The Association between Ethanol Elimination Rate and Hangover Severity
Abstract
1. Introduction
2. Methods
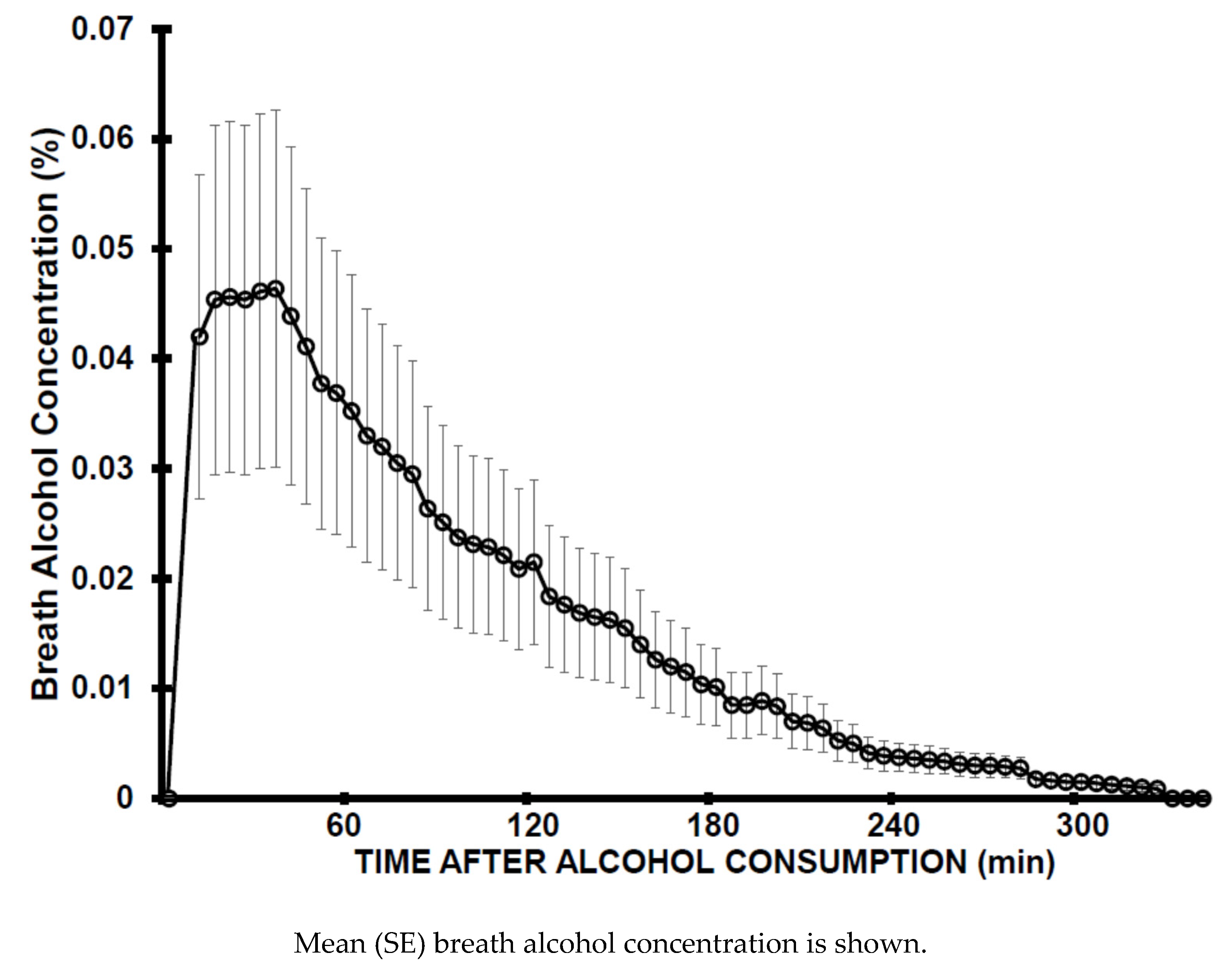
2.1. Study 1. Assessment of Ethanol Elimination Rate—Acute Study
2.2. Study 2. Assessment of Hangover Severity—Hangover Study
2.3. Statistical Analysis
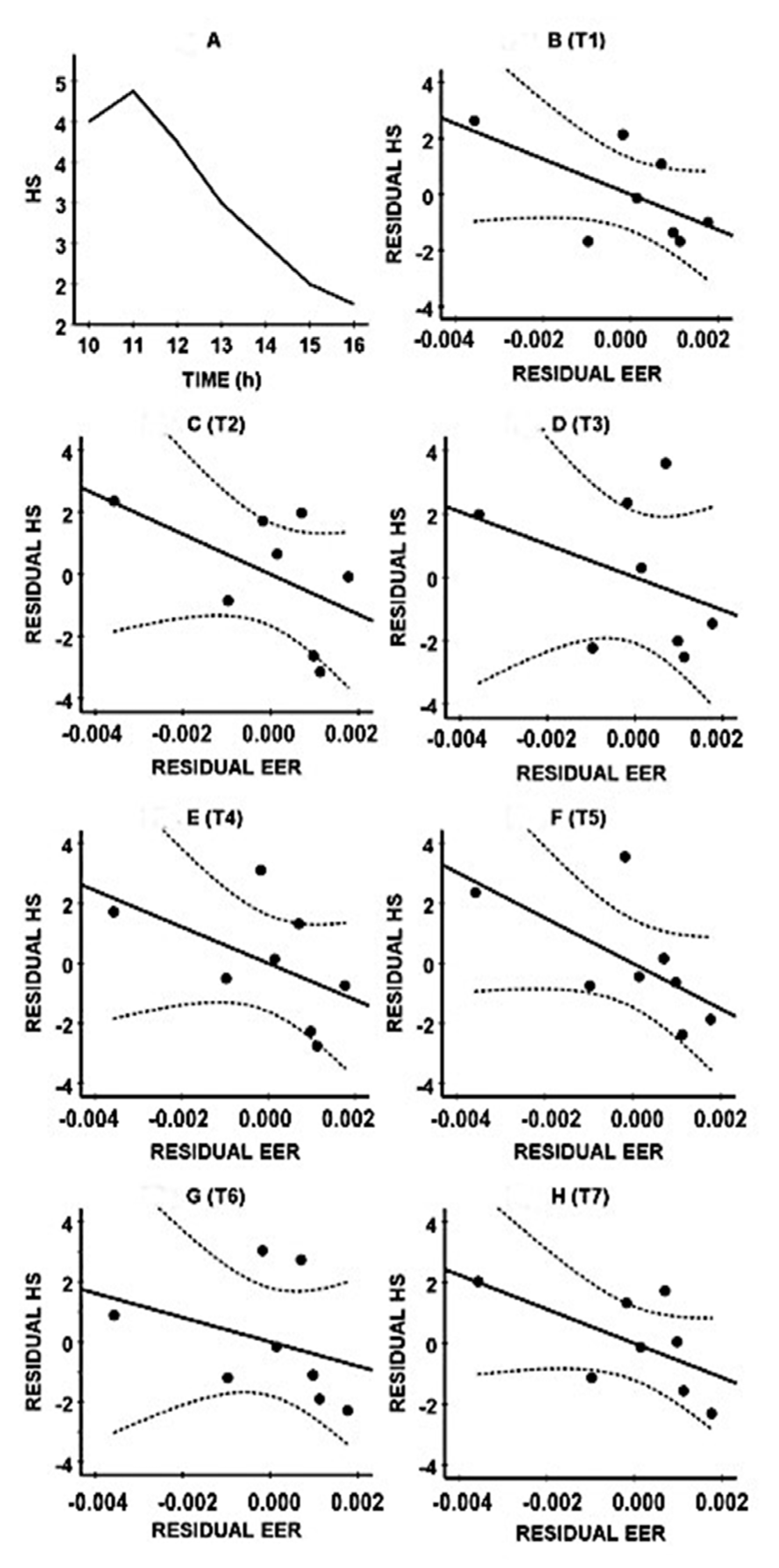
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Van Schrojenstein Lantman, M.; van de Loo, A.J.; Mackus, M.; Verster, J.C. Development of a definition for the alcohol hangover: Consumer descriptions and expert consensus. Curr. Drug Abuse Rev. 2016, 9, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Verster, J.C.; Scholey, A.; van de Loo, A.J.A.E.; Benson, S.; Stock, A.K. Updating the definition of the alcohol hangover. J. Clin. Med. 2020, 9, 823. [Google Scholar] [CrossRef] [PubMed]
- Palmer, E.; Tyacke, R.; Sastre, M.; Lingford-Hughes, A.; Nutt, D.; Ward, R.J. Alcohol Hangover: Underlying Biochemical, Inflammatory and Neurochemical Mechanisms. Alcohol Alcohol. 2019, 54, 196–203. [Google Scholar] [CrossRef]
- Penning, R.; van Nuland, M.; Fliervoet, L.A.L.; Olivier, B.; Verster, J.C. The pathology of alcohol hangover. Curr. Drug Abuse Rev. 2010, 3, 68–75. [Google Scholar] [CrossRef]
- Tipple, C.; Benson, S.; Scholey, A. A review of the physiological factors associated with alcohol hangover. Curr. Drug Abuse Rev. 2016, 9, 93–98. [Google Scholar] [CrossRef]
- Mackus, M.; van Schrojenstein Lantman, M.; van de Loo, A.J.A.E.; Nutt, D.J.; Verster, J.C. An effective hangover treatment: Friend or foe? Drug Sci. Policy Law 2017, 3. [Google Scholar] [CrossRef]
- Pittler, M.H.; Verster, J.C.; Ernst, E. Interventions for preventing or treating alcohol hangover: Systematic review of randomized trials. Br. Med. J. 2005, 331, 1515–1518. [Google Scholar] [CrossRef]
- Verster, J.C.; Penning, R. Treatment and prevention of alcohol hangover. Curr. Drug Abuse Rev. 2010, 3, 103–109. [Google Scholar] [CrossRef]
- Jayawardena, R.; Thejani, T.; Ranasinghe, P.; Fernando, D.; Verster, J.C. Interventions for treatment and/or prevention of alcohol hangover: Systematic review. Hum. Psychopharmacol. 2017, 32, e2600. [Google Scholar] [CrossRef]
- Van Schrojenstein Lantman, M.; Mackus, M.; van de Loo, A.J.A.E.; Verster, J.C. The impact of alcohol hangover symptoms on cognitive and physical functioning, and mood. Hum. Psychopharmacol. 2017, 32, e2623. [Google Scholar] [CrossRef]
