Nanotoxicology and Nanosafety: Safety-by-Design and Testing at a Glance
Abstract
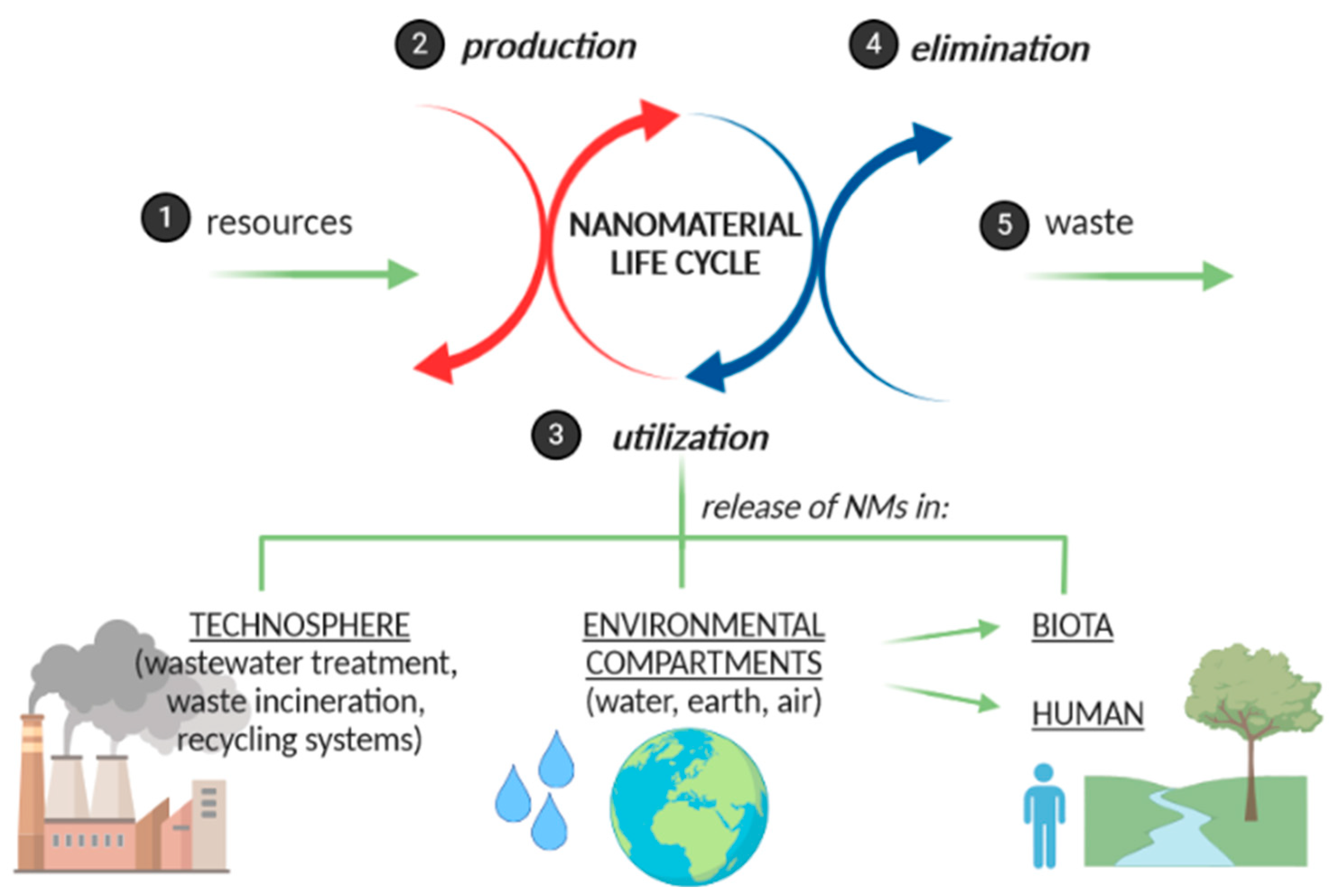
:1. Introduction
2. Formulating Nanomaterials in Innovative Products
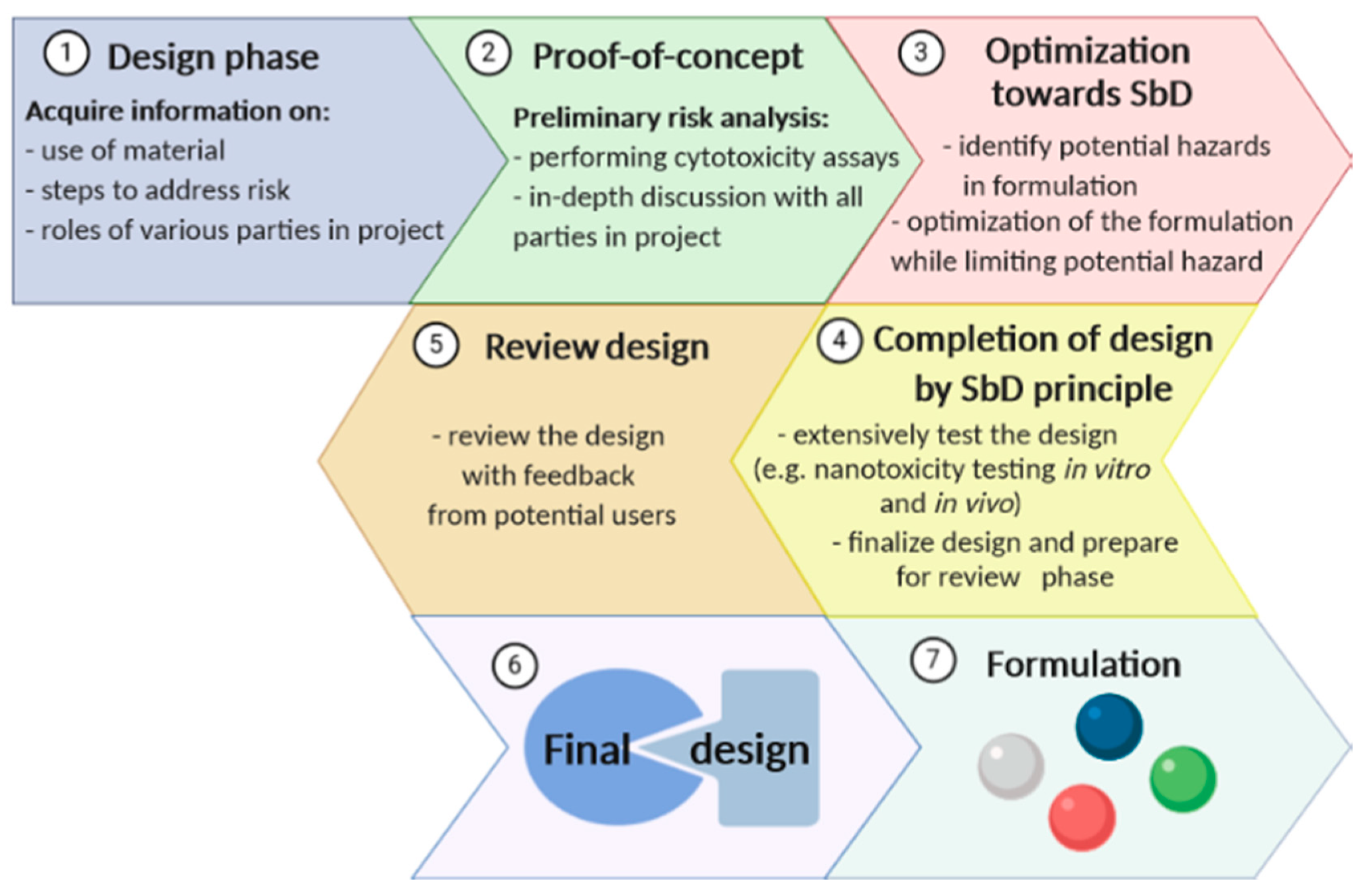
3. “Safety-by-Design” of Nanomaterials
4. Nanotoxicology: From Past Lights and Shadows to Current Concerns
5. Toxicity Tests
5.1. In Vitro
5.1.1. 2D Models
5.1.2. 3D Models
5.2. In Vivo
5.2.1. Aquatic Models
5.2.2. Small Rodents
6. The Impact of Nanoparticles: In Vitro and In Vivo Studies
6.1. Titanium Dioxide Nanoparticles
6.2. Silver Nanoparticles
7. The Importance of Nanosafety
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Boverhof, D.R.; Bramante, C.M.; Butala, J.H.; Clancy, S.F.; Lafranconi, M.; West, J.; Gordon, S.C. Comparative assessment of nanomaterial definitions and safety evaluation considerations. Regul. Toxicol. Pharmacol. 2015, 73, 137–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Severino, P.; Fangueiro, J.F.; Chaud, M.V.; Cordeiro, J.; Silva, A.M.; Souto, E.B. Advances in nanobiomaterials for topical administrations: New galenic and cosmetic formulations. In Nanobiomaterials in Galenic Formulations and Cosmetics; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–23. [Google Scholar]
- Committee, E.S. Guidance on the risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain. EFSA J. 2011, 9, 2140. [Google Scholar] [CrossRef]
- Li, X.; Liu, W.; Sun, L.; Aifantis, K.E.; Yu, B.; Fan, Y.; Feng, Q.; Cui, F.; Watari, F. Effects of physicochemical properties of nanomaterials on their toxicity. J. Biomed. Mater. Res. Part A 2015, 103, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.W.; Song, I.H.; Um, S.H. Role of physicochemical properties in nanoparticle toxicity. Nanomaterials 2015, 5, 1351–1365. [Google Scholar] [CrossRef] [Green Version]
- Hobson, D.W.; Roberts, S.M.; Shvedova, A.A.; Warheit, D.B.; Hinkley, G.K.; Guy, R.C. Applied Nanotoxicology. Int. J. Toxicol. 2016, 35, 5–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maynard, A.D. Don’t define nanomaterials. Nature 2011, 475, 31. [Google Scholar] [CrossRef]
- Salieri, B.; Turner, D.A.; Nowack, B.; Hischier, R. Life cycle assessment of manufactured nanomaterials: Where are we? NanoImpact 2018, 10, 108–120. [Google Scholar] [CrossRef]
- Wang, S.; Lu, W.; Tovmachenko, O.; Rai, U.S.; Yu, H.; Ray, P.C. Challenge in understanding size and shape dependent toxicity of gold nanomaterials in human skin keratinocytes. Chem. Phys. Lett. 2008, 463, 145–149. [Google Scholar] [CrossRef] [Green Version]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Schwirn, K.; Voelker, D.; Galert, W.; Quik, J.; Tietjen, L. Environmental Risk Assessment of Nanomaterials in the light of new obligations under the REACH regulation-Which challenges remain and how to approach them? Integr. Environ. Assess. Manag. 2020, 3. [Google Scholar] [CrossRef] [Green Version]
- Boros, B.-V.; Ostafe, V. Evaluation of Ecotoxicology Assessment Methods of Nanomaterials and Their Effects. Nanomaterials 2020, 10, 610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Tang, K.; Harper, S.; Harper, B.; Steevens, J.A.; Xu, R. Predictive modeling of nanomaterial exposure effects in biological systems. Int. J. Nanomed. 2013, 8, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofmann-Amtenbrink, M.; Grainger, D.W.; Hofmann, H. Nanoparticles in medicine: Current challenges facing inorganic nanoparticle toxicity assessments and standardizations. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1689–1694. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wang, Z.; Dhankher, O.P.; Xing, B. Nanotechnology as a new sustainable approach for controlling crop diseases and increasing agricultural production. J. Exp. Bot. 2020, 71, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Advanced analytical techniques for the measurement of nanomaterials in food and agricultural samples: A review. Environ. Eng. Sci. 2013, 30, 118–125. [Google Scholar] [CrossRef]
- Boateng, E.; Chen, A. Recent advances in nanomaterial-based solid-state hydrogen storage. Mater. Today Adv. 2020, 6, 100022. [Google Scholar] [CrossRef]
- Khan, N.I.; Song, E. Lab-on-a-Chip Systems for Aptamer-Based Biosensing. Micromachines 2020, 11, 220. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Yadava, R. Nanosensors for health care. In Nanosensors for Smart Cities; Elsevier: Amsterdam, The Netherlands, 2020; pp. 433–450. [Google Scholar]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [Green Version]
