Translation of Exposure and Epidemiology for Risk Assessment: A Shifting Paradigm
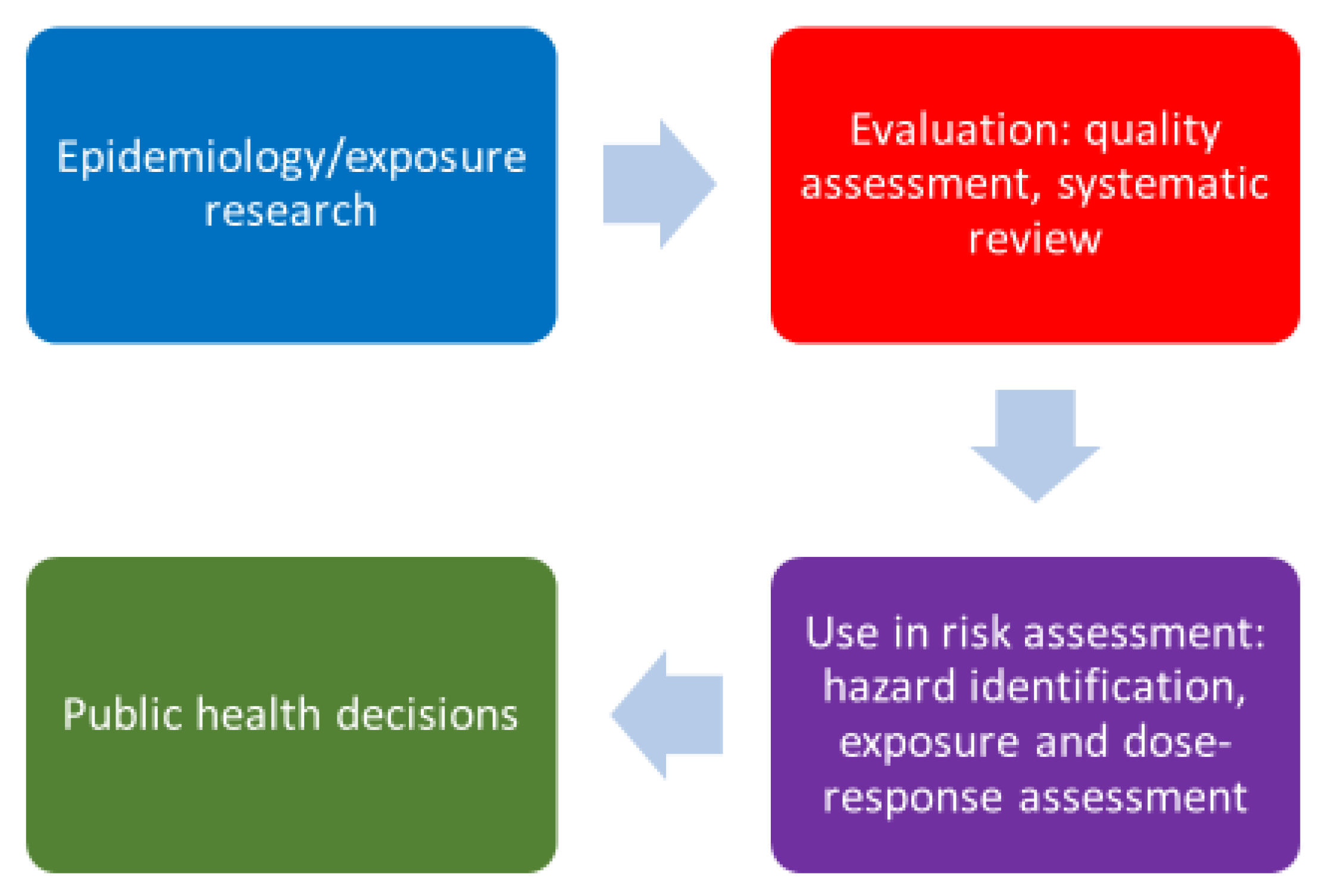
1. Background
2. Example Exposure Assessments and Quality Evaluations
- Does the exposure measure capture the variability in exposure among the participants, considering intensity, frequency, and duration of exposure?
