Modeling Healthcare Costs Attributable to Secondhand Smoke Exposure at Home among South Korean Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Model Overview
2.2. Target Population
2.3. Input Variables
2.4. Markov Model
2.5. Sensitivity Analysis
3. Results
3.1. Input Data
3.2. Base-Case Analysis
3.3. One-Way Sensitivity Analysis
3.4. Probabilistic Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moritsugu, K.P. The 2006 Report of the Surgeon General: The Health Consequences of Involuntary Exposure to Tobacco Smoke. Am. J. Prev. Med. 2007, 32, 542–543. [Google Scholar] [CrossRef]
- Oberg, M.; Jaakkola, M.S.; Woodward, A.; Peruga, A.; Pruss-Ustun, A. Worldwide burden of disease from exposure to second-hand smoke: A retrospective analysis of data from 192 countries. Lancet 2011, 377, 139–146. [Google Scholar] [CrossRef]
- Lippi, G.; Mattiuzzi, C. Secondhand smoke in childhood: The world-wide burden of a major public health-care problem. J. Paediatr. Child Health 2019, 55, 1397–1398. [Google Scholar] [CrossRef]
- Yao, T.; Sung, H.Y.; Wang, Y.; Lightwood, J.; Max, W. Healthcare Costs of Secondhand Smoke Exposure at Home for U.S. Children. Am. J. Prev. Med. 2019, 56, 281–287. [Google Scholar] [CrossRef]
- OECD Health Statistics 2019. Adult Population Smoking Daily, 2007 and 2017 (or Nearest Years). Available online: https://www.oecd-ilibrary.org/sites/21ac51dd-en/index.html?itemId=/content/component/21ac51dd-en#figure-d1e17082 (accessed on 14 May 2020).
- Ashley, M.J.; Ferrence, R. Reducing children’s exposure to environmental tobacco smoke in homes: Issues and strategies. Tob. Control 1998, 7, 61–65. [Google Scholar] [CrossRef]
- Youth Health Survey (YHS). Available online: http://www.cdc.go.kr/yhs/ (accessed on 27 May 2020).
- Oberg, M.; Jaakkola, M.S.; Pruss-Ustun, A.; Peruga, A.; Woodward, A. ; World Health Organization. Global Estimate of the Burden of Disease from Second-Hand Smoke; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Waters, H.R.; Foldes, S.S.; Alesci, N.L.; Samet, J. The economic impact of exposure to secondhand smoke in Minnesota. Am. J. Public Health 2009, 99, 754–759. [Google Scholar] [CrossRef]
- Plescia, M.; Wansink, D.; Waters, H.R.; Herndon, S. Medical costs of secondhand-smoke exposure in North Carolina. N. C. Med. J. 2011, 72, 7–12. [Google Scholar]
- Hill, S.C.; Liang, L. Smoking in the home and children’s health. Tob. Control 2008, 17, 32–37. [Google Scholar] [CrossRef]
- Max, W.; Sung, H.Y.; Shi, Y. The cost of secondhand smoke exposure at home in California. Tob. Control 2015, 24, 205–210. [Google Scholar] [CrossRef]
- Briggs, A.H.; Sculpher, M.; Claxton, K. Decision Modelling for Health Economic Evaluation; OUP: Oxford, UK, 2006. [Google Scholar]
- Lee, J.; Han, A.R.; Choi, D.; Lim, K.-M.; Bae, S. Modeling lifetime costs and health outcomes attributable to secondhand smoke exposure at home among Korean adult women. BMJ Open 2017, 7, e013292. [Google Scholar] [CrossRef]
- Gretchen, B.; Chapman, F.A.S. Decision Making in Health Care; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- The Korean Otologic Society. Pediatrics Otitis Media Clinical Practice Guideline; The Korean Otologic Society: Seoul, Korea, 2014. [Google Scholar]
- Hay, A.D.; Wilson, A.D. The natural history of acute cough in children aged 0 to 4 years in primary care: A systematic review. Br. J. Gen. Pract. 2002, 52, 401–409. [Google Scholar] [PubMed]
- Ostapchuk, M.; Roberts, D.M.; Haddy, R. Community-acquired pneumonia in infants and children. Am. Fam. Physician 2004, 70, 899–908. [Google Scholar] [PubMed]