- Attaallah, R.; Antonacci, A.; Arduini, F.; Amine, A.; Scognamiglio, V. Nanobiosensors for Bioclinical Applications: Pros and Cons. In Green Nanoparticles; Springer: Berlin/Heidelberg, Germany, 2020; pp. 117–149. [Google Scholar]
- Patil, S.B.; Annese, V.F.; Cumming, D.R. Commercial Aspects of Biosensors for Diagnostics and Environmental Monitoring. In Advances in Nanosensors for Biological and Environmental Analysis; Elsevier: Amsterdam, The Netherlands, 2019; pp. 133–142. [Google Scholar]
- Raki, L.; Beaudoin, J.; Alizadeh, R.; Makar, J.; Sato, T. Cement and concrete nanoscience and nanotechnology. Materials 2010, 3, 918–942. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.; Jo, B.W.; Yoon, Y.-S. Development of nano cement concrete by top-down and bottom-up nanotechnology concept. In Smart Nanoconcretes and Cement-Based Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 183–213. [Google Scholar]
- Yang, H.Y.; Han, Z.J.; Yu, S.F.; Pey, K.L.; Ostrikov, K.; Karnik, R. Carbon nanotube membranes with ultrahigh specific adsorption capacity for water desalination and purification. Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Diallo, M.; Brinker, C.J. Nanotechnology for sustainability: Environment, water, food, minerals, and climate. In Nanotechnology Research Directions for Societal Needs in 2020; Springer: Berlin/Heidelberg, Germany, 2011; pp. 221–259. [Google Scholar]
- Yeung, A.W.K.; Souto, E.B.; Durazzo, A.; Lucarini, M.; Novellino, E.; Tewari, D.; Wang, D.; Atanasov, A.G.; Santini, A. Big impact of nanoparticles: Analysis of the most cited nanopharmaceuticals and nanonutraceuticals research. Curr. Res. Biotechnol. 2020, 2, 53–63. [Google Scholar] [CrossRef]
- Durazzo, A.; Nazhand, A.; Lucarini, M.; Atanasov, A.G.; Souto, E.B.; Novellino, E.; Capasso, R.; Santini, A. An Updated Overview on Nanonutraceuticals: Focus on Nanoprebiotics and Nanoprobiotics. Int. J. Mol. Sci. 2020, 21, 2285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucarini, M.; Durazzo, A.; Kiefer, J.; Santini, A.; Lombardi-Boccia, G.; Souto, E.B.; Romani, A.; Lampe, A.; Ferrari Nicoli, S.; Gabrielli, P.; et al. Grape Seeds: Chromatographic Profile of Fatty Acids and Phenolic Compounds and Qualitative Analysis by FTIR-ATR Spectroscopy. Foods 2019, 9, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, J.R.; Severino, P.; Ferreira, C.S.; Zielinska, A.; Santini, A.; Souto, S.B.; Souto, E.B. Linseed Essential Oil - Source of Lipids as Active Ingredients for Pharmaceuticals and Nutraceuticals. Curr. Med. Chem. 2019, 26, 4537–4558. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Zielinska, A.; Souto, S.B.; Durazzo, A.; Lucarini, M.; Santini, A.; Silva, A.M.; Atanasov, A.G.; Marques, C.; Andrade, L.N.; et al. (+)-Limonene 1,2-epoxide-loaded SLN: Evaluation of drug release, antioxidant activity and cytotoxicity in HaCaT cell line. Int. J. Mol. Sci. 2020, 21, E1449. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.V.; Laux, P.; Luch, A.; Sudrik, C.; Wiehr, S.; Wild, A.-M.; Santomauro, G.; Bill, J.; Sitti, M. Review of emerging concepts in nanotoxicology: Opportunities and challenges for safer nanomaterial design. Toxicol. Mech. Methods 2019, 29, 378–387. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Hou, Y.; Sun, S. Magnetic core/shell Fe3O4/Au and Fe3O4/Au/Ag nanoparticles with tunable plasmonic properties. J. Am. Chem. Soc. 2007, 129, 8698–8699. [Google Scholar] [CrossRef]
- Kong, B.; Seog, J.H.; Graham, L.M.; Lee, S.B. Experimental considerations on the cytotoxicity of nanoparticles. Nanomedicine 2011, 6, 929–941. [Google Scholar] [CrossRef] [Green Version]
- Yemmireddy, V.K.; Hung, Y.C. Using photocatalyst metal oxides as antimicrobial surface coatings to ensure food safety—Opportunities and challenges. Compr. Rev. Food Sci. Food Saf. 2017, 16, 617–631. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Ji, Z.; Xia, T.; Meng, H.; Low-Kam, C.; Liu, R.; Pokhrel, S.; Lin, S.; Wang, X.; Liao, Y.-P. Use of metal oxide nanoparticle band gap to develop a predictive paradigm for oxidative stress and acute pulmonary inflammation. ACS Nano 2012, 6, 4349–4368. [Google Scholar] [CrossRef]
- Guerrini, L.; Alvarez-Puebla, R.A.; Pazos-Perez, N. Surface modifications of nanoparticles for stability in biological fluids. Materials 2018, 11, 1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braakhuis, H.M.; Park, M.V.; Gosens, I.; De Jong, W.H.; Cassee, F.R. Physicochemical characteristics of nanomaterials that affect pulmonary inflammation. Part. Fibre Toxicol. 2014, 11, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukhanova, A.; Bozrova, S.; Sokolov, P.; Berestovoy, M.; Karaulov, A.; Nabiev, I. Dependence of nanoparticle toxicity on their physical and chemical properties. Nanoscale Res. Lett. 2018, 13, 44. [Google Scholar] [CrossRef] [Green Version]
