Investigation of Measles Outbreak among Thai and Migrant Workers in Two Factories in Nakhon Pathom, Thailand, 2019
Abstract
:1. Introduction
2. Methods
2.1. Overview of the Study
2.2. Data Collection
2.3. Data Analysis
2.4. Environmental Survey
2.5. Ethics Clearance
3. Results
3.1. Setting Overview
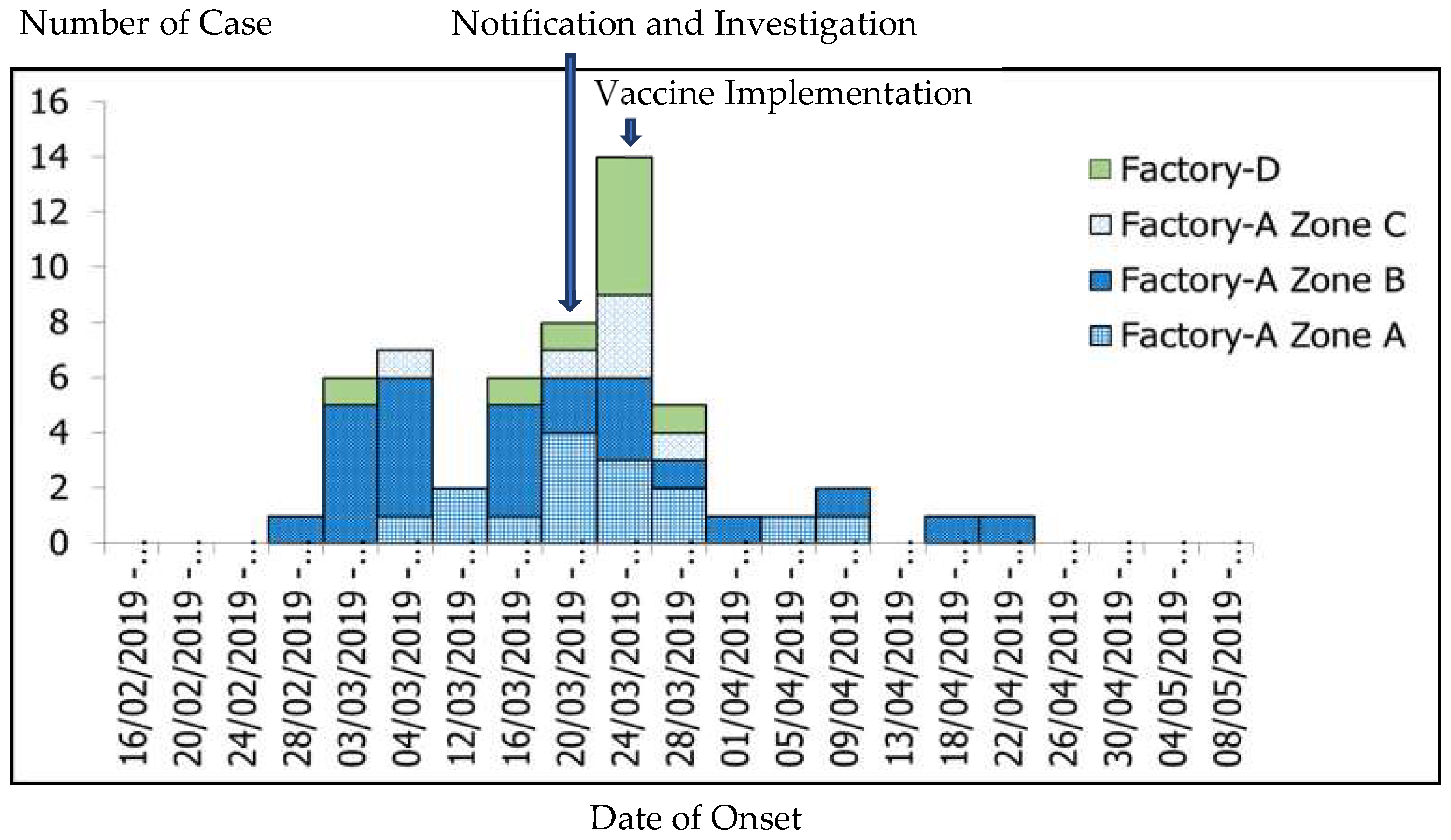
3.2. Outbreak Description
3.3. Laboratory Study
3.4. Environmental Study
3.5. Social Network Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Quadros, C.A. Can measles be eradicated globally? Bull. World Health Organ. 2004, 82, 134–138. [Google Scholar] [PubMed]
- Wallinga, J.; Heijne, J.C.M.; Kretzschmar, M. A measles epidemic threshold in a highly vaccinated population. PLoS Med. 2005, 2, e316. [Google Scholar] [CrossRef] [PubMed]
- Verguet, S.; Jassat, W.; Hedberg, C.; Tollman, S.; Jamison, D.T.; Hofman, K.J. Measles control in Sub-Saharan Africa: South Africa as a case study. Vaccine 2012, 30, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Bjørnstad, O.N.; Grenfell, B. Hazards, spatial transmission and timing of outbreaks in epidemic metapopulations. Environ. Ecol. Stat. 2008, 15, 265–277. [Google Scholar] [CrossRef]
- Ministry of Public Health (Thailand). Situation of Measles/Rubella/CRS 2019 Thailand [Internet]. 2020. Available online: https://apps.boe.moph.go.th/measles/ (accessed on 30 January 2020).