- Bae, S.; Lee, S.; Bae, E.Y.; Jang, S. Korean Guidelines for Pharmacoeconomic Evaluation (Second and Updated Version). PharmacoEconomics 2013, 31, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.C.; Siegel, J.E.; Gold, M.R.; Kamlet, M.S.; Russell, L.B. Recommendations of the Panel on Cost-Effectiveness in Health and Medicine. JAMA 1996, 276, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Gergen, P.J.; Fowler, J.A.; Maurer, K.R.; Davis, W.W.; Overpeck, M.D. The burden of environmental tobacco smoke exposure on the respiratory health of children 2 months through 5 years of age in the United States: Third National Health and Nutrition Examination Survey, 1988 to 1994. Pediatrics 1998, 101, E8. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, W.; Yu, S.; Qian, W. Chang-Ning Epidemiological Study of Children’s Health: I: Passive Smoking and Children’s Respiratory Diseases. Int. J. Epidemiol. 1988, 17, 348–355. [Google Scholar] [CrossRef]
- Magnusson, L.L.; Olesen, A.B.; Wennborg, H.; Olsen, J. Wheezing, asthma, hayfever, and atopic eczema in childhood following exposure to tobacco smoke in fetal life. Clin. Exp. Allergy 2005, 35, 1550–1556. [Google Scholar] [CrossRef]
- Mitchell, E.A.; Milerad, J. Smoking and the sudden infant death syndrome. Rev. Environ. Health 2006, 21, 81–103. [Google Scholar] [CrossRef]
- Strachan, D.P.; Cook, D.G. Health effects of passive smoking. 1. Parental smoking and lower respiratory illness in infancy and early childhood. Thorax 1997, 52, 905. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, K.F. What’s the Relative Risk?A Method of Correcting the Odds Ratio in Cohort Studies of Common Outcomes. JAMA 1998, 280, 1690–1691. [Google Scholar] [CrossRef]
- Cheol Seong, S.; Kim, Y.Y.; Khang, Y.H.; Heon Park, J.; Kang, H.J.; Lee, H.; Do, C.H.; Song, J.S.; Hyon Bang, J.; Ha, S.; et al. Data Resource Profile: The National Health Information Database of the National Health Insurance Service in South Korea. Int. J. Epidemiol. 2017, 46, 799–800. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Yoon, S.; Kim, L.Y.; Kim, D.S. Towards Actualizing the Value Potential of Korea Health Insurance Review and Assessment (HIRA) Data as a Resource for Health Research: Strengths, Limitations, Applications, and Strategies for Optimal Use of HIRA Data. J. Korean Med. Sci. 2017, 32, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-C.; Chang, Y.-H.; Chuang, L.-J.; Su, H.-F.; Li, C.-Y. Incidence and recurrence of acute otitis media in Taiwan’s pediatric population. Clinics 2011, 66, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Vital Statistics Korea. Available online: http://kosis.kr/index/index.do (accessed on 2 September 2019).
- Briggs, A.H.; Weinstein, M.C.; Fenwick, E.A.; Karnon, J.; Sculpher, M.J.; Paltiel, A.D. Model parameter estimation and uncertainty analysis: A report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-6. Med. Decis. Mak. 2012, 32, 722–732. [Google Scholar] [CrossRef] [PubMed]
- RCGP (Royal College of General Practitioners) Research and Surveillance Centre. Weekly Returns Service Annual Report 2016–2017; RCGP (Royal College of General Practitioners) Research and Surveillance Centre: London, UK, 2017. [Google Scholar]
- Winer, R.A.; Qin, X.; Harrington, T.; Moorman, J.; Zahran, H. Asthma Incidence among Children and Adults: Findings from the Behavioral Risk Factor Surveillance System Asthma Call-back Survey—United States, 2006–2008. J. Asthma 2012, 49, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Healthcare Bigdata Hub. Available online: https://opendata.hira.or.kr (accessed on 10 January 2020).