- Souto, E.B.; Campos, J.R.; Da Ana, R.; Martins-Gomes, C.; Silva, A.M.; Souto, S.B.; Lucarini, M.; Durazzo, A.; Santini, A. Ocular Cell Lines and Genotoxicity Assessment. Int. J. Environ. Res. Public Health 2020, 17, 2046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doktorovova, S.; Souto, E.B.; Silva, A.M. Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers - a systematic review of in vitro data. Eur. J. Pharm. Biopharm. 2014, 87, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jun, B.-H. Silver Nanoparticles: Synthesis and application for nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef] [Green Version]
- Bajaj, S.; Haverty, M.G.; Arróyave, R.; Shankar, S. Phase stability in nanoscale material systems: Extension from bulk phase diagrams. Nanoscale 2015, 7, 9868–9877. [Google Scholar] [CrossRef] [Green Version]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.; Fu, Z.Y.; Tan, H.R.; Tan, J.P.Y.; Ng, S.C.; Teo, E. Hydrothermal synthesis of CeO2 nanocrystals: Ostwald ripening or oriented attachment? Cryst. Growth Des. 2012, 12, 3296–3303. [Google Scholar] [CrossRef]
- Doktorovova, S.; Kovacevic, A.B.; Garcia, M.L.; Souto, E.B. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: Current evidence from in vitro and in vivo evaluation. Eur. J. Pharm. Biopharm 2016, 108, 235–252. [Google Scholar] [CrossRef]
- Doktorovova, S.; Silva, A.M.; Gaivao, I.; Souto, E.B.; Teixeira, J.P.; Martins-Lopes, P. Comet assay reveals no genotoxicity risk of cationic solid lipid nanoparticles. J. Appl. Toxicol. 2014, 34, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, H.; Pojana, G.; Zuin, S.; Jacobsen, N.R.; Møller, P.; Loft, S.; Semmler-Behnke, M.; McGuiness, C.; Balharry, D.; Marcomini, A. Engineered nanomaterial risk. Lessons learnt from completed nanotoxicology studies: Potential solutions to current and future challenges. Crit. Rev. Toxicol. 2013, 43, 1–20. [Google Scholar] [CrossRef]
- Warheit, D.B. Hazard and risk assessment strategies for nanoparticle exposures: How far have we come in the past 10 years? F1000Research 2018, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borm, P.J.; Robbins, D.; Haubold, S.; Kuhlbusch, T.; Fissan, H.; Donaldson, K.; Schins, R.; Stone, V.; Kreyling, W.; Lademann, J. The potential risks of nanomaterials: A review carried out for ECETOC. Part. Fibre Toxicol. 2006, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Hjorth, R.; van Hove, L.; Wickson, F. What can nanosafety learn from drug development? The feasibility of "safety by design". Nanotoxicology 2017, 11, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Kraegeloh, A.; Suarez-Merino, B.; Sluijters, T.; Micheletti, C. Implementation of safe-by-design for nanomaterial development and safe innovation: Why we need a comprehensive approach. Nanomaterials 2018, 8, 239. [Google Scholar] [CrossRef] [Green Version]
- Gottardo, S.; Alessandrelli, M.; Amenta, V.; Atluri, R.; Barberio, G.; Bekker, C.; Bergonzo, P.; Bleeker, E.; Booth, A.; Borges, T. NANoREG Framework for the Safety Assessment of Nanomaterials; European Commission Joint Research Centre: Ispra, Italy, 2017. [Google Scholar]
- Mohs, R.C.; Greig, N.H. Drug discovery and development: Role of basic biological research. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2017, 3, 651–657. [Google Scholar]
- Ahuja, V.; Sharma, S. Drug safety testing paradigm, current progress and future challenges: An overview. J. Appl. Toxicol. 2014, 34, 576–594. [Google Scholar] [CrossRef]
- Merlot, C. Computational toxicology—A tool for early safety evaluation. Drug Discov. Today 2010, 15, 16–22. [Google Scholar] [CrossRef]
- Siramshetty, V.B.; Nickel, J.; Omieczynski, C.; Gohlke, B.-O.; Drwal, M.N.; Preissner, R. WITHDRAWN—A resource for withdrawn and discontinued drugs. Nucleic Acids Res. 2016, 44, D1080–D1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shvedova, A.; Pietroiusti, A.; Kagan, V. Nanotoxicology ten years later: Lights and shadows. Toxicol. Appl. Pharmacol. 2016, 299, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oberdörster, G. Safety assessment for nanotechnology and nanomedicine: Concepts of nanotoxicology. J. Intern. Med. 2010, 267, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Gaur, N.; Sharma, N.; Dahiya, A.; Yadav, P.; Ojha, H.; Goyal, R.K.; Sharma, R.K. Toxicity and Regulatory Concerns for Nanoformulations in Medicine. ELSI Handb. Nanotechnol. 2020, 5, 333–357. [Google Scholar] [CrossRef]
