Anti-Inflammatory Potential of Cultured Ginseng Roots Extract in Lipopolysaccharide-Stimulated Mouse Macrophages and Adipocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Sample Preparation
2.3. Total Polyphenol and Flavonoid Contents of GR Extract
2.4. High-Performance Liquid Chromatography (HPLC) Analyses of GRE for Determining Ginsenoside Content
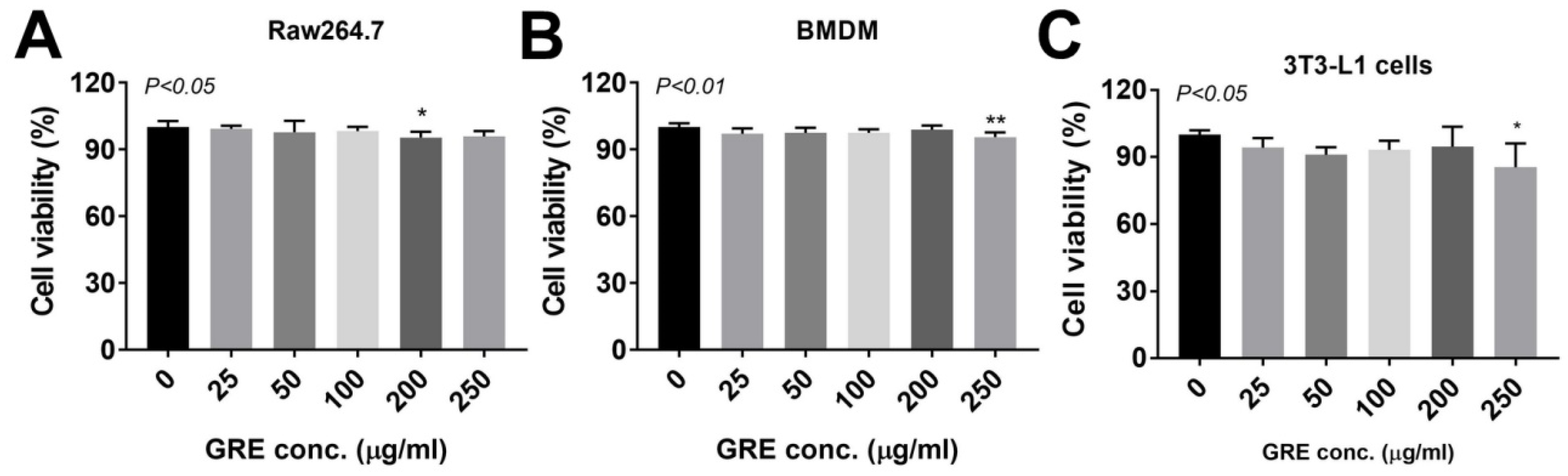
2.5. Cell Culture
2.6. Total RNA Extraction and qPCR
2.7. Western Blot Analysis
2.8. Inflammation Cytokine Detection by ELISA
2.9. Reactive Oxygen Species (ROS) Detection
2.10. Oxygen Consumption Rate (OCR) by Seahorse
2.11. Statistical Analysis
3. Results
3.1. Total Polyphenol, Flavonoid, and Ginsenoside Contents of GR Extract
3.2. GR Extract Attenuated LPS-Induced Inflammatory Responses, ER Stress, and Reactive Oxygen Species in Mouse Macrophage Cell Line, RAW264.7 Cells
3.3. GR Extract Attenuated LPS-Induced Inflammatory Responses and ER Stress while Increasing Antioxidant Gene Expression in Mouse BMDMs
3.4. GR Extract Protected Mitochondrial Function and Energy Metabolism in LPS-Induced Inflamed Macrophages
3.5. GR Extract Attenuated LPS-Mediated Adipocyte Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cho, I.H. Effects of Panax ginseng in Neurodegenerative Diseases. J. Ginseng. Res. 2012, 36, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Kim, J.H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J. Ginseng. Res. 2014, 38, 161–166. [Google Scholar] [CrossRef]
- Vuksan, V.; Sung, M.K.; Sievenpiper, J.L.; Stavro, P.M.; Jenkins, A.L.; Di Buono, M.; Lee, K.S.; Leiter, L.A.; Nam, K.Y.; Arnason, J.T.; et al. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: Results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Yoon, H.; Park, H.M.; Song, B.C.; Yeum, K.J. Implications of red Panax ginseng in oxidative stress associated chronic diseases. J. Ginseng. Res. 2017, 41, 113–119. [Google Scholar] [CrossRef]
- Qi, H.Y.; Li, L.; Ma, H. Cellular stress response mechanisms as therapeutic targets of ginsenosides. Med. Res. Rev. 2018, 38, 625–654. [Google Scholar] [CrossRef]
- Paek, K.Y.; Murthy, H.N.; Hahn, E.J.; Zhong, J.J. Large scale culture of ginseng adventitious roots for production of ginsenosides. Adv. Biochem. Eng. Biotechnol. 2009, 113, 151–176. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.P. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv. Food. Nutr. Res. 2009, 55, 1–99. [Google Scholar] [CrossRef]
- Wu, J.; Zhong, J.J. Production of ginseng and its bioactive components in plant cell culture: Current technological and applied aspects. J. Biotechnol. 1999, 68, 89–99. [Google Scholar] [CrossRef]