- Anderson, H.R.; Cook, D.G. Passive smoking and sudden infant death syndrome: Review of the epidemiological evidence. Thorax 1997, 52, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.L.; Hassanien, A.; Cook, D.G.; Britton, J.; Leonardi-Bee, J. Parental smoking and the risk of middle ear disease in children: A systematic review and meta-analysis. Arch. Pediatr. Adolesc. Med. 2012, 166, 18–27. [Google Scholar] [CrossRef]
- Jones, L.L.; Hashim, A.; McKeever, T.; Cook, D.G.; Britton, J.; Leonardi-Bee, J. Parental and household smoking and the increased risk of bronchitis, bronchiolitis and other lower respiratory infections in infancy: Systematic review and meta-analysis. Respir. Res. 2011, 12, 5. [Google Scholar] [CrossRef]
- Burke, H.; Leonardi-Bee, J.; Hashim, A.; Pine-Abata, H.; Chen, Y.; Cook, D.G.; Britton, J.R.; McKeever, T.M. Prenatal and passive smoke exposure and incidence of asthma and wheeze: Systematic review and meta-analysis. Pediatrics 2012, 129, 735–744. [Google Scholar] [CrossRef]
- Park, C.S.; Kang, H.Y.; Kwon, I.; Kang, D.R.; Jung, H.Y. Cost-of-illness Study of Asthma in Korea: Estimated from the Korea National Health Insurance Claims Database. J. Prev. Med. Public Health 2006, 39, 397–403. [Google Scholar]
- You Jin Jung, W.M.Y. Analysis of Korean Medical status of Acute Bronchitis, Chronic Bronchitis and Allergic Rhinitis patients. J. Korean Med. 2019, 40, 87–98. [Google Scholar] [CrossRef]
- Chun, B.C.; Sohn, W.Y.; Jung, W.; Lee, H.J. Economic burden of otitis media and a survey of physicians for its practice and claim codes in Korea. J. Korean Med. Assoc. 2013, 56, 62–71. [Google Scholar] [CrossRef]
- Consumer Price Index. Available online: http://kosis.kr/index/index.do (accessed on 20 December 2019).
- Exchange Rate Statistics by Currency. Available online: http://www.index.go.kr/main.do?cate=1 (accessed on 2 September 2019).
- Bae, S.J.; Paltiel, A.D.; Fuhlbrigge, A.L.; Weiss, S.T.; Kuntz, K.M. Modeling the Potential Impact of a Prescription Drug Copayment Increase on the Adult Asthmatic Medicaid Population. Value Health 2008, 11, 110–118. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fuhlbrigge, A.L.; Bae, S.J.; Weiss, S.T.; Kuntz, K.M.; Paltiel, A.D. Cost-effectiveness of inhaled steroids in asthma: Impact of effect on bone mineral density. J. Allergy Clin. Immunol. 2006, 117, 359–366. [Google Scholar] [CrossRef]
- Suzuki, M.; Thiem, V.D.; Yanai, H.; Matsubayashi, T.; Yoshida, L.-M.; Tho, L.H.; Minh, T.T.; Anh, D.D.; Kilgore, P.E.; Ariyoshi, K. Association of environmental tobacco smoking exposure with an increased risk of hospital admissions for pneumonia in children under 5 years of age in Vietnam. Thorax 2009, 64, 484–489. [Google Scholar] [CrossRef][Green Version]
- Bentdal, Y.E.; Karevold, G.; Nafstad, P.; Kvaerner, K.J. Early acute otitis media: Predictor for AOM and respiratory infections in schoolchildren? Int. J. Pediatr. Otorhinolaryngol. 2007, 71, 1251–1259. [Google Scholar] [CrossRef]
- Health Spending. Available online: https://data.oecd.org/healthres/health-spending.htm (accessed on 20 March 2020).