- de Souza, A.L.; Andreani, T.; de Oliveira, R.N.; Kiill, C.P.; dos Santos, F.K.; Allegretti, S.M.; Chaud, M.V.; Souto, E.B.; Silva, A.M.; Gremiao, M.P. In vitro evaluation of permeation, toxicity and effect of praziquantel-loaded solid lipid nanoparticles against Schistosoma mansoni as a strategy to improve efficacy of the schistosomiasis treatment. Int. J. Pharm. 2014, 463, 31–37. [Google Scholar] [CrossRef]
- Diniz, F.R.; Maia, R.C.A.P.; Rannier, L.; Andrade, L.N.; Chaud, M.V.; da Silva, C.F.; Corrêa, C.B.; de Albuquerque Junior, R.L.C.; da Costa, L.P.; Souto, E.B.; et al. Silver nanoparticles-composing alginate/gelatin hydrogel improves wound healing in vivo. Nanomaterials 2020, 10, 390. [Google Scholar] [CrossRef] [Green Version]
- Hissae Yassue-Cordeiro, P.; Zandonai, C.H.; Pereira Genesi, B.; Santos Lopes, P.; Sanchez-Lopez, E.; Garcia, M.L.; Camargo Fernandes-Machado, N.R.; Severino, P.; Souto, E.B.; Ferreira da Silva, C. Development of Chitosan/Silver Sulfadiazine/Zeolite Composite Films for Wound Dressing. Pharmaceutics 2019, 11, 535. [Google Scholar] [CrossRef] [Green Version]
- Severino, P.; Silveira, E.F.; Loureiro, K.; Chaud, M.V.; Antonini, D.; Lancellotti, M.; Sarmento, V.H.; da Silva, C.F.; Santana, M.H.A.; Souto, E.B. Antimicrobial activity of polymyxin-loaded solid lipid nanoparticles (PLX-SLN): Characterization of physicochemical properties and in vitro efficacy. Eur. J. Pharm. Sci. 2017, 106, 177–184. [Google Scholar] [CrossRef]
- Doktorovova, S.; Santos, D.L.; Costa, I.; Andreani, T.; Souto, E.B.; Silva, A.M. Cationic solid lipid nanoparticles interfere with the activity of antioxidant enzymes in hepatocellular carcinoma cells. Int. J. Pharm. 2014, 471, 18–27. [Google Scholar] [CrossRef]
- Souto, E.B.; da Ana, R.; Souto, S.B.; Zielińska, A.; Marques, C.; Andrade, L.N.; Horbańczuk, O.K.; Atanasov, A.G.; Lucarini, M.; Durazzo, A.; et al. In Vitro Characterization, Modelling, and Antioxidant Properties of Polyphenon-60 from Green Tea in Eudragit S100-2 Chitosan Microspheres. Nutrients 2020, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giner-Casares, J.J.; Henriksen-Lacey, M.; Coronado-Puchau, M.; Liz-Marzán, L.M. Inorganic nanoparticles for biomedicine: Where materials scientists meet medical research. Mater. Today 2016, 19, 19–28. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanoparticle Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Lee, J.; Mahendra, S.; Alvarez, P.J. Nanomaterials in the construction industry: A review of their applications and environmental health and safety considerations. ACS Nano 2010, 4, 3580–3590. [Google Scholar] [CrossRef]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Bian, W.-P.; Pei, D.-S. Zebrafish model for safety and toxicity testing of nutraceuticals. In Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2016; pp. 333–339. [Google Scholar]
- Hadrup, N.; Loeschner, K.; Mandrup, K.; Ravn-Haren, G.; Frandsen, H.L.; Larsen, E.H.; Lam, H.R.; Mortensen, A. Subacute oral toxicity investigation of selenium nanoparticles and selenite in rats. Drug Chem. Toxicol. 2019, 42, 76–83. [Google Scholar] [CrossRef]
- Pamies, D.; Martínez, C.E.; Sogorb, M.A.; Vilanova, E. Mechanism-based models in reproductive and developmental toxicology. In Reproductive and Developmental Toxicology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 135–146. [Google Scholar]
- Savage, D.T.; Hilt, J.Z.; Dziubla, T.D. In Vitro Methods for Assessing Nanoparticle Toxicity. In Nanotoxicity; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–29. [Google Scholar]
- van Gestel, C.A.; Loureiro, S. Terrestrial isopods as model organisms in soil ecotoxicology: A review. ZooKeys 2018, 6. [Google Scholar] [CrossRef]
- DeLoid, G.M.; Cohen, J.M.; Pyrgiotakis, G.; Demokritou, P. Preparation, characterization, and in vitro dosimetry of dispersed, engineered nanomaterials. Nat. Protoc. 2017, 12, 355–371. [Google Scholar] [CrossRef]
- Spyrogianni, A.; Herrmann, I.K.; Lucas, M.S.; Leroux, J.-C.; Sotiriou, G.A. Quantitative analysis of the deposited nanoparticle dose on cell cultures by optical absorption spectroscopy. Nanomedicine 2016, 11, 2483–2496. [Google Scholar] [CrossRef]
- Cho, E.C.; Zhang, Q.; Xia, Y. The effect of sedimentation and diffusion on cellular uptake of gold nanoparticles. Nat. Nanotechnol. 2011, 6, 385–391. [Google Scholar] [CrossRef]
- Watson, C.Y.; DeLoid, G.M.; Pal, A.; Demokritou, P. Buoyant Nanoparticles: Implications for Nano-Biointeractions in Cellular Studies. Small 2016, 12, 3172–3180. [Google Scholar] [CrossRef] [Green Version]