- Gunn, C.; Mackus, M.; Griffin, C.; Munafò, M.R.; Adams, S. A systematic review of the next-day effects of heavy alcohol consumption on cognitive performance. Addiction 2018, 113, 2182–2193. [Google Scholar] [CrossRef] [PubMed]
- Verster, J.C.; Bervoets, A.C.; de Klerk, S.; Vreman, R.A.; Olivier, B.; Roth, T.; Brookhuis, K.A. Effects of alcohol hangover on simulated highway driving performance. Psychopharmacology 2014, 231, 2999–3008. [Google Scholar] [CrossRef] [PubMed]
- Alford, C.; Broom, C.; Carver, H.; Johnson, S.J.; Reece, R.; Lands, S.; Verster, J.C. The impact of alcohol hangover on simulated driving performance during a ‘commute to work’—Zero and residual alcohol effects compared. J. Clin. Med. 2020, 9, 1435. [Google Scholar] [CrossRef] [PubMed]
- Frone, M.R. Employee psychoactive substance involvement: Historical context, key findings, and future directions. Annu. Rev. Organ. Psychol. Organ. Behav. 2019, 6, 273–297. [Google Scholar] [CrossRef]
- Bhattacharya, A. Financial Headache. The Cost of Workplace Hangovers and Intoxication to the UK Economy; Institute of Alcohol Studies: London, UK, 2019. [Google Scholar]
- Sacks, J.J.; Gonzales, K.R.; Bouchery, E.E.; Tomedi, L.E.; Brewer, R.D. 2010 National and State Costs of Excessive Alcohol Consumption. Am. J. Prev. Med. 2015, 49, e73–e79. [Google Scholar] [CrossRef]
- Howland, J.; Rohsenow, D.J.; Edwards, E.M. Are some drinkers resistant to hangover? A literature review. Curr. Drug Abuse Rev. 2008, 1, 42–46. [Google Scholar] [CrossRef]
- Verster, J.C.; de Klerk, S.; Bervoets, A.C.; Kruisselbrink, L.D. Can hangover immunity really be claimed? Curr. Drug Abuse Rev. 2013, 6, 253–254. [Google Scholar] [CrossRef] [PubMed]
- Kruisselbrink, L.D.; Bervoets, A.C.; de Klerk, S.; van de Loo, A.J.A.E.; Verster, J.C. Hangover resistance in a Canadian university student population. Addict. Behav. Rep. 2017, 5, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Mackus, M.; van de Loo, A.J.A.E.; Raasveld, S.J.; Hogewoning, A.; Sastre Toraño, J.; Flesch, F.M.; Korte-Bouws, G.A.H.; Van Neer, R.H.P.; Wang, X.; Nguyen, T.T.; et al. Biomarkers of the alcohol hangover state: Ethyl glucuronide (EtG) and ethyl sulfate (EtS). Hum. Psychopharmacol. 2017, 32, e2624. [Google Scholar] [CrossRef] [PubMed]
