis. Patient Educ. Couns. 2017, 100, 1432–1446. [Google Scholar] [CrossRef]
- Wegesser, T.C.; Pinkerton, K.E.; Last, J.A. California wildfires of 2008: Coarse and find particulate matter toxicity. Environ. Health Perspect. 2009, 117, 893–897. [Google Scholar] [CrossRef]
- Verma, V.; Polidori, A.; Schauer, J.J.; Shafer, M.M.; Cassee, F.R.; Sioutas, C.P. Physicochemical and toxicological profiles of particulate matter in Los Angeles during the October 2007 southern California wildfires. Environ. Sci. Technol. 2009, 43, 954–960. [Google Scholar] [CrossRef] [PubMed]

| Variable | Intervention Group (n = 25) | Control Group (n = 38) | p |
|---|---|---|---|
| Male sex | 22 (88.0) | 35 (92.1) | 0.674 |
| Age, years | 72.8 ± 7.6 | 71.6 ± 8.7 | 0.585 |
| Education level, years | 6.3 ± 3.8 | 7.3 ± 3.6 | 0.298 |
| Living status | 0.506 | ||
| Living alone | 5 (20.0) | 5 (13.5) | |
| Living with someone | 20 (80.0) | 32 (86.5) | |
| Smoking | 0.807 | ||
| Never | 3 (12.0) | 5 (13.5) | |
| Quit | 17 (68.0) | 22 (59.5) | |
| Current | 5 (20.0) | 10 (27.0) | |
| Charlson Comorbidity Index | 5.4 ± 1.5 | 5.1 ± 1.2 | 0.381 |
| COPD duration, years | 7.5 ± 4.6 | 6.0 ± 4.7 | 0.231 |
| Burning incenses | 1.000 | ||
| None | 3 (12.0) | 4 (10.8) | |
| Occasionally | 5 (20.0) | 7 (18.9) | |
| Everyday | 17 (68.0) | 26 (70.3) | |
| Environmental status at baseline | |||
| Raining | 2 (8.0) | 7 (18.9) | 0.292 |