- Lison, D.; Huaux, F. Ups and downs of cellular uptake. Nat. Nanotechnol. 2011, 6, 332–333. [Google Scholar] [CrossRef]
- Clippinger, A.J.; Ahluwalia, A.; Allen, D.; Bonner, J.C.; Casey, W.; Castranova, V.; David, R.M.; Halappanavar, S.; Hotchkiss, J.A.; Jarabek, A.M.; et al. Expert consensus on an in vitro approach to assess pulmonary fibrogenic potential of aerosolized nanomaterials. Arch. Toxicol. 2016, 90, 1769–1783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fröhlich, E. Comparison of conventional and advanced in vitro models in the toxicity testing of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1091–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geiser, M.; Jeannet, N.; Fierz, M.; Burtscher, H. Evaluating Adverse Effects of Inhaled Nanoparticles by Realistic In Vitro Technology. Nanomaterials 2017, 7, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, N.; Wills, J.W.; Doak, S.H. Three-dimensional Models for In vitro Nanotoxicity Testing. In Nanotoxicology: Experimental and Computational Perspectives; The Royal Society of Chemistry: London, UK, 2018; Chapter 10; pp. 248–275. [Google Scholar]
- Joris, F.; Manshian, B.B.; Peynshaert, K.; De Smedt, S.C.; Braeckmans, K.; Soenen, S.J. Assessing nanoparticle toxicity in cell-based assays: Influence of cell culture parameters and optimized models for bridging the in vitro-in vivo gap. Chem. Soc. Rev. 2013, 42, 8339–8359. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Gabrielson, N.P.; Uckun, F.M.; Fan, T.M.; Cheng, J. Size-Dependent Tumor Penetration and In Vivo Efficacy of Monodisperse Drug–Silica Nanoconjugates. Mol. Pharm. 2013, 10, 883–892. [Google Scholar] [CrossRef] [Green Version]
- Kermanizadeh, A.; Løhr, M.; Roursgaard, M.; Messner, S.; Gunness, P.; Kelm, J.M.; Møller, P.; Stone, V.; Loft, S. Hepatic toxicology following single and multiple exposure of engineered nanomaterials utilising a novel primary human 3D liver microtissue model. Part. Fibre Toxicol. 2014, 11, 56. [Google Scholar] [CrossRef] [Green Version]
- Broutier, L.; Andersson-Rolf, A.; Hindley, C.J.; Boj, S.F.; Clevers, H.; Koo, B.-K.; Huch, M. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat. Protoc. 2016, 11, 1724–1743. [Google Scholar] [CrossRef]
- Young, E.W.K.; Beebe, D.J. Fundamentals of microfluidic cell culture in controlled microenvironments. Chem. Soc. Rev. 2010, 39, 1036–1048. [Google Scholar] [CrossRef]
- Fierz, M.; Houle, C.; Steigmeier, P.; Burtscher, H. Design, Calibration, and Field Performance of a Miniature Diffusion Size Classifier. Aerosol Sci. Technol. 2011, 45, 1–10. [Google Scholar] [CrossRef]
- Hartung, T.; Hoffmann, S.; Stephens, M. Mechanistic validation. ALTEX 2013, 30, 119–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauenstein, L.; Switalla, S.; Prenzler, F.; Seehase, S.; Pfennig, O.; Förster, C.; Fieguth, H.; Braun, A.; Sewald, K. Assessment of immunotoxicity induced by chemicals in human precision-cut lung slices (PCLS). Toxicol. In Vitro 2014, 28, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Semmler-Behnke, M.; Kreyling, W.G.; Lipka, J.; Fertsch, S.; Wenk, A.; Takenaka, S.; Schmid, G.; Brandau, W. Biodistribution of 1.4- and 18-nm Gold Particles in Rats. Small 2008, 4, 2108–2111. [Google Scholar] [CrossRef] [PubMed]
- Sauer, U.G.; Vogel, S.; Aumann, A.; Hess, A.; Kolle, S.N.; Ma-Hock, L.; Wohlleben, W.; Dammann, M.; Strauss, V.; Treumann, S.; et al. Applicability of rat precision-cut lung slices in evaluating nanomaterial cytotoxicity, apoptosis, oxidative stress, and inflammation. Toxicol. Appl. Pharmacol. 2014, 276, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dragoni, S.; Franco, G.; Regoli, M.; Bracciali, M.; Morandi, V.; Sgaragli, G.; Bertelli, E.; Valoti, M. Gold Nanoparticles Uptake and Cytotoxicity Assessed on Rat Liver Precision-Cut Slices. Toxicol. Sci. 2012, 128, 186–197. [Google Scholar] [CrossRef]
- Baun, A.; Hartmann, N.B.; Grieger, K.; Kusk, K.O. Ecotoxicity of engineered nanoparticles to aquatic invertebrates: A brief review and recommendations for future toxicity testing. Ecotoxicology 2008, 17, 387–395. [Google Scholar] [CrossRef]
- Villanueva-Flores, F.; Castro-Lugo, A.; Ramírez, O.T.; Palomares, L.A. Understanding cellular interactions with nanomaterials: Towards a rational design of medical nanodevices. Nanotechnology 2020, 31, 132002. [Google Scholar] [CrossRef]