- Mackus, M.; van de Loo, A.J.A.E.; Korte-Bouws, G.A.H.; van Neer, R.H.P.; Wang, X.; Nguyen, T.T.; Brookhuis, K.A.; Garssen, J.; Verster, J.C. Urine methanol concentration and alcohol hangover severity. Alcohol 2017, 59, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Van Schrojenstein Lantman, M.; van de Loo, A.J.A.E.; Mackus, M.; Brookhuis, K.A.; Kraneveld, A.D.; Garssen, J.; Verster, J.C. Susceptibility to alcohol hangovers: Not just a matter of being resilient. Alcohol Alcohol. 2018, 53, 241–244. [Google Scholar] [CrossRef]
- Hogewoning, A.; van de Loo, A.J.A.E.; Mackus, M.; Raasveld, S.J.; de Zeeuw, R.; Bosma, E.R.; Bouwmeester, N.H.; Brookhuis, K.A.; Garssen, J.; Verster, J.C. Characteristics of social drinkers with and without a hangover after heavy alcohol consumption. Subst. Abuse Rehabil. 2016, 7, 161–167. [Google Scholar] [CrossRef]
- Van de Loo, A.J.A.E.; Mackus, M.; van Schrojenstein Lantman, M.; Kraneveld, A.D.; Garssen, J.; Scholey, A.; Verster, J.C. Susceptibility to alcohol hangovers: The association with self-reported immune status. Int. J. Environ. Res. Public Health 2018, 15, 1286. [Google Scholar] [CrossRef] [PubMed]
- Van de Loo, A.J.A.E.; van Schrojenstein Lantman, M.; Mackus, M.; Scholey, A.; Verster, J.C. Impact of mental resilience and perceived immune functioning on the severity of alcohol hangover. BMC Res. Notes 2018, 11, 526. [Google Scholar] [CrossRef] [PubMed]
- Van de Loo, A.J.A.E.; Mackus, M.; Korte-Bouws, G.A.H.; Brookhuis, K.A.; Garssen, J.; Verster, J.C. Urine ethanol concentration and alcohol hangover severity. Psychopharmacology 2017, 234, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Mackus, M.; van Schrojenstein Lantman, M.; van de Loo, A.J.A.E.; Brookhuis, K.A.; Kraneveld, A.D.; Garssen, J.; Verster, J.C. Alcohol metabolism in hangover sensitive versus hangover resistant social drinkers. Drug Alcohol Depend. 2018, 185, 351–355. [Google Scholar] [CrossRef]
- Friel, P.N.; Logan, B.K.; O’Malley, D.; Baer, J.S. Development of dosing guidelines for reaching selected target breath alcohol concentrations. J. Stud. Alcohol 1999, 60, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Verster, J.C.; van de Loo, A.J.A.E.; Adams, S.; Stock, A.-K.; Benson, S.; Alford, C.; Scholey, A.; Bruce, G. Naturalistic study design in alcohol hangover research: Advantages, limitations, and solutions. J. Clin. Med. 2019, 8, 2160. [Google Scholar] [CrossRef]