- Park, B.G.; Jung, H.J.; Cho, Y.W.; Lim, H.W.; Lim, C.J. Potentiation of antioxidative and anti-inflammatory properties of cultured wild ginseng root extract through probiotic fermentation. J. Pharm. Pharmacol. 2013, 65, 457–464. [Google Scholar] [CrossRef]
- Yu, G.J.; Choi, I.W.; Kim, G.Y.; Kim, B.W.; Park, C.; Hong, S.H.; Moon, S.K.; Cha, H.J.; Chang, Y.C.; Paek, K.Y.; et al. Anti-inflammatory potential of saponins derived from cultured wild ginseng roots in lipopolysaccharide-stimulated RAW 264.7 macrophages. Int. J. Mol. Med. 2015, 35, 1690–1698. [Google Scholar] [CrossRef]
- Oberg, B.P.; McMenamin, E.; Lucas, F.L.; McMonagle, E.; Morrow, J.; Ikizler, T.A.; Himmelfarb, J. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004, 65, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Shaul, M.E.; Bennett, G.; Strissel, K.J.; Greenberg, A.S.; Obin, M.S. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet--induced obesity in mice. Diabetes 2010, 59, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, K.; Takeuchi, O.; Kawai, T.; Sanjo, H.; Ogawa, T.; Takeda, Y.; Takeda, K.; Akira, S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J. Immunol. 1999, 162, 3749–3752. [Google Scholar]
- Malinin, N.L.; Boldin, M.P.; Kovalenko, A.V.; Wallach, D. MAP3K-related kinase involved in NF-kappaB induction by TNF, CD95 and IL-1. Nature 1997, 385, 540–544. [Google Scholar] [CrossRef]
- Seo, S.H.; Jo, S.M.; Kim, J.; Lee, M.; Lee, Y.; Kang, I. Peanut Sprout Extracts Attenuate Triglyceride Accumulation by Promoting Mitochondrial Fatty Acid Oxidation in Adipocytes. Int. J. Mol. Sci. 2019, 20, 1216. [Google Scholar] [CrossRef]
- Kang, O.H.; Shon, M.Y.; Kong, R.; Seo, Y.S.; Zhou, T.; Kim, D.Y.; Kim, Y.S.; Kwon, D.Y. Anti-diabetic effect of black ginseng extract by augmentation of AMPK protein activity and upregulation of GLUT2 and GLUT4 expression in db/db mice. BMC Complementary Altern. Med. 2017, 17, 341. [Google Scholar] [CrossRef]
- Kim, Y.; Gromovsky, A.D.; Brown, J.M.; Chung, S. Gamma-tocotrienol attenuates the aberrant lipid mediator production in NLRP3 inflammasome-stimulated macrophages. J. Nutr. Biochem. 2018, 58, 169–177. [Google Scholar] [CrossRef]
- Benz, R.; McLaughlin, S. The molecular mechanism of action of the proton ionophore FCCP (carbonylcyanide p-trifluoromethoxyphenylhydrazone). Biophys. J. 1983, 41, 381–398. [Google Scholar] [CrossRef]
- Cheng, C.; Zou, Y.; Peng, J. Oregano Essential Oil Attenuates RAW264.7 Cells from Lipopolysaccharide-Induced Inflammatory Response through Regulating NADPH Oxidase Activation-Driven Oxidative Stress. Molecules (Basel, Switz.) 2018, 2, 1857. [Google Scholar] [CrossRef]