- Was exposure assessed using methods known or suspected to have poor validity?
- Do the data reported address exposure scenarios (e.g., sources, pathways, routes, receptors) that are relevant to the assessment? Are the data and supporting information accessible and clearly documented? Do the data describe variability and uncertainty (quantitative and qualitative) or are the procedures, measures, methods, or models evaluated and characterized?
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Windle, M.; Lee, H.D.; Cherng, S.T.; Lesko, C.R.; Hanrahan, C.F.; Jackson, J.W.; McAdams-DeMarco, M.; Ehrhardt, S.; Baral, S.D.; D’Souza, G.; et al. From Epidemiologic Knowledge to Improved Health: A Vision for Translational Epidemiology. Am. J. Epidemiol. 2019, 188, 2049–2060. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.J.; LaKind, J.S.; Mattison, D.R.; Alcala, C.S.; Branch, F.; Castillo, J.; Clark, A.; Clougherty, J.E.; Darney, S.P.; Erickson, H.; et al. A matrix for bridging the epidemiology and risk assessment gap. Glob. Epidemiol. 2019, 1, 100005. [Google Scholar] [CrossRef]
- Amler, R.W.; Barone, S.; Belger, A.; Berlin, C.M.; Cox, C.; Frank, H.; Goodman, M.; Harry, J.; Hooper, S.R.; Ladda, R.; et al. Hershey Medical Center Technical Workshop Report: Optimizing the design and interpretation of epidemiologic studies for assessing neurodevelopmental effects from in utero chemical exposure. Neurotoxicology 2006, 27, 861–874. [Google Scholar] [CrossRef]
- LaKind, J.S.; Sobus, J.R.; Goodman, M.; Barr, D.B.; Fürst, P.; Albertini, R.J.; Arbuckle, T.E.; Schoeters, G.; Tan, Y.-M.; Teeguarden, J.; et al. A proposal for assessing study quality: Biomonitoring, Environmental Epidemiology, and Short-lived Chemicals (BEES-C) instrument. Environ. Int. 2014, 73, 195–207. [Google Scholar] [CrossRef] [PubMed]
- LaKind, J.S.; Goodman, M.; Barr, D.B.; Weisel, C.P.; Schoeters, G. Lessons learned from the application of BEES-C: Systematic assessment of study quality of epidemiologic research on BPA, neurodevelopment, and respiratory health. Environ. Int. 2015, 80, 41–71. [Google Scholar] [CrossRef]
- LaKind, J.S.; O’Mahony, C.; Armstrong, T.; Tibaldi, R.; Blount, B.C.; Naiman, D.Q. ExpoQual: Evaluating measured and modeled human exposure data. Environ. Res. 2019, 171, 302–312. [Google Scholar] [CrossRef]
- Morgan, R.L.; Thayer, K.A.; Santesso, N.; Holloway, A.C.; Blain, R.; Eftim, S.E.; Goldstone, A.E.; Ross, P.; Guyatt, G.H.; Schünemann, H.J. Evaluation of the risk of bias in non-randomized studies of interventions (ROBINS-I) and the ‘target experiment’ concept in studies of exposures: Rationale and preliminary instrument development. Environ. Int. 2018, 120, 382–387. [Google Scholar] [CrossRef]
- NTP (National Toxicology Program). Handbook for Conducting a Literature-Based Health Assessment Using OHAT Approach for Systematic Review and Evidence Integration. U.S. Dept. of Health and Human Services, National Toxicology Program; 2015. Available online: https://ntp.niehs.nih.gov/ntp/ohat/pubs/handbookjan2015_508.pdf (accessed on 6 June 2020).