- Lofland, J.H.; Locklear, J.C.; Frick, K.D. Different approaches to valuing the lost productivity of patients with migraine. Pharmacoeconomics 2001, 19, 917–925. [Google Scholar] [CrossRef]
- Weinstein, M.C.; Siegel, J.E.; Garber, A.M.; Lipscomb, J.; Luce, B.R.; Manning, W.G., Jr.; Torrance, G.W. Productivity costs, time costs and health-related quality of life: A response to the Erasmus Group. Health Econ. 1997, 6, 505–510. [Google Scholar] [CrossRef]
- Gamble, J.E.; Bizal, J.A.; Daetwyler, E.P. Otitis media and chronic middle ear effusion in the asthmatic pediatric patient. Ear Nose Throat J. 1992, 71, 397–399. [Google Scholar] [CrossRef]
- Eisner, M.D.; Klein, J.; Hammond, S.K.; Koren, G.; Lactao, G.; Iribarren, C. Directly measured second hand smoke exposure and asthma health outcomes. Thorax 2005, 60, 814–821. [Google Scholar] [CrossRef]
- Winickoff, J.P.; Friebely, J.; Tanski, S.E.; Sherrod, C.; Matt, G.E.; Hovell, M.F.; McMillen, R.C. Beliefs about the health effects of "thirdhand" smoke and home smoking bans. Pediatrics 2009, 123, e74–e79. [Google Scholar] [CrossRef] [PubMed]

| Variables | Distribution | |
|---|---|---|
| Odds ratio of disease | AOM first time | Lognormal distribution |
| AOM recurrence | Lognormal distribution | |
| Acute bronchitis | Lognormal distribution | |
| Pneumonia | Lognormal distribution | |
| Asthma | Lognormal distribution | |
| SIDS | Lognormal distribution | |
| Cost | AOM week 1 | Gamma distribution |
| Acute bronchitis week 1 | Gamma distribution | |
| Pneumonia | Gamma distribution | |
| Asthma | Gamma distribution | |
| Asthma exacerbation | Gamma distribution | |
| Disease | Age | Incidence Rate | Ref | Age | Mortality Rate | Ref | Age | Odds Ratio | Ref |
|---|---|---|---|---|---|---|---|---|---|
| SIDS | 0 | 0.0002 | [30] | - | - | - | - | 1.94 (1.55–2.43) | [35] |
| AOM first time | 0–2 | 0.0717 | [29] | - | - | - | - | 1.32 (1.20–1.45) | [36] |
| 3–5 | 0.1588 | ||||||||
| 6–19 | 0.041 | ||||||||
| AOM recurrence | 0–2 | 0.412 | [29] | - | - | - | - | 1.32 (1.14–1.52) | [1] |
| 3–5 | 0.381 | ||||||||
| 6–19 | 0.267 | ||||||||