- Nishimura, Y.; Inoue, A.; Sasagawa, S.; Koiwa, J.; Kawaguchi, K.; Kawase, R.; Maruyama, T.; Kim, S.; Tanaka, T. Using zebrafish in systems toxicology for developmental toxicity testing. Congenit. Anom. 2016, 56, 18–27. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.-S. Zebrafish: A complete animal model to enumerate the nanoparticle toxicity. J. Nanobiotechnol. 2016, 14, 65. [Google Scholar] [CrossRef] [Green Version]
- Haque, E.; Ward, A.C. Zebrafish as a Model to Evaluate Nanoparticle Toxicity. Nanomaterials 2018, 8, 561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubinstein, A.L. Zebrafish assays for drug toxicity screening. Expert Opin. Drug Metab. Toxicol. 2006, 2, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Spitsbergen, J.M.; Kent, M.L. The state of the art of the zebrafish model for toxicology and toxicologic pathology research-advantages and current limitations. Toxicol. Pathol. 2003, 31, 62–87. [Google Scholar] [CrossRef]
- Richmond, J. Refinement, Reduction, and Replacement of Animal Use for Regulatory Testing: Future Improvements and Implementation Within the Regulatory Framework. ILAR J. 2002, 43, S63–S68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstead, A.L.; Li, B. Nanotoxicity: Emerging concerns regarding nanomaterial safety and occupational hard metal (WC-Co) nanoparticle exposure. Int. J. Nanomed. 2016, 11, 6421–6433. [Google Scholar] [CrossRef] [Green Version]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Küchler, S.; Radowski, M.R.; Blaschke, T.; Dathe, M.; Plendl, J.; Haag, R.; Schäfer-Korting, M.; Kramer, K.D. Nanoparticles for skin penetration enhancement–A comparison of a dendritic core-multishell-nanotransporter and solid lipid nanoparticles. Eur. J. Pharm. Biopharm. 2009, 71, 243–250. [Google Scholar] [CrossRef]
- Paranjpe, M.; Müller-Goymann, C.C. Nanoparticle-mediated pulmonary drug delivery: A review. Int. J. Mol. Sci. 2014, 15, 5852–5873. [Google Scholar] [CrossRef]
- Oberdörster, G.; Kuhlbusch, T.A. In vivo effects: Methodologies and biokinetics of inhaled nanomaterials. NanoImpact 2018, 10, 38–60. [Google Scholar] [CrossRef]
- Bermudez, E.; Mangum, J.B.; Wong, B.A.; Asgharian, B.; Hext, P.M.; Warheit, D.B.; Everitt, J.I. Pulmonary responses of mice, rats, and hamsters to subchronic inhalation of ultrafine titanium dioxide particles. Toxicol. Sci. 2004, 77, 347–357. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K.; Kubota, H.; Hojo, R.; Miyagawa, M. Effective dispersal of titanium dioxide nanoparticles for toxicity testing. J. Toxicol. Sci. 2019, 44, 515–521. [Google Scholar] [CrossRef] [Green Version]
- Uboldi, C.; Urbán, P.; Gilliland, D.; Bajak, E.; Valsami-Jones, E.; Ponti, J.; Rossi, F. Role of the crystalline form of titanium dioxide nanoparticles: Rutile, and not anatase, induces toxic effects in Balb/3T3 mouse fibroblasts. Toxicol. In Vitro 2016, 31, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Oberbek, P.; Kozikowski, P.; Czarnecka, K.; Sobiech, P.; Jakubiak, S.; Jankowski, T. Inhalation exposure to various nanoparticles in work environment—contextual information and results of measurements. J. Nanoparticle Res. 2019, 21, 222. [Google Scholar] [CrossRef] [Green Version]
- Skocaj, M.; Filipic, M.; Petkovic, J.; Novak, S. Titanium dioxide in our everyday life; is it safe? Radiol. Oncol. 2011, 45, 227–247. [Google Scholar] [CrossRef]
- Wong, S.W.; Leung, P.T.; Djurišić, A.; Leung, K.M. Toxicities of nano zinc oxide to five marine organisms: Influences of aggregate size and ion solubility. Anal. Bioanal. Chem. 2010, 396, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Nguyen, D.L.T.; Nguyen, V.-H.; Le, T.-H.; Vo, D.-V.N.; Trinh, Q.T.; Bae, S.-R.; Chae, S.Y.; Kim, S.Y.; Le, Q.V. Recent Advances in TiO2-Based Photocatalysts for Reduction of CO2 to Fuels. Nanomaterials 2020, 10, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lekamge, S.; Miranda, A.F.; Ball, A.S.; Shukla, R.; Nugegoda, D. The toxicity of coated silver nanoparticles to Daphnia carinata and trophic transfer from alga Raphidocelis subcapitata. PLoS ONE 2019, 14. [Google Scholar] [CrossRef]
- Bai, C.; Tang, M. Toxicological study of metal and metal oxide nanoparticles in zebrafish. J. Appl. Toxicol. 2020, 40, 37–63. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-J.; He, Z.-Z.; Fang, Y.-W.; Xu, Y.; Chen, Y.-N.; Wang, G.-Q.; Yang, Y.-Q.; Yang, Z.; Li, Y.-H. Effect of titanium dioxide nanoparticles on zebrafish embryos and developing retina. Int. J. Ophthalmol. 2014, 7, 917. [Google Scholar]
- Ramsden, C.; Henry, T.; Handy, R. Sub-lethal effects of titanium dioxide nanoparticles on the physiology and reproduction of zebrafish. Aquat. Toxicol. 2013, 126, 404–413. [Google Scholar] [CrossRef]