- Rhule, A.; Navarro, S.; Smith, J.R.; Shepherd, D.M. Panax notoginseng attenuates LPS-induced pro-inflammatory mediators in RAW264.7 cells. J. Ethnopharmacol. 2006, 106, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Farruggia, C.; Kim, M.B.; Bae, M.; Lee, Y.; Pham, T.X.; Yang, Y.; Han, M.J.; Park, Y.K.; Lee, J.Y. Astaxanthin exerts anti-inflammatory and antioxidant effects in macrophages in NRF2-dependent and independent manners. J. Nutr. Biochem. 2018, 62, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Kang, I.; Fang, X.; Wang, W.; Lee, M.A.; Hollins, R.R.; Marshall, M.R.; Chung, S. Gamma-tocotrienol attenuates high-fat diet-induced obesity and insulin resistance by inhibiting adipose inflammation and M1 macrophage recruitment. Int. J. Obes. (Lond.) 2015, 39, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Venuprasad, K.; Elly, C.; Gao, M.; Salek-Ardakani, S.; Harada, Y.; Luo, J.L.; Yang, C.; Croft, M.; Inoue, K.; Karin, M.; et al. Convergence of Itch-induced ubiquitination with MEKK1-JNK signaling in Th2 tolerance and airway inflammation. J. Clin. Investig. 2006, 116, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Barry, S.P.; Roth, R.J.; Wu, J.J.; Jones, E.A.; Bennett, A.M.; Flavell, R.A. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 2274–2279. [Google Scholar] [CrossRef]
- Motterlini, R.; Foresti, R.; Bassi, R.; Green, C.J. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic. Biol. Med. 2000, 28, 1303–1312. [Google Scholar] [CrossRef]
- Ye, J.; Keller, J.N. Regulation of energy metabolism by inflammation: A feedback response in obesity and calorie restriction. Aging (Albany NY) 2010, 2, 361–368. [Google Scholar] [CrossRef]
- Ye, L.; Kleiner, S.; Wu, J.; Sah, R.; Gupta, R.K.; Banks, A.S.; Cohen, P.; Khandekar, M.J.; Bostrom, P.; Mepani, R.J.; et al. TRPV4 is a regulator of adipose oxidative metabolism, inflammation, and energy homeostasis. Cell 2012, 151, 96–110. [Google Scholar] [CrossRef]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Suganami, T.; Tanimoto-Koyama, K.; Nishida, J.; Itoh, M.; Yuan, X.; Mizuarai, S.; Kotani, H.; Yamaoka, S.; Miyake, K.; Aoe, S.; et al. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 84–91. [Google Scholar] [CrossRef]
- Blaser, H.; Dostert, C.; Mak, T.W.; Brenner, D. TNF and ROS Crosstalk in Inflammation. Trends Cell Biol. 2016, 26, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Hoshino, K.; Kawai, T.; Sanjo, H.; Takada, H.; Ogawa, T.; Takeda, K.; Akira, S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 1999, 11, 443–451. [Google Scholar] [CrossRef]