| Acute Bronchitis | 0 | 0.0048 | [32] | 0 | 0.000003 | [30] | - | 1.58 (1.27–1.98) | [37] |
| 1–4 | 0.0259 | 1–19 | 0 | ||||||
| 5–14 | 0.0113 | ||||||||
| 15–19 | 0.0107 | ||||||||
| Pneumonia | 0–4 | 0.6920 | [34] | 0 | 0.000015 | [30] | - | 1.43 (0.93–2.21) | [37] |
| 5–9 | 0.1599 | 1–14 | 0.000001 | ||||||
| 10–14 | 0.0416 | 15–19 | 0.000002 | ||||||
| 15–19 | 0.0179 | ||||||||
| Asthma | 0–4 | 0.0234 | [33] | - | - | - | 0–2 | 1.14 (0.94–1.38) | [38] |
| 5–11 | 0.0111 | 3–4 | 1.21 (1.00–1.47) | ||||||
| 12–19 | 0.0044 | 5–19 | 1.30 (1.04–1.62) | ||||||
| Baseline mortality | - | - | - | 0 | 0.002807 | [30] | - | - | - |
| 1–4 | 0.00013 | ||||||||
| 5–9 | 0.000076 | ||||||||
| 10–14 | 0.000095 | ||||||||
| 15–19 | 0.000216 |
| State | Age | Weekly Healthcare Costs | Ref |
|---|---|---|---|
| Event free, Post-OM | 0–19 | 0 | - |
| AOM week 1 | 0–4 | 14.7 | [34] |
| 5–9 | 15.0 | ||
| 10–14 | 17.2 | ||
| 15–19 | 21.4 | ||
| AOM week 2 | 0–19 | 0 | - |
| (Post-OM) Bronchitis week 1 | 0–19 | 14.4 | [40] |
| (Post-OM) Bronchitis week 2 | 0–19 | 0 | - |
| (Post-OM) Pneumonia | 0–4 | 104.2 | [34] |
| 5–9 | 78.8 | ||
| 10–14 | 85.3 | ||
| 15–19 | 90.3 | ||
| Asthma | 0–14 | 2.7 | [39] |
| 15–19 | 3.1 | ||
| Asthma exacerbation | 0–14 | 65.4 | [39] |
| 15–19 | 99.2 |
| Parameters | SHS Exposure States | Direct Healthcare Costs (USD) | |
|---|---|---|---|
| Total | Incremental (%) | ||
| Base-case | |||
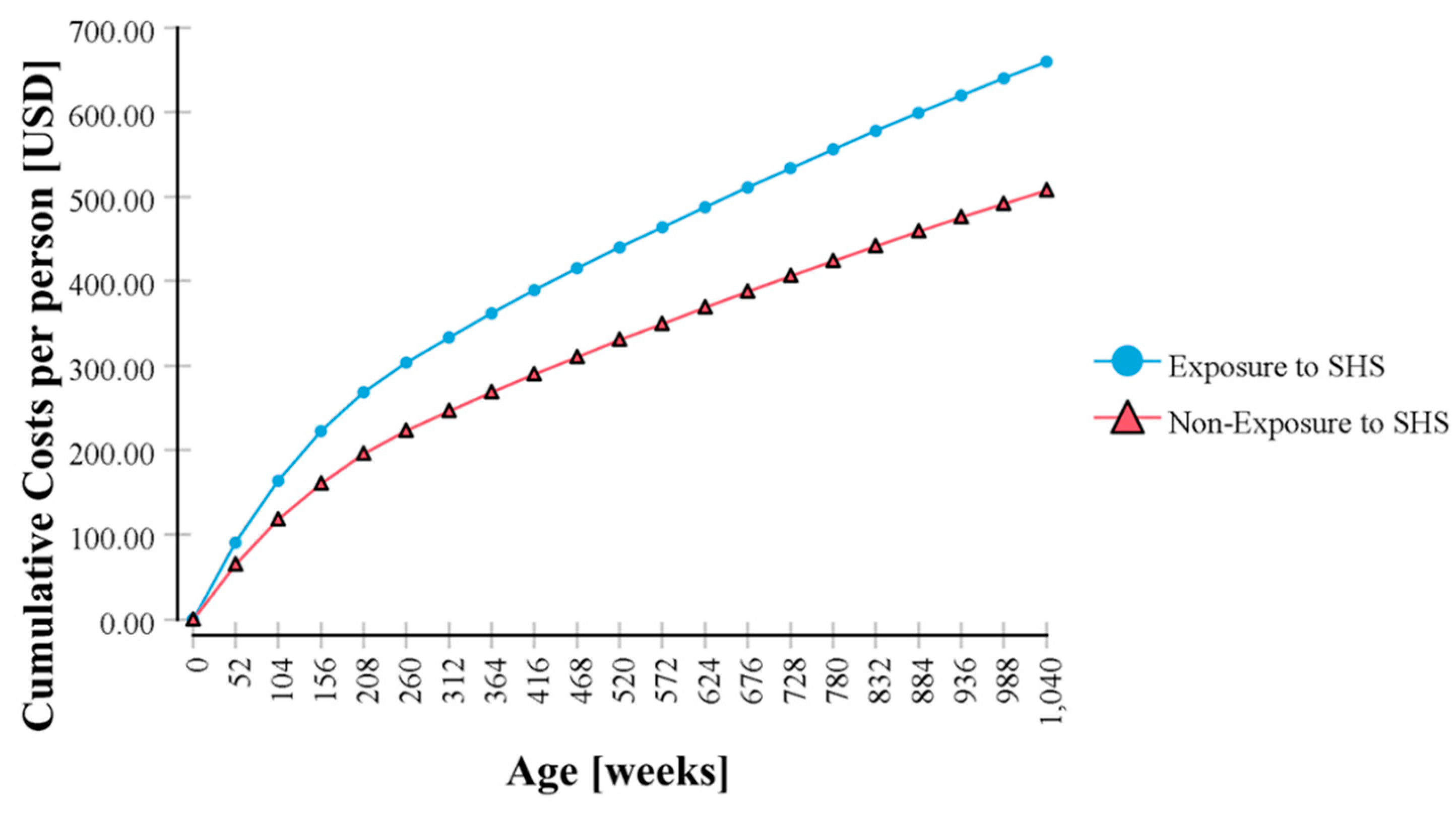
| No | 507.32 | ||
| Yes | 659.61 | 30.02 | |
| Discount rate | |||
| 0% | No | 769.24 | |
| Yes | 987.69 | 28.40 | |
| 3% | No | 591.46 | |
| Yes | 765.19 | 29.37 | |
| 7.50% | No | 428.02 | |
| Yes | 559.87 | 30.80 | |
| Exposure period | |||
| 0–9 years old | No | 507.32 | |
| Yes | 639.18 | 25.99 | |
| 10–19 years old | No | 507.32 | |
| Yes | 522.64 | 3.02 | |
| Odds ratio for SIDS mortality | |||
| Upper 95% CI | No | 507.32 | |
| Yes | 659.64 | 30.02 | |
| Lower 95% CI | No | 507.32 | |
| Yes | 659.58 | 30.01 | |
| Odds ratio for AOM morbidity | |||
| Upper 95% CI | No | 507.32 | |
| Yes | 657.49 | 29.60 | |