- Ottoni, C.; Neto, M.L.; Léo, P.; Ortolan, B.; Barbieri, E.; De Souza, A. Environmental impact of biogenic silver nanoparticles in soil and aquatic organisms. Chemosphere 2020, 239, 124698. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, G.P.; Debone, H.S.; Severino, P.; Souto, E.B.; da Silva, C.F. Design and characterization of chitosan/zeolite composite films—Effect of zeolite type and zeolite dose on the film properties. Mater. Sci. Eng. C 2016, 60, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.C.; Carbone, C.; Sousa, M.C.; Espina, M.; Garcia, M.L.; Sanchez-Lopez, E.; Souto, E.B. Nanomedicines for the Delivery of Antimicrobial Peptides (AMPs). Nanomaterials 2020, 10, 560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [Green Version]
- Marin, S.; Mihail Vlasceanu, G.; Elena Tiplea, R.; Raluca Bucur, I.; Lemnaru, M.; Minodora Marin, M.; Mihai Grumezescu, A. Applications and toxicity of silver nanoparticles: A recent review. Curr. Top. Med. Chem. 2015, 15, 1596–1604. [Google Scholar] [CrossRef]
- Wei, L.; Lu, J.; Xu, H.; Patel, A.; Chen, Z.-S.; Chen, G. Silver nanoparticles: Synthesis, properties, and therapeutic applications. Drug Discov. Today 2015, 20, 595–601. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and cytotoxic properties of silver nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef] [Green Version]
- Calderón-Jiménez, B.; Johnson, M.E.; Montoro Bustos, A.R.; Murphy, K.E.; Winchester, M.R.; Vega Baudrit, J.R. Silver nanoparticles: Technological advances, societal impacts, and metrological challenges. Front. Chem. 2017, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Griffitt, R.J.; Luo, J.; Gao, J.; Bonzongo, J.-C.; Barber, D.S. Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ. Toxicol. Chem. 2008, 27, 1972–1978. [Google Scholar] [CrossRef]
- Kim, Y.S.; Song, M.Y.; Park, J.D.; Song, K.S.; Ryu, H.R.; Chung, Y.H.; Chang, H.K.; Lee, J.H.; Oh, K.H.; Kelman, B.J. Subchronic oral toxicity of silver nanoparticles. Part. Fibre Toxicol. 2010, 7, 20. [Google Scholar] [CrossRef] [Green Version]
- Pinzaru, I.; Coricovac, D.; Dehelean, C.; Moacă, E.-A.; Mioc, M.; Baderca, F.; Sizemore, I.; Brittle, S.; Marti, D.; Calina, C.D. Stable PEG-coated silver nanoparticles–A comprehensive toxicological profile. Food Chem. Toxicol. 2018, 111, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.M.; Fadeel, B. Emerging systems biology approaches in nanotoxicology: Towards a mechanism-based understanding of nanomaterial hazard and risk. Toxicol. Appl. Pharmacol. 2016, 299, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Kahru, A.; Dubourguier, H.-C. From ecotoxicology to nanoecotoxicology. Toxicology 2010, 269, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Krug, H.F. Nanosafety research--are we on the right track? Angew Chem. Int. Ed. Engl. 2014, 53, 12304–12319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oberdörster, G.; Castranova, V.; Asgharian, B.; Sayre, P. Inhalation exposure to carbon nanotubes (CNT) and carbon nanofibers (CNF): Methodology and dosimetry. J. Toxicol. Environ. Health Part B 2015, 18, 121–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatkhutdinova, L.; Khaliullin, T.; Shvedova, A. Carbon nanotubes exposure risk assessment: From toxicology to epidemiologic studies (overview of the current problem). Nanotechnologies Russia 2015, 10, 501–509. [Google Scholar] [CrossRef] [Green Version]
- Aschberger, K.; Johnston, H.J.; Stone, V.; Aitken, R.J.; Tran, C.L.; Hankin, S.M.; Peters, S.A.; Christensen, F.M. Review of fullerene toxicity and exposure–Appraisal of a human health risk assessment, based on open literature. Regul. Toxicol. Pharmacol. 2010, 58, 455–473. [Google Scholar] [CrossRef]
- Krug, H.F.; Nau, K. Reliability for Nanosafety Research–Considerations on the Basis of a Comprehensive Literature Review. ChemBioEng Rev. 2017, 4, 331–338. [Google Scholar] [CrossRef]
- Henschler, D. Toxicological problems relating to changes in the environment. Angew. Chem. Int. Ed. Engl. 1973, 12, 274–283. [Google Scholar] [CrossRef]
- Ray, P.C.; Yu, H.; Fu, P.P. Toxicity and environmental risks of nanomaterials: Challenges and future needs. J. Environ. Sci. Health Part C 2009, 27, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Ge, X.; Ke, D.; Tang, H.; Zhang, J.; Calvaresi, M.; Gao, B.; Sun, L.; Su, Q.; Wang, H. The Bioavailability, Biodistribution and Toxic Effects of Silica-coated Upconversion Nanoparticles in Vivo. Front. Chem. 2019, 7, 218. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Panariti, A.; Miserocchi, G.; Rivolta, I. The effect of nanoparticle uptake on cellular behavior: Disrupting or enabling functions? Nanotechnol. Sci. Appl. 2012, 5, 87. [Google Scholar] [PubMed] [Green Version]