- Radke, E.G.; Glenn, B.; Galizia, A.; Persad, A.; Nachman, R.; Bateson, T.; Wright, J.M.; Navas-Acien, A.; Arroyave, W.D.; Puett, R.C.; et al. Development of outcome-specific criteria for study evaluation in systematic reviews of epidemiology studies. Environ. Int. 2019, 130, 104884. [Google Scholar] [CrossRef]
- Rooney, A.A.; Cooper, G.S.; Jahnke, G.D.; Lam, J.; Morgan, R.L.; Boyles, A.; Ratcliffe, J.M.; Kraft, A.D.; Schünemann, H.J.; Schwingl, P.; et al. How credible are the study results? Evaluating and applying internal validity tools to literature-based assessments of environmental health hazards. Environ. Int. 2016, 92–93, 617–629. [Google Scholar] [CrossRef]
- Sterne, J.; Hernan, M.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, U.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Youngstrom, E.A.; Kenworthy, L.; Lipkin, P.H.; Goodman, M.; Squibb, K.; Mattison, D.R.; Anthony, L.G.; Makris, S.L.; Bale, A.S.; Raffaele, K.C.; et al. A proposal to facilitate weight-of-evidence assessments: Harmonization of Neurodevelopmental Environmental Epidemiology Studies (HONEES). Neurotoxicol. Teratol. 2011, 33, 354–359. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Allen, M.T.; Levy, L.S. Parkinson’s disease and pesticide exposure-a new assessment. Crit. Rev. Toxicol. 2013, 43, 515–534. [Google Scholar] [CrossRef] [PubMed]
- Checkoway, H.; Boffetta, P.; Mundt, D.J.; Mundt, K.A. Critical review and synthesis of the epidemiologic evidence on formaldehyde exposure and risk of leukemia and other lymphohematopoietic malignancies. Cancer Causes Control 2012, 23, 1747–1766. [Google Scholar] [CrossRef] [PubMed]
- Goodman, M.; LaKind, J.S.; Mattison, D.R. Do phthalates act as obesogens in humans? A systematic review of the epidemiological literature. Crit. Rev. Toxicol. 2014, 44, 151–175. [Google Scholar] [CrossRef] [PubMed]
- Lock, E.A.; Zhang, J.; Checkoway, H. Solvents and Parkinson disease: A systematic review of toxicological and epidemiological evidence. Toxicol. Appl. Pharmacol. 2013, 266, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Peretz, J.; Vrooman, L.; Ricke, W.A.; Hunt, P.A.; Ehrlich, S.; Hauser, R.; Padmanabhan, V.; Taylor, H.S.; Swan, S.; Vandevoort, C.A.; et al. Bisphenol a and reproductive health: Update of experimental and human evidence, 2007–2013. Environ. Health Perspect. 2014, 122, 775–786. [Google Scholar] [CrossRef]
- Environmental Protection Agency (US EPA). SAB Advice on Approaches to Derive a Maximum Contaminant Level Goal for Perchlorate. EPA-SAB-13-004; 2013. Available online: http://yosemite.epa.gov/sab/sabproduct.nsf/d21b76bff879fa0a8525735a00766807/d3bb75d4297ca4698525794300522ace!OpenDocument (accessed on 6 June 2020).
- U.S. Environmental Protection Agency (US EPA). Application of Systematic Review in TSCA Risk Evaluations. (740-P1-8001); U.S. Environmental Protection Agency, Office of Chemical Safety and Pollution Prevention: Washington, DC, USA, 2018.
- U.S. Environmental Protection Agency (US EPA). ORD Staff Handbook for Developing IRIS Assessments, Version 1.0; Integrated Risk Information System, National Center for Environmental Assessment, Office of Research and Development: Washington, DC, USA, 2019.