- Verster, J.C.; van de Loo, A.J.A.E.; Benson, S.; Scholey, A.; Stock, A.-K. The assessment of overall hangover severity. J. Clin. Med. 2020, 9, 786. [Google Scholar] [CrossRef]
- Watson, P.E.; Watson, I.D.; Batt, R.D. Prediction of blood alcohol concentrations in human subjects. Updating the Widmark Equation. J. Stud. Alcohol Drugs 1981, 42, 547–556. [Google Scholar] [CrossRef]
- Efron, B. Bootstrap methods: Another look at the jackknife. Ann. Stat. 1979, 7, 1–26. [Google Scholar] [CrossRef]
- Efron, B.; Tibshirani, R. An Introduction to the Bootstrap; Chapman & Hall/CRC: Boca Raton, FL, USA, 1993. [Google Scholar]
- Rahman, S.; Majumber, A.K. Use of Bootstrapping in Hypothesis Testing: Bootstrapping for Estimation and Hypothesis Testing; LAP LAMBERT Academic Publishing: Riga, Latvia, 2013. [Google Scholar]
- Sideridis, G.D.; Simos, P. What is the actual correlation between expressive and receptive measures of vocabulary? Approximating the sampling distribution of the correlation coefficient using the bootstrapping method. Int. J. Educ. Psychol. Assess. 2010, 5, 117–133. [Google Scholar]
- Efron, B. Better bootstrap confidence intervals. J. Am. Stat. Assoc. 1987, 82, 171–185. [Google Scholar] [CrossRef]
- Fiorentino, D.D.; Moskowitz, H. Breath alcohol elimination rate as a function of age, gender, and drinking practice. Forensic Sci. Int. 2013, 233, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.W. Evidence-based survey of the elimination rates of ethanol from blood with applications in forensic casework. Forensic Sci. Int. 2010, 200, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ramchandani, V.A.; O’Connor, S. Studying Alcohol Elimination Using the Alcohol Clamp Method. Available online: https://pubs.niaaa.nih.gov/publications/arh294/286-290.htm (accessed on 3 May 2020).
- Keiding, S.; Christensen, N.J.; Damgaard, S.E.; Dejgård, A.; Iversen, H.L.; Jacobsen, A.; Johansen, S.; Lundquist, F.; Rubinstein, E.; Winkler, K. Ethanol metabolism in heavy drinkers after massive and moderate alcohol intake. Biochem. Pharmacol. 1983, 32, 3097–3102. [Google Scholar] [CrossRef]