- Ikebe, M.; Kitaura, Y.; Nakamura, M.; Tanaka, H.; Yamasaki, A.; Nagai, S.; Wada, J.; Yanai, K.; Koga, K.; Sato, N.; et al. Lipopolysaccharide (LPS) increases the invasive ability of pancreatic cancer cells through the TLR4/MyD88 signaling pathway. J. Surg. Oncol. 2009, 100, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.J.; Bae, G.S.; Park, J.H.; Song, T.H.; Choi, A.; Ryu, B.Y.; Pang, M.G.; Kim, E.J.; Yoon, M.; Chang, M.B. Antioxidant effects of cultured wild ginseng root extracts on the male reproductive function of boars and guinea pigs. Anim. Reprod. Sci. 2016, 170, 51–60. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar]
- Skenderidis, P.; Kerasioti, E.; Karkanta, E.; Stagos, D.; Kouretas, D.; Petrotos, K.; Hadjichristodoulou, C.; Tsakalof, A. Assessment of the antioxidant and antimutagenic activity of extracts from goji berry of Greek cultivation. Toxicol. Rep. 2018, 5, 251–257. [Google Scholar] [CrossRef]
- Wei, Y.H.; Lu, C.Y.; Wei, C.Y.; Ma, Y.S.; Lee, H.C. Oxidative stress in human aging and mitochondrial disease-consequences of defective mitochondrial respiration and impaired antioxidant enzyme system. Chin. J. Physiol. 2001, 44, 1–11. [Google Scholar]
- Zou, C.; Synan, M.J.; Li, J.; Xiong, S.; Manni, M.L.; Liu, Y.; Chen, B.B.; Zhao, Y.; Shiva, S.; Tyurina, Y.Y.; et al. LPS impairs oxygen utilization in epithelia by triggering degradation of the mitochondrial enzyme Alcat1. J. Cell Sci. 2016, 129, 51–64. [Google Scholar] [CrossRef]
- Pang, C.; Gao, Z.; Yin, J.; Zhang, J.; Jia, W.; Ye, J. Macrophage infiltration into adipose tissue may promote angiogenesis for adipose tissue remodeling in obesity. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E313–E322. [Google Scholar] [CrossRef]
- Oh, J.; Lee, H.; Park, D.; Ahn, J.; Shin, S.S.; Yoon, M. Ginseng and Its Active Components Ginsenosides Inhibit Adipogenesis in 3T3-L1 Cells by Regulating MMP-2 and MMP-9. Evid.-Based Complement. Altern. Med. 2012, 2012, 265023. [Google Scholar] [CrossRef]
- Hwang, J.T.; Kim, S.H.; Lee, M.S.; Kim, S.H.; Yang, H.J.; Kim, M.J.; Kim, H.S.; Ha, J.; Kim, M.S.; Kwon, D.Y. Anti-obesity effects of ginsenoside Rh2 are associated with the activation of AMPK signaling pathway in 3T3-L1 adipocyte. Biochem. Biophys. Res. Commun. 2007, 364, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.T.; Lee, M.S.; Kim, H.J.; Sung, M.J.; Kim, H.Y.; Kim, M.S.; Kwon, D.Y. Antiobesity effect of ginsenoside Rg3 involves the AMPK and PPAR-gamma signal pathways. Phytother. Res. 2009, 23, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Kim, K.A.; Kim, D.H. Ginsenoside Rh1 ameliorates high fat diet-induced obesity in mice by inhibiting adipocyte differentiation. Biol. Pharm. Bull. 2013, 36, 102–107. [Google Scholar] [CrossRef] [PubMed]