| Outdoor temperature, °C | 28.6 ± 5.9 | 27.3 ± 3.4 | 0.310 |
| Outdoor humidity, % | 68.0 ± 11.1 | 70.5 ± 11.1 | 0.393 |
| Outdoor wind velocity, km/s. | 0.47 ± 0.46 | 0.54 ± 0.47 | 0.551 |
| Indoor temperature, °C | 28.3 ± 5.3 | 27.4 ± 3.1 | 0.404 |
| Indoor humidity, % | 68.6 ± 9.3 | 68.9 ± 12.1 | 0.897 |
| Indoor wind velocity, km/s. | 0.19 ± 0.17 | 0.24 ± 0.19 | 0.301 |
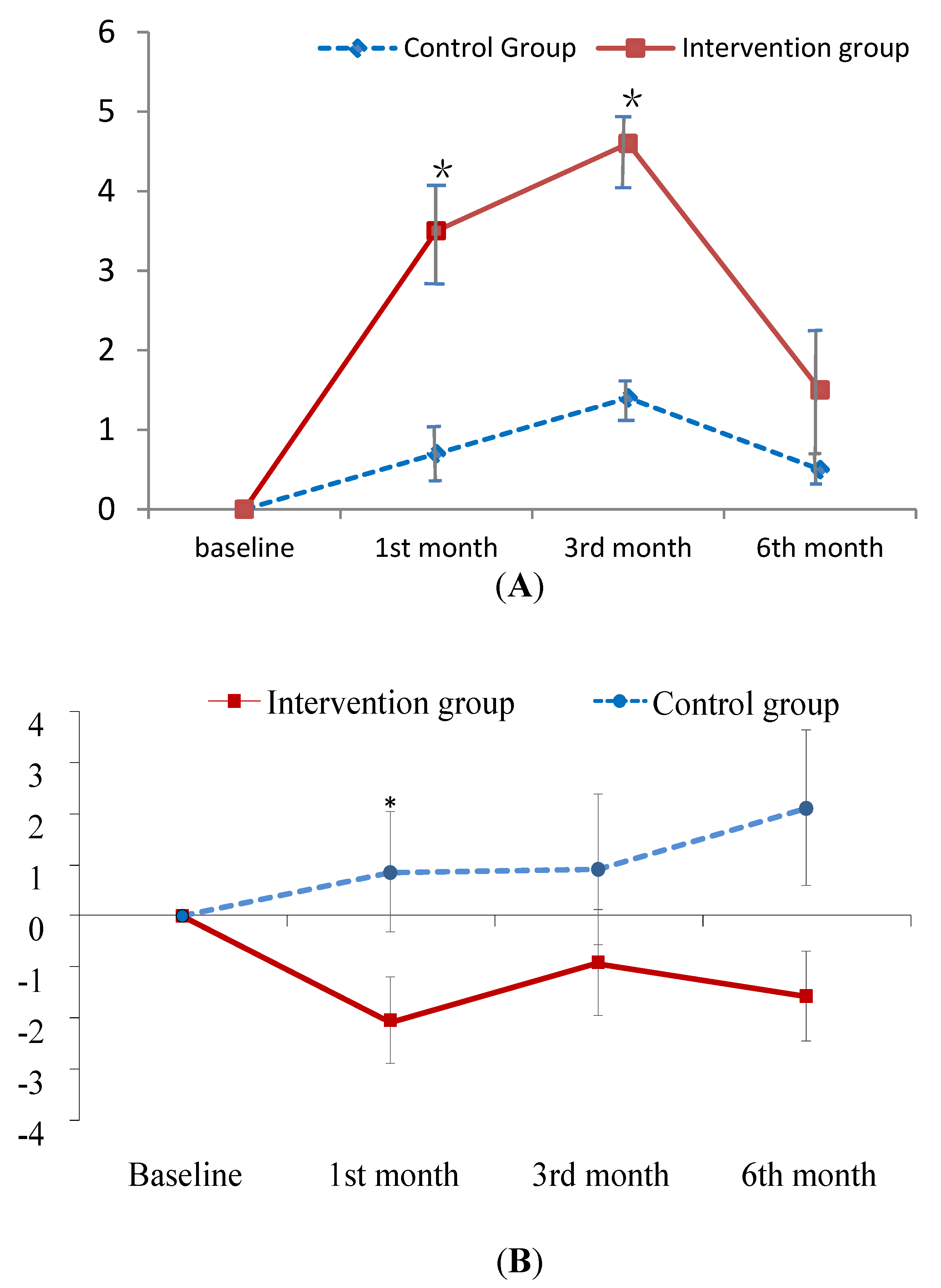
| Self-care knowledge of PM | 3.7 ± 3.6 | 9.0 ± 1.7 | <0.001 |
| FEV1 predicted value, % | 40.1 ± 15.1 | 49.4 ± 22.7 | 0.076 |
| Variable | Intervention Group (n = 25) | Control Group (n = 38) | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 1st Month | 3rd Month | 6th Month | Baseline | 1st Month | 3rd Month | 6th Month | |
| PM2.5, μg/m3 | ||||||||
| Outdoor | 102.1 ± 71.4 | 76.8 ± 49.5 | 86.7 ± 77.3 | 88.7 ± 72.4 | 82.5 ± 60.3 | 85.9 ± 84.9 | 83.1 ± 61.7 | 111.5 ± 69.4 |
| Living room | 103.6 ± 75.5 | 115.0 ± 191.1 | 99.6 ± 98.2 | 91.7 ± 70.5 | 82.8 ± 58.3 | 82.3 ± 83.1 | 91.3 ± 73.2 | 100.7 ± 58.0 |
| Kitchen | 109.9 ± 78.8 | 116.2 ± 171.8 | 90.8 ± 77.3 | 94.3 ± 72.2 | 96.7 ± 69.2 | 89.2 ± 83.8 | 87.2 ± 64.5 | 116.9 ± 74.9 |
| Bedroom | 101.5 ± 78.1 | 113.1 ± 201.5 | 94.7 ± 75.0 | 87.4 ± 65.9 | 95.0 ± 114.5 | 91.8 ± 87.2 | 93.2 ± 67.5 | 106.7 ± 80.7 |
| Worship hall | 164.4 ± 199.6 | 135.2 ± 209.8 | 131.2 ± 200.9 | 204.5 ± 303.3 | 108.3 ± 82.2 | 299.0 ± 504.8 | 246.3 ± 503.7 | 252.1 ± 380.9 |