- Haseba, T.; Kameyama, K.; Mashimo, K.; Ohno, Y. Dose-dependent change in elimination kinetics of ethanol due to shift of dominant metabolizing enzyme from ADH 1 (Class I) to ADH 3 (Class III) in mouse. Int. J. Hepatol. 2012, 2012. [Google Scholar] [CrossRef]
- Fujimiya, T.; Yamaoka, K.; Fukui, Y. Parallel first-order and Michaelis-Menten elimination kinetics of ethanol. Respective role of alcohol dehydrogenase (ADH), non-ADH and first-order pathways. J. Pharmacol. Exp. Ther. 1989, 249, 311–317. [Google Scholar]
- Vankirk, K.; Sassin, J.F. Technique for serial blood sampling during sleep recording. Amer. J. EEG Technol. 1969, 9, 143–146. [Google Scholar] [CrossRef]
- Agnew, H.W., Jr.; Webb, W.B.; Williams, R.L. The first night effect: An EEG study of sleep. Psychophysiol. 1966, 2, 263–266. [Google Scholar] [CrossRef]
- Van Schrojenstein Lantman, M.; Mackus, M.; Roth, T.; Verster, J.C. Total sleep time, alcohol consumption, and the duration and severity of alcohol hangover. Nat. Sci. Sleep 2017, 9, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Devenney, L.E.; Coyle, K.B.; Roth, T.; Verster, J.C. Sleep after heavy alcohol consumption and physical activity levels during alcohol hangover. J. Clin. Med. 2019, 8, 752. [Google Scholar] [CrossRef] [PubMed]
- Van Lawick van Pabst, A.E.; Devenney, L.E.; Verster, J.C. Sex differences in the presence and severity of alcohol hangover symptoms. J. Clin. Med. 2019, 8, 867. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Kwak, J.H.; Jeon, G.; Lee, J.W.; Seo, J.H.; Lee, H.S.; Lee, J.H. Red ginseng relieves the effects of alcohol consumption and hangover symptoms in healthy men: A randomized crossover study. Food Funct. 2014, 5, 528–534. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.J.; Jeong, H.Y.; Kim, J.Y.; Choi, E.K.; Chae, S.W.; Kwon, O. A standardized extract of the fruit of Hovenia dulcis alleviated alcohol-induced hangover in healthy subjects with heterozygous ALDH2: A randomized, controlled, crossover trial. J. Ethnopharmacol. 2017, 209, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Mammen, R.R.; Natinga Mulakal, J.; Mohanan, R.; Maliakel, B.; Krishnakumar, I.M. Clove bud polyphenols alleviate alterations in inflammation and oxidative stress markers associated with binge drinking: A randomized double-blinded placebo-controlled crossover study. J. Med. Food 2018, 21, 1188–1196. [Google Scholar] [CrossRef]
| Demographics | Acute Study (2016) | Hangover Study (2015) | p-Value |