- Division of Epidemiology. R506 Database—Number of Cases and Deaths by Month and Province. Available online: http://www.boe.moph.go.th/boedb/surdata/disease.php?dcontent=old&ds=21 (accessed on 3 August 2019).
- International Organization for Migration. Thailand Migration Report 2011; International Organization for Migration: Bangkok, Thailand, 2011. [Google Scholar]
- Ministry of Health the Republic of the Union of Myanmar. Expanded Programme on Immunization Myanmar Multiyear Plan 2012–2016; Ministry of Health: Naypytaw, Myanmar, 2011.
- Thar, A.M.C.; Wai, K.T.; Harries, A.D.; Show, K.L.; Mon, L.L.; Lin, H.H. Reported measles cases, measles-related deaths and measles vaccination coverage in Myanmar from 2014 to 2018. Trop. Med. Health 2020, 48. [Google Scholar] [CrossRef] [PubMed]
- Kaji, A.; Parker, D.; Chu, C.S.; Thayatkawin, W.; Suelaor, J.; Charatrueangrongkun, R. Immunization coverage in migrant school children along the Thailand-Myanmar border. J. Immigr. Minority Health 2016, 18, 1038–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention. Measles: Specimen Collection, Storage, and Shipment Collecting and Shipping Specimens for Suspected Measles Cases. 2018. Available online: https://www.cdc.gov/measles/lab-tools/rt-pcr.html (accessed on 4 January 2020).
- Manual for the Laboratory Diagnosis of Measles and Rubella. Available online: https://www.who.int/ihr/elibrary/manual_diagn_lab_mea_rub_en.pdf (accessed on 4 January 2020).
- Stattner, E.; Vidot, N. Social network analysis in epidemiology: Current trends and perspectives. In Proceedings of the 2011 Fifth International Conference on Research Challenges in Information Science, Gosier, France, 19 May 2011. [Google Scholar]
- World Health Organization. Measles and Rubella Surveillance Data. 2020. Available online: https://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/active/measles_monthlydata/en/ (accessed on 13 January 2020).
- World Health Organization. Measles. Available online: https://www.who.int/immunization/monitoring_surveillance/burden/vpd/WHO_SurveillanceVaccinePreventable_11_Measles_R1.pdf?ua=1 (accessed on 25 April 2020).
- Centers for Disease Control and Prevention. Transmission of Measles. Available online: https://www.cdc.gov/measles/transmission.html (accessed on 23 June 2020).