- Shin, T.H.; Lee, D.Y.; Lee, H.S.; Park, H.J.; Jin, M.S.; Paik, M.J.; Manavalan, B.; Mo, J.S.; Lee, G. Integration of metabolomics and transcriptomics in nanotoxicity studies. BMB Rep. 2018, 51, 14–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Type | Advantages | Drawbacks | References |
|---|---|---|---|
| 2D cell culture | |||
| Upright NM exposure | Easy experiment set-up; Can be used for virtually all 2D cell cultures; | Agglomeration of nanoparticles; Inconsistent protocols between studies; Inhomogeneous distribution over time | [78] |
| Surface-based NM presentation | Exact NM/µm; No agglomeration of particles; Homogeneous distribution over time Easy monitoring of uptake and toxicity | NM–substrate interactions influence internalization and toxicity results; Only static conditions can be tested. | [99] |
| Inverted cell culture | Assessment of buoyant NM nanotoxicity | Limited use for larger-sized or insoluble NMs | [79,80,81,82] |
| Air liquid interface | More physiologically relevant; Cheaper than in vivo studies; Range of commercially devices available | Limited to airborne NMs; Only relevant to nanotoxicity studies related to inhalation | [83,85] |
| 3D cell culture | |||
| Co-culture | Promotes in vivo-like cell–cell interactions; More relevant than 2D nanotoxicity platforms; | Still lacks 3D microenvironment | [86] |
| Spheroids and organoids | More in vivo-like complexity; Oxygen and nutrient gradient; Barrier to NMs distribution and nanotoxicity; Easy-to-use protocols | Heterogeneity; Lower reproducibility; Simplified 3D architecture; No high throughput | [86,87,89,90] |
| Organ-on-Chip | High throughput; Low cost; Physiologically relevant microenvironment; Precise control over NM presentation and dosimetry | Surface effects stemming from small dimensions; Little mixing of solutions; Difficult integration of sensors; | [91,92] |
| Precision-cut tissue slices | Compatible with a range of tissue samples and animal species; High reproducibility; Quickly obtainable; Retain the tissue native architecture | Tissue damage due to slicing; Limited number of slices per organ | [93,94,95,96,97] |
| Type | Advantages | Drawbacks | Source(s) |
|---|---|---|---|
| Aquatic models | |||
| Planktonic crustaceans | Standardized protocols and guidelines; Easy implementation | Primarily used as pre-screening method; Large biological difference to humans | [98] |
| Zebrafish | High throughput; Similar genome; Rapid developmental process; Low cost; Easy monitoring of embryogenesis; ADME effects studied | Difficult monitoring of rapid developmental process; Ethical concerns; Species to species variation | [100,101,104] |
| Mammal models | |||
| Small rodents | Multiple routes of exposure; Guidelines exist to evaluate nanotoxicity; ADME effects studied; Chronic effects studied | Ethical concerns; Species to species variation; Expertise necessary; High cost | [105,106] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielińska, A.; Costa, B.; Ferreira, M.V.; Miguéis, D.; Louros, J.M.S.; Durazzo, A.; Lucarini, M.; Eder, P.; V. Chaud, M.; Morsink, M.; et al. Nanotoxicology and Nanosafety: Safety-by-Design and Testing at a Glance. Int. J. Environ. Res. Public Health 2020, 17, 4657. https://doi.org/10.3390/ijerph17134657
Zielińska A, Costa B, Ferreira MV, Miguéis D, Louros JMS, Durazzo A, Lucarini M, Eder P, V. Chaud M, Morsink M, et al. Nanotoxicology and Nanosafety: Safety-by-Design and Testing at a Glance. International Journal of Environmental Research and Public Health. 2020; 17(13):4657. https://doi.org/10.3390/ijerph17134657
Chicago/Turabian StyleZielińska, Aleksandra, Beatriz Costa, Maria V. Ferreira, Diogo Miguéis, Jéssica M. S. Louros, Alessandra Durazzo, Massimo Lucarini, Piotr Eder, Marco V. Chaud, Margreet Morsink, and et al. 2020. "Nanotoxicology and Nanosafety: Safety-by-Design and Testing at a Glance" International Journal of Environmental Research and Public Health 17, no. 13: 4657. https://doi.org/10.3390/ijerph17134657
APA StyleZielińska, A., Costa, B., Ferreira, M. V., Miguéis, D., Louros, J. M. S., Durazzo, A., Lucarini, M., Eder, P., V. Chaud, M., Morsink, M., Willemen, N., Severino, P., Santini, A., & Souto, E. B. (2020). Nanotoxicology and Nanosafety: Safety-by-Design and Testing at a Glance. International Journal of Environmental Research and Public Health, 17(13), 4657. https://doi.org/10.3390/ijerph17134657