- U.S. Environmental Protection Agency (US EPA). Benzidine. CASRN 92-87-5; In Integrated Risk Information System (IRIS) Assessment Summary; National Center for Environmental Assessment: Washington, DC, USA, 1987. [Google Scholar]
- U.S. Environmental Protection Agency (US EPA). Nickel subsulfide. CASRN 12035-72-2; In Integrated Risk Information System (IRIS) Assessment Summary; National Center for Environmental Assessment: Washington, DC, USA, 1987. [Google Scholar]
- U.S. Environmental Protection Agency (US EPA). Arsenic, Inorganic. CASRN 7440-38-2; In Integrated Risk Information System (IRIS) Assessment Summary; National Center for Environmental Assessment: Washington, DC, USA, 1995. [Google Scholar]
- U.S. Environmental Protection Agency (US EPA). Bis(Chloromethyl)Ether (BCME). CASRN 542-88-1; In Integrated Risk Information System (IRIS) Assessment Summary; National Center for Environmental Assessment: Washington, DC, USA, 2002. [Google Scholar]
- U.S. Environmental Protection Agency (US EPA). 1,3-Butadiene. CASRN 106-99-0; In Integrated Risk Information System (IRIS) Assessment Summary; National Center for Environmental Assessment: Washington, DC, USA, 2002. [Google Scholar]
- U.S. Environmental Protection Agency (US EPA). Benzene. CASRN 71-43-2; In Integrated Risk Information System (IRIS) Assessment Summary; National Center for Environmental Assessment: Washington, DC, USA, 2003. [Google Scholar]
- U.S. Environmental Protection Agency (US EPA). Trichloroethylene. CASRN 79-01-6; In Integrated Risk Information System (IRIS) Assessment Summary; National Center for Environmental Assessment: Washington, DC, USA, 2011. [Google Scholar]
- U.S. Environmental Protection Agency (US EPA). Toxicological Review of Trichloroethylene Appendices (CAS No. 79-01-6). In Support of Summary Information on the Integrated Risk Information System (IRIS); EPA/635/R-09/011F; US Environmental Protection Agency: Washington, DC, USA, 2011. [Google Scholar]
- U.S. Environmental Protection Agency (US EPA). Evaluation of the Inhalation Carcinogenicity of Ethylene Oxide (CASRN 75-21-8). In Support of Summary Information on the Integrated Risk Information System (IRIS); EPA/635/R-16/350Fa; US Environmental Protection Agency: Washington, DC, USA, 2016. [Google Scholar]
- Case, R.; Hosker, M.E.; McDonald, D.B.; Pearson, J.T. Tumours of the urinary bladder in workmen engaged in the manufacture and use of certain dyestuff intermediates in the British chemical industry. Part, I. The role of aniline, benzidine, alpha-naphthylamine, and beta-naphthylamine. Br. J. Ind. Med. 1954, 11, 75. [Google Scholar] [CrossRef]
- Chovil, A.; Sutherland, R.; Halliday, M. Respiratory cancer in a cohort of nickel sinter plant workers. Occup. Environ. Med. 1981, 38, 327–333. [Google Scholar] [CrossRef]
- Figueroa, W.G.; Raszkowski, R.; Weiss, W. Lung cancer in chloromethyl methyl ether workers. N. Engl. J. Med. 1973, 288, 1096–1097. [Google Scholar] [CrossRef]
- Lemen, R.A.; Johnson, W.M.; Wagoner, J.K.; Archer, V.E.; Saccomanno, G. Cytologic observations and cancer incidence following exposure to BCME. Ann. N. Y. Acad. Sci. 1976, 271, 71–80. [Google Scholar] [CrossRef]
- Magnus, K.; Andersen, A.; Hogetveit, A.C. Cancer of respiratory organs among workers at a nickel refinery in Norway. Int. J. Cancer 1982, 30, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Peto, J.; Cuckle, H.; Doll, R.; Hermon, C.; Morgan, L.G. Respiratory cancer mortality of Welsh nickel refinery workers. IARC Sci. Publ. 1984, 53, 37–46. [Google Scholar]