- Long Ngoc, V.; Niramitsantipon, A.; Jiraphongsa, C.; Attawong, B.; Khuankaw, W.; Tipsriraj, S.; Pattamadilok, S. Investigation on measles outbreak among university students in Phrae Province, Thailand, 2008: Risk factors and seroprevalence of antibodies to measles. OSIR J. 2011, 4, 6–12. [Google Scholar]
- Tipayapatanakul, S.; Sangkerd, U.; Chookaew, A.; Darakai, S.; Jaturabandit, N.; Kantanon, O. A measles outbreak in a Myanmar migrant worker camps, Bang Wan sub-district, Kuraburi district, Phang-nga, Thailand, May–June 2012. WESR Thail. 2014, 45, 13–22. [Google Scholar]
- Piyawatweala, S.; Kudwonsa, W.; Sriwirat, S.; Suttisa, A.; Sukhuna, C. Measles outbreak among male prisoners in Maha Sarakham province, Thailand, March–June 2013. WESR Thail. 2014, 45, 753–760. [Google Scholar]
- Grais, R.; Conlan, A.; Ferrari, M.; Djibo, A.; Menach, A.L.; Bjørnstad, O. Time is of the essence: Exploring a measles outbreak response vaccination in Niamey, Niger. J. R. Soc. Interface 2007, 5, 67–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marinović, A.A.B.; Swaan, C.; Wichmann, O.; Steenbergen, J.V.; Kretzschmar, M. Effectiveness and timing of vaccination during school measles outbreak. Emerg. Infect. Dis. 2012, 18, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Kantayaporn, T.; Archavanitkul, K.; Peerapatanapokin, W.; Disthawong, N.; Singkul, N.; Sinvuttaya, S.; Chinvarasopak, P.; Panatanasan, K. Expanded program on immunization (EPI) for children of Myanmar migrants living in Bangkok, Thailand. J. Popul. Soc. Stud. 2013, 21, 227–242. [Google Scholar]
- Aaby, P. Malnutrition and overcrowding/intensive exposure in severe measles infection: Review of community studies. Rev. Infect. Dis. 1988, 10, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Gayer, M.; Legros, D.; Formenty, P.; Connolly, M.A. Conflict and emerging infectious diseases. Emerg. Infect. Dis. 2007, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Ongwandee, S.; Boonmadam, U.; Mongkol, A. Measles outbreak in Mae La Oon temporary shelter, Maehongson, April–August 2008. Wkly. Epidemiol. Surveill. Rep. 2009, 40, 205–209. [Google Scholar]
- Marin, N.; Chanachai, K.; Phutap, J.; Luckpanalee, R.; Chalamat, M.; Kudthasrima, N.; Thammawijaya, P.; Pattamadilok, S.; Iamsirithaworn, S. Measles outbreak in a mountainous area, Mae-suay district, Chiangrai province, Thailand, December 2006–February 2007. Wkly. Epidemiol. Surveill. Rep. 2009, 40, 58–61. [Google Scholar]
- Division of Epidemiology. Measles Elimination Program. Available online: https://apps.boe.moph.go.th/measles/ (accessed on 3 September 2009).
- Werber, D.; Hoffmann, A.; Santibanez, S.; Mankertz, A.; Sagebiel, D. Large measles outbreak introduced by asylum seekers and spread among the insufficiently vaccinated resident population, Berlin, October 2014 to August 2015. Eurosurveillance 2017, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giambi, C.; Del Manso, M.; Marchetti, G.; Olsson, K.; Adel Ali, K.; Declich, S. Immunisation of Migrants in EU/EEA Countries: Policies and Practices. Vaccine 2019, 37, 5439–5451, U.S. National Library of Medicine. Available online: https://www.ncbi.nlm.nih.gov/pubmed/31296374 (accessed on 13 January 2020). [CrossRef] [PubMed]
- Mellou, K.; Silvestros, C.; Saranti-Papasaranti, E.; Koustenis, A.; Pavlopoulou, I.D.; Georgakopoulou, T. Increasing childhood vaccination coverage of the refugee and migrant population in Greece through the European programme PHILOS, April 2017 to April 2018. Eurosurveillance 2019, 24. [Google Scholar] [CrossRef] [PubMed]
- Division of Health Administration, Thai Ministry of Public Health. Migrant Worker Manual. Available online: https://phdb.moph.go.th/main/index/download/533 (accessed on 12 December 2019).
- Gastañaduy, P.A.; Banerjee, E.; Debolt, C.; Bravo-Alcántara, P.; Samad, S.A.; Pastor, D. Public health responses during measles outbreaks in elimination settings: Strategies and challenges. Hum. Vaccines Immunother. 2018, 14, 2222–2238. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization. Electronic Immunization Registry: Practical Considerations for Planning, Development, Implementation and Evaluation. PAHO/WHO IRIS. PAHO. 2018. Available online: http://iris.paho.org/xmlui/handle/123456789/34865 (accessed on 13 January 2020).