| PM10, μg/m3 | ||||||||
| Outdoor | 112.3 ± 85.0 | 82.4 ± 55.6 | 89.9 ± 64.0 | 89.9 ± 70.3 | 88.2 ± 61.2 | 101.4 ± 86.1 | 96.4 ± 62.0 | 129.9 ± 83.6 |
| Living room | 106.7 ± 74.9 | 94.5 ± 84.1 | 97.4 ± 71.9 | 91.0 ± 69.6 | 88.7 ± 62.2 | 90.6 ± 76.6 | 91.0 ± 62.4 | 121.5 ± 72.9 |
| Kitchen | 117.2 ± 78.7 | 115.2 ± 152.8 | 97.0 ± 71.3 | 97.9 ± 73.7 | 104.7 ± 74.9 | 100.1 ± 84.0 | 93.6 ± 62.4 | 133.3 ± 86.7 |
| Bedroom | 107.5 ± 71.2 | 83.9 ± 73.9 | 96.5 ± 67.8 | 89.7 ± 69.9 | 86.3 ± 62.5 | 104.6 ± 97.0 | 96.7 ± 72.2 | 126.1 ± 82.5 |
| Worship hall | 187.2 ± 334.6 | 110.9 ± 98.3 | 129.5 ± 148.7 | 298.1 ± 607.3 | 168.4 ± 311.9 | 187.4 ± 197.2 | 455.5 ± 1516.9 | 275.7 ± 398.5 |
| CAT scale | ||||||||
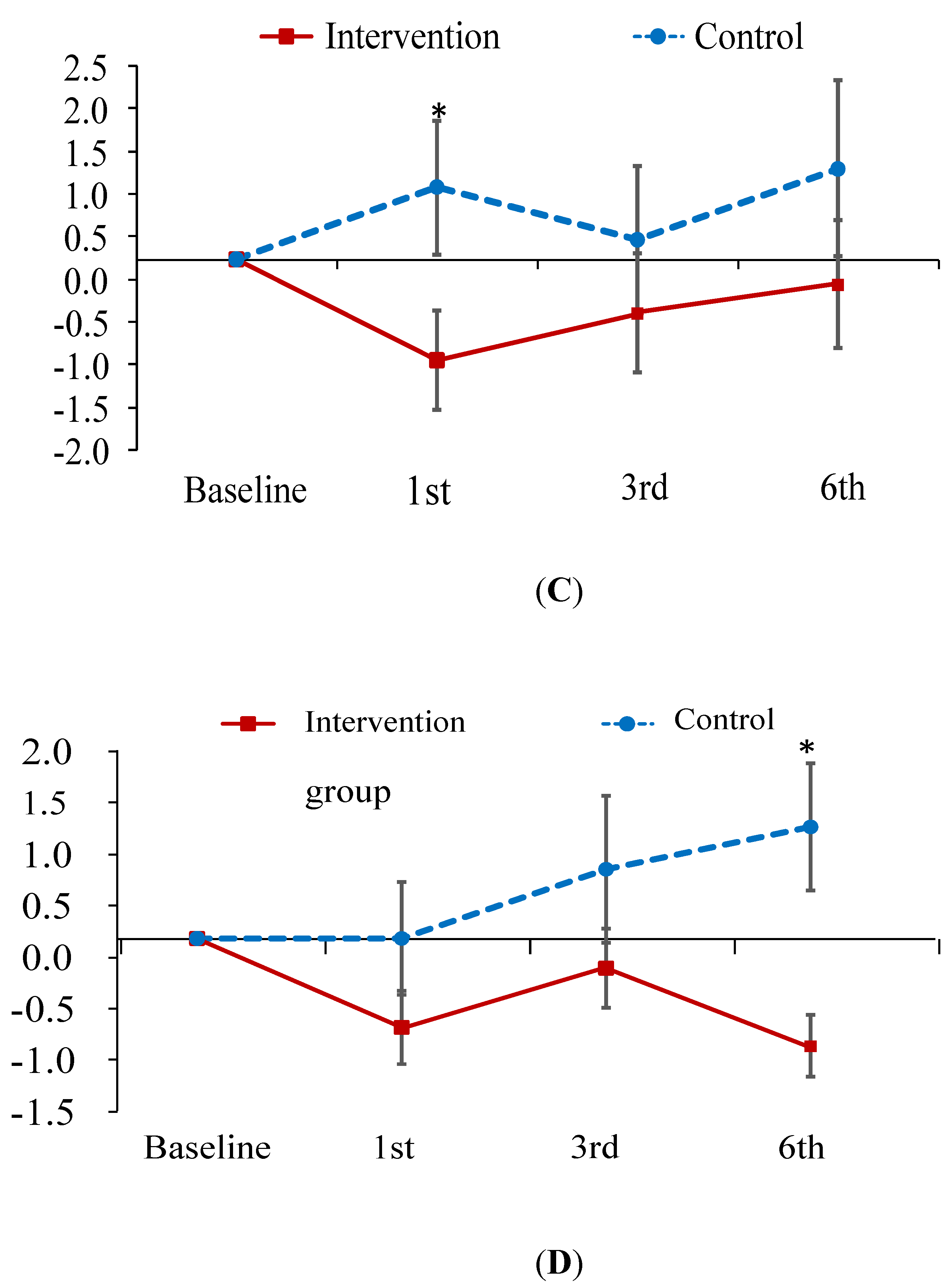
| Total | 9.7 ± 6.0 | 7.7 ± 4.8 | 8.8 ± 5.9 | 8.1 ± 5.0 | 9.6 ± 6.2 | 10.4 ± 5.5 | 10.5 ± 7.2 | 11.7 ± 7.4 |
| Physical | 7.1 ± 3.7 | 5.9 ± 3.3 | 6.4 ± 4.0 | 6.8 ± 4.3 | 6.5 ± 4.2 | 7.4 ± 3.7 | 6.8 ± 4.2 | 7.6 ± 5.1 |
| Psychological | 2.6 ± 2.7 | 1.8 ± 2.0 | 2.4 ± 2.2 | 1.6 ± 1.7 | 3.0 ± 2.4 | 3.0 ± 2.6 | 3.7 ± 3.5 | 4.1 ± 3.0 |
| mMRC | 2.0 ± 1.5 | 1.7 ± 1.4 | 1.9 ± 1.4 | 1.6 ± 1.4 | 1.4 ± 1.0 | 1.4 ± 1.2 | 1.4 ± 1.1 | 1.3 ± 1.1 |
| FEV1 predicted value, % | 40.1 ± 15.1 | 41.0 ± 14.2 | 39.6 ± 16.6 | 39.2 ± 16.3 | 49.4 ± 22.7 | 45.8 ± 20.1 | 49.2 ± 21.0 | 51.3 ± 23.0 |
| Self-care knowledge of PM | 3.7 ± 3.6 | 7.2 ± 3.2 | 8.1 ± 2.1 | 5.2 ± 4.2 | 9.0 ± 1.7 | 9.7 ± 1.5 | 10.4 ± 1.1 | 9.5 ± 1.5 |