- Roberts, R.S.; Julian, J.A.; Muir, D.C.; Shannon, H.S. Cancer mortality associated with the high-temperature oxidation of nickel subsulfide. IARC Sci. Publ. 1984, 53, 23–35. [Google Scholar]
- Hornung, R.W.; Greife, A.L.; Stayner, L.T.; Steenland, N.K.; Herrick, R.F.; Elliott, L.J.; Ringenburg, V.L.; Morawetz, J. Statistical model for prediction of retrospective exposure to ethylene oxide in an occupational mortality study. Am. J. Ind. Med. 1994, 25, 825–836. [Google Scholar] [CrossRef]
- Macaluso, M.; Larson, R.; Delzell, E.; Sathiakumar, N.; Hovinga, M.; Julian, J.; Muir, D.; Cole, P. Leukemia and cumulative exposure to butadiene, styrene and benzene among workers in the synthetic rubber industry. Toxicology 1996, 113, 190–202. [Google Scholar] [CrossRef]
- Enterline, P.E.; Marsh, G.M. Cancer among workers exposed to arsenic and other substances in a copper smelter. Am. J. Epidemiol. 1982, 116, 895–911. [Google Scholar] [CrossRef]
- Zavon, M.R.; Hoegg, U.; Bingham, E. Benzidine exposure as a cause of bladder tumors. Arch. Environ. Health 1973, 27, 1–7. [Google Scholar] [CrossRef]
- Cebrian, M.E.; Albores, A.; Aguilar, M.; Blakely, E. Chronic arsenic poisoning in the north of Mexico. Hum. Toxicol. 1983, 2, 121–133. [Google Scholar] [CrossRef]
- Chen, C.-J.; Wang, C.-J. Ecological correlation between arsenic level in well water and age-adjusted mortality from malignant neoplasms. Cancer Res. 1990, 50, 5470–5474. [Google Scholar]
- Infante, P.F.; Rinsky, R.A.; Wagoner, J.K.; Young, R.J. Leukaemia in benzene workers. Lancet 1977, 2, 76–78. [Google Scholar] [CrossRef]
- Rencher, A.C.; Carter, M.W.; McKee, D.W. A retrospective epidemiological study of mortality at a large western copper smelter. J. Occup. Med. 1977, 19, 754–758. [Google Scholar] [PubMed]
- EFSA (European Food Safety Authority). Application of systematic review methodology to food and feed safety assessments to support decision making. EFSA J. 2010, 8, 1637. [Google Scholar] [CrossRef]
- Hardy, A.; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, H.K.; More, S.; Naegeli, H.; Noteborn, H.; Ockleford, C.; EFSA (European Food Safety Authority) Committee; et al. Guidance on the use of the weight of evidence approach in scientific assessments. EFSA J. 2017, 15, 4971. Available online: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2017.4971 (accessed on 6 June 2020).
- Uman, L.S. Systematic reviews and meta-analyses. J. Can. Acad. Child Adolesc. Psychiatry 2011, 20, 57–59. [Google Scholar] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
LaKind, J.S.; Naiman, J.; Burns, C.J. Translation of Exposure and Epidemiology for Risk Assessment: A Shifting Paradigm. Int. J. Environ. Res. Public Health 2020, 17, 4220. https://doi.org/10.3390/ijerph17124220
LaKind JS, Naiman J, Burns CJ. Translation of Exposure and Epidemiology for Risk Assessment: A Shifting Paradigm. International Journal of Environmental Research and Public Health. 2020; 17(12):4220. https://doi.org/10.3390/ijerph17124220
Chicago/Turabian StyleLaKind, Judy S., Joshua Naiman, and Carol J. Burns. 2020. "Translation of Exposure and Epidemiology for Risk Assessment: A Shifting Paradigm" International Journal of Environmental Research and Public Health 17, no. 12: 4220. https://doi.org/10.3390/ijerph17124220
APA StyleLaKind, J. S., Naiman, J., & Burns, C. J. (2020). Translation of Exposure and Epidemiology for Risk Assessment: A Shifting Paradigm. International Journal of Environmental Research and Public Health, 17(12), 4220. https://doi.org/10.3390/ijerph17124220