| Lower 95% CI | No | 507.32 | |
| Yes | 661.69 | 30.43 | |
| Odds ratio for recurrent AOM morbidity | |||
| Upper 95% CI | No | 507.32 | |
| Yes | 654.96 | 29.10 | |
| Lower 95% CI | No | 507.32 | |
| Yes | 664.77 | 31.04 | |
| Odds ratio for acute bronchitis morbidity | |||
| Upper 95% CI | No | 507.32 | |
| Yes | 659.1 | 29.92 | |
| Lower 95% CI | No | 507.32 | |
| Yes | 660.27 | 30.15 | |
| Odds ratio for pneumonia morbidity | |||
| Upper 95% CI | No | 507.32 | |
| Yes | 559.98 | 10.38 | |
| Lower 95% CI | No | 507.32 | |
| Yes | 812.81 | 60.22 | |
| Probability of asthma exacerbation | |||
| +20% | No | 492.52 | |
| Yes | 641.85 | 30.32 | |
| −20% | No | 522.03 | |
| Yes | 677.28 | 29.74 | |
| Statistic | Costs | |
|---|---|---|
| SHS Non-Exposed | SHS Exposed | |
| Mean | 491.77 | 648.49 |
| Standard deviation | 58.99 | 141.17 |
| Minimum | 306.31 | 314.65 |
| 2.5% | 384.08 | 430.40 |
| 10% | 417.72 | 487.44 |
| Median | 489.97 | 629.75 |
| 90% | 567.82 | 833.63 |
| 97.5% | 614.76 | 976.57 |
| Maximum | 751.07 | 1464.70 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Bae, S. Modeling Healthcare Costs Attributable to Secondhand Smoke Exposure at Home among South Korean Children. Int. J. Environ. Res. Public Health 2020, 17, 4496. https://doi.org/10.3390/ijerph17124496
Park J, Bae S. Modeling Healthcare Costs Attributable to Secondhand Smoke Exposure at Home among South Korean Children. International Journal of Environmental Research and Public Health. 2020; 17(12):4496. https://doi.org/10.3390/ijerph17124496
Chicago/Turabian StylePark, Jeewon, and SeungJin Bae. 2020. "Modeling Healthcare Costs Attributable to Secondhand Smoke Exposure at Home among South Korean Children" International Journal of Environmental Research and Public Health 17, no. 12: 4496. https://doi.org/10.3390/ijerph17124496
APA StylePark, J., & Bae, S. (2020). Modeling Healthcare Costs Attributable to Secondhand Smoke Exposure at Home among South Korean Children. International Journal of Environmental Research and Public Health, 17(12), 4496. https://doi.org/10.3390/ijerph17124496