| Time | Interaction of Study Group by Time Points | |||
|---|---|---|---|---|
| Outcome | 1st Month | 3rd Month | 6th Month | |
| PM2.5, μg/m3 | ||||
| Outdoor a | −33.42 (21.77) | −3.94 (12.77) | −23.87 (12.90) | |
| Living room b | 20.11 (36.43) | 4.94 (29.97) | −15.23 (26.93) | |
| Kitchen b | 20.91 (34.78) | 8.64 (28.18) | −23.52 (32.91) | |
| Bedroom b | 26.71 (40.91) | 18.32 (33.98) | 4.67 (33.96) | |
| Worship hall b | −242.96 (106.82) * | −131.27 (118.01) | −58.73 (74.34) | |
| PM10, μg/m3 | ||||
| Outdoor c | −29.15 (14.94) | −18.32 (8.89) * | −16.77 (8.60) | |
| Living room b | −8.15 (21.32) | 4.91 (25.16) | −21.23 (14.67) | |
| Kitchen b | 9.81 (31.71) | 7.97 (29.13) | −22.56 (20.37) | |
| Bedroom b | −31.92 (22.65) | −1.64 (25.55) | −25.12 (17.12) | |
| Worship Hall b | −153.67 (75.99) * | −341.13 (267.71) | 5.98 (100.24) | |
| CAT scale d | ||||
| Total | −2.17 (1.04) * | 1.61 (1.42) | 3.22 (1.66) | |
| Physical | −1.55 (0.75) * | 0.72 (0.86) | 1.12 (1.03) | |
| Psychological | 0.63 (0.51) | 0.89 (0.76) | −2.89 (0.82) * | |
| mMRC d | −0.49 (0.26) | −0.18 (0.26) | −0.30 (0.31) | |
| FEV1 predicted value, % e | 3.73 (2.90) | −1.46 (3.34) | −4.67 (3.21) | |