| Zone | Population (N) | Screen (n) | Percent Screening | Number of Case | AR by Population (%) | AR by Screening (%) |
|---|---|---|---|---|---|---|
| Factory-S | 2890 | 1251 | 43.28 | 47 | 1.63 | 3.76 |
| • Zone A | 859 | 407 | 47.37 | 15 | 1.75 | 3.69 |
| • Zone B | 633 | 355 | 56.08 | 25 | 3.94 | 7.04 |
| • Zone C | 711 | 350 | 49.23 | 6 | 0.84 | 1.88 |
| • Warehouse | 189 | 139 | 73.54 | 1 | 0.53 | 0.79 |
| Factory-D | 2691 | 921 | 34.22 | 9 | 0.33 | 1.00 |
| Total | 5581 | 2172 | 39.80 | 56 | 1.00 | 2.58 |
| Age Group—Years | Population—n | Case—n | Attack Rate (%) |
|---|---|---|---|
| 16–19 | 131 | 0 | 0 |
| 20–24 | 1039 | 23 | 2.21 |
| 25–29 | 1397 | 19 | 1.36 |
| 30–34 | 103 | 10 | 0.97 |
| 35–39 | 840 | 1 | 0.01 |
| >39 | 643 | 2 | 0.03 |
| Unknown | 1 | - | |
| Total | 5581 | 56 | 1.00 |
| Thai | Migrant Worker | Total | |
|---|---|---|---|
| No Measles Vaccination | 3 (11.6%) | 11 (36.7%) | 14 (25.0%) |
| 1 Dose of Measles Vaccination | 3 (11.6%) | 0 (0%) | 3 (5.4%) |
| 2 Doses of Measles Vaccination | 4 (15.5%) | 0 (0%) | 4 (7.1%) |
| Can’t Remember | 16 (61.3%) | 19 (63.3%) | 35(62.5%) |
| Total | 26 (100%) | 30 (100%) | 56 (100%) |
| Antigen | Laboratory Test | Specimen | Sample | Result—n (%) | ||
|---|---|---|---|---|---|---|
| Positive | Borderline | Negative | ||||
| Measles and Rubella | IgM | Serum | 17 | 8 (53%) | 3 | 6 |
| PCR | TS+NPS | 8 | 5 (genotype D8) (62%) | 0 | 3 | |
| CR for Zika | PCR | Urine | 9 | 0 | 0 | 9 |
| PCR | EDTABlood | 4 | 0 | 0 | 4 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wongsanuphat, S.; Thitichai, P.; Jaiyong, R.; Plernprom, P.; Thintip, K.; Jitpeera, C.; Suphanchaimat, R. Investigation of Measles Outbreak among Thai and Migrant Workers in Two Factories in Nakhon Pathom, Thailand, 2019. Int. J. Environ. Res. Public Health 2020, 17, 4627. https://doi.org/10.3390/ijerph17134627
Wongsanuphat S, Thitichai P, Jaiyong R, Plernprom P, Thintip K, Jitpeera C, Suphanchaimat R. Investigation of Measles Outbreak among Thai and Migrant Workers in Two Factories in Nakhon Pathom, Thailand, 2019. International Journal of Environmental Research and Public Health. 2020; 17(13):4627. https://doi.org/10.3390/ijerph17134627
Chicago/Turabian StyleWongsanuphat, Suphanat, Phanthanee Thitichai, Rungrot Jaiyong, Patchanee Plernprom, Kanthika Thintip, Charuttaporn Jitpeera, and Rapeepong Suphanchaimat. 2020. "Investigation of Measles Outbreak among Thai and Migrant Workers in Two Factories in Nakhon Pathom, Thailand, 2019" International Journal of Environmental Research and Public Health 17, no. 13: 4627. https://doi.org/10.3390/ijerph17134627
APA StyleWongsanuphat, S., Thitichai, P., Jaiyong, R., Plernprom, P., Thintip, K., Jitpeera, C., & Suphanchaimat, R. (2020). Investigation of Measles Outbreak among Thai and Migrant Workers in Two Factories in Nakhon Pathom, Thailand, 2019. International Journal of Environmental Research and Public Health, 17(13), 4627. https://doi.org/10.3390/ijerph17134627