| Gene | Forward/Reverse | Sequence (5′–3′) |
|---|---|---|
| mGPx | Forward | AGTCCACCGTGTATGCCTTCT |
| Reverse | GAGACGCGACATTCTCAATGA | |
| mHO-1 | Forward | CAGGTGATGCTGACAGAGGA |
| Reverse | TCTCTGCAGGGGCAGTATCT | |
| mHPRT | Forward | TTGCTCGAGATGTCATGAAGGA |
| Reverse | AGCAGGTCAGCAAAGAACTTATAGC | |
| mIL-1β | Forward | AAATACCTGTGGCCTTGGGC |
| Reverse | CTTGGGATCCACACTCTCCAG | |
| mIL-6 | Forward | CTGCAAGAGACTTCCATCCAGTT |
| Reverse | AGGGAAGGCCGTGGTTGT | |
| mMCP1 | Forward | AGGTCCCTGTCATGCTTCTG |
| Reverse | GCTGCTGGTGATCCTCTTGT | |
| mSOD1 | Forward | AACCAGTTGTGTTGTCAGGAC |
| Reverse | CCACCATGTTTCTTAGAGTGAGG | |
| mSOD2 | Forward | CAGACCTGCCTTACGACTATGG |
| Reverse | CTCGGTGGCGTTGAGATTGTT | |
| mTNFα | Forward | GGCTGCCCCGACTACGT |
| Reverse | ACTTTCTCCTGGTATGAGATAGCAAAT | |
| m36B4 | Forward | GGATCTGCTGCATCTGCTTG |
| Reverse | GGCGACCTGGAAGTCCAACT |
| GRE (mg/mL) | |
|---|---|
| Total polyphenols (mg gallic acid/extract g) | 10.87 ± 0.11 |
| Total flavonoids (mg catechin/extract g) | 3.79 ± 0.14 1 |
| Rg1 | 0.11 ± 0.00 |
| Re | 0.27 ± 0.01 |
| Rh1 (S) | 0.02 ± 0.00 |
| Rg2 (S) | 0.08 ± 0.00 |
| Rg2 (R) | 0.06 ± 0.00 |
| Rb1 | 0.16 ± 0.01 |
| Rc | 0.05 ± 0.00 |
| Rb2 | 0.04 ± 0.00 |
| Rg6 | 0.01 ± 0.00 |
| Rg3 (S) | 0.01 ± 0.00 |
| Rg3 (R) | 0.03 ± 0.001 |
| Rhs (S) | Non-detected |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.J.; Jo, S.-M.; Seo, S.H.; Lee, M.; Lee, Y.; Kang, I. Anti-Inflammatory Potential of Cultured Ginseng Roots Extract in Lipopolysaccharide-Stimulated Mouse Macrophages and Adipocytes. Int. J. Environ. Res. Public Health 2020, 17, 4716. https://doi.org/10.3390/ijerph17134716
Park HJ, Jo S-M, Seo SH, Lee M, Lee Y, Kang I. Anti-Inflammatory Potential of Cultured Ginseng Roots Extract in Lipopolysaccharide-Stimulated Mouse Macrophages and Adipocytes. International Journal of Environmental Research and Public Health. 2020; 17(13):4716. https://doi.org/10.3390/ijerph17134716
Chicago/Turabian StylePark, Hyun Ju, Sang-Mi Jo, Seok Hee Seo, Myoungsook Lee, Yunkyoung Lee, and Inhae Kang. 2020. "Anti-Inflammatory Potential of Cultured Ginseng Roots Extract in Lipopolysaccharide-Stimulated Mouse Macrophages and Adipocytes" International Journal of Environmental Research and Public Health 17, no. 13: 4716. https://doi.org/10.3390/ijerph17134716
APA StylePark, H. J., Jo, S.-M., Seo, S. H., Lee, M., Lee, Y., & Kang, I. (2020). Anti-Inflammatory Potential of Cultured Ginseng Roots Extract in Lipopolysaccharide-Stimulated Mouse Macrophages and Adipocytes. International Journal of Environmental Research and Public Health, 17(13), 4716. https://doi.org/10.3390/ijerph17134716