| Self-care knowledge of PM f | 2.73 (0.81) *** | 3.00 (0.81) *** | 1.06 (1.02) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.-E.; Chi, M.-C.; Hwang, S.-L.; Lin, C.-M.; Lin, Y.-C. Effects of Particulate Matter Education on Self-Care Knowledge Regarding Air Pollution, Symptom Changes, and Indoor Air Quality among Patients with Chronic Obstructive Pulmonary Disease. Int. J. Environ. Res. Public Health 2020, 17, 4103. https://doi.org/10.3390/ijerph17114103
Guo S-E, Chi M-C, Hwang S-L, Lin C-M, Lin Y-C. Effects of Particulate Matter Education on Self-Care Knowledge Regarding Air Pollution, Symptom Changes, and Indoor Air Quality among Patients with Chronic Obstructive Pulmonary Disease. International Journal of Environmental Research and Public Health. 2020; 17(11):4103. https://doi.org/10.3390/ijerph17114103
Chicago/Turabian StyleGuo, Su-Er, Miao-Ching Chi, Su-Lun Hwang, Chieh-Mo Lin, and Yu-Ching Lin. 2020. "Effects of Particulate Matter Education on Self-Care Knowledge Regarding Air Pollution, Symptom Changes, and Indoor Air Quality among Patients with Chronic Obstructive Pulmonary Disease" International Journal of Environmental Research and Public Health 17, no. 11: 4103. https://doi.org/10.3390/ijerph17114103
APA StyleGuo, S.-E., Chi, M.-C., Hwang, S.-L., Lin, C.-M., & Lin, Y.-C. (2020). Effects of Particulate Matter Education on Self-Care Knowledge Regarding Air Pollution, Symptom Changes, and Indoor Air Quality among Patients with Chronic Obstructive Pulmonary Disease. International Journal of Environmental Research and Public Health, 17(11), 4103. https://doi.org/10.3390/ijerph17114103