|---|---|---|---|
| Age (years) | 21.4 (2.4) | 20.4 (2.4) | 0.005 * |
| Height (m) | 1.78 (0.06) | 1.78 (0.06) | 0.685 |
| Weight (kg) | 67.6 (8.7) | 67.1 (7.6) | 0.499 |
| Body Mass Index (BMI, kg/m2) | 21.5 (2.6) | 21.3 (2.5) | 0.469 |
| Usual Number of Drinks on Evening Out (units) | 7.8 (2.9) | 8.8 (3.2) | 0.351 |
| Number of Hangovers Per Month | 2.4 (1.8) | 2.1 (2.0) | 0.345 |
| Assessment | Original Sample | Bootstrapping Results | ||||||
|---|---|---|---|---|---|---|---|---|
| Time | rP | p-Value | Bias | SE | rPB | Lower CIB Limit | Upper CIB Limit | |
| 10.00 (T1) | −0.607 | 0.148 | 0.069 | 0.403 | −0.676 | −0.952 | +0.219 | |
| 11.00 (T2) | −0.519 | 0.233 | −0.009 | 0.308 | −0.510 * | −0.857 | −0.127 | |
| 12.00 (T3) | −0.362 | 0.425 | −0.020 | 0.386 | −0.342 | −0.921 | +0.249 | |
| 13.00 (T4) | −0.508 | 0.244 | −0.036 | 0.272 | −0.472 * | −0.922 | −0.221 | |
| 14.00 (T5) | −0.629 | 0.130 | −0.021 | 0.272 | −0.608 * | −0.973 | −0.302 | |
| 15.00 (T6) | −0.332 | 0.467 | −0.066 | 0.366 | −0.266 | −0.972 | +0.109 | |
| 16.00 (T7) | −0.586 | 0.166 | 0.026 | 0.353 | −0.612 | −0.973 | +0.050 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mackus, M.; van de Loo, A.J.; Garssen, J.; Kraneveld, A.D.; Scholey, A.; Verster, J.C. The Association between Ethanol Elimination Rate and Hangover Severity. Int. J. Environ. Res. Public Health 2020, 17, 4324. https://doi.org/10.3390/ijerph17124324
Mackus M, van de Loo AJ, Garssen J, Kraneveld AD, Scholey A, Verster JC. The Association between Ethanol Elimination Rate and Hangover Severity. International Journal of Environmental Research and Public Health. 2020; 17(12):4324. https://doi.org/10.3390/ijerph17124324
Chicago/Turabian StyleMackus, Marlou, Aurora JAE van de Loo, Johan Garssen, Aletta D Kraneveld, Andrew Scholey, and Joris C Verster. 2020. "The Association between Ethanol Elimination Rate and Hangover Severity" International Journal of Environmental Research and Public Health 17, no. 12: 4324. https://doi.org/10.3390/ijerph17124324
APA StyleMackus, M., van de Loo, A. J., Garssen, J., Kraneveld, A. D., Scholey, A., & Verster, J. C. (2020). The Association between Ethanol Elimination Rate and Hangover Severity. International Journal of Environmental Research and Public Health, 17(12), 4324. https://doi.org/10.3390/ijerph17124324






