Fungal Contaminants in Energy Efficient Dwellings: Impact of Ventilation Type and Level of Urbanization
Abstract
1. Introduction
2. Materials and Methods
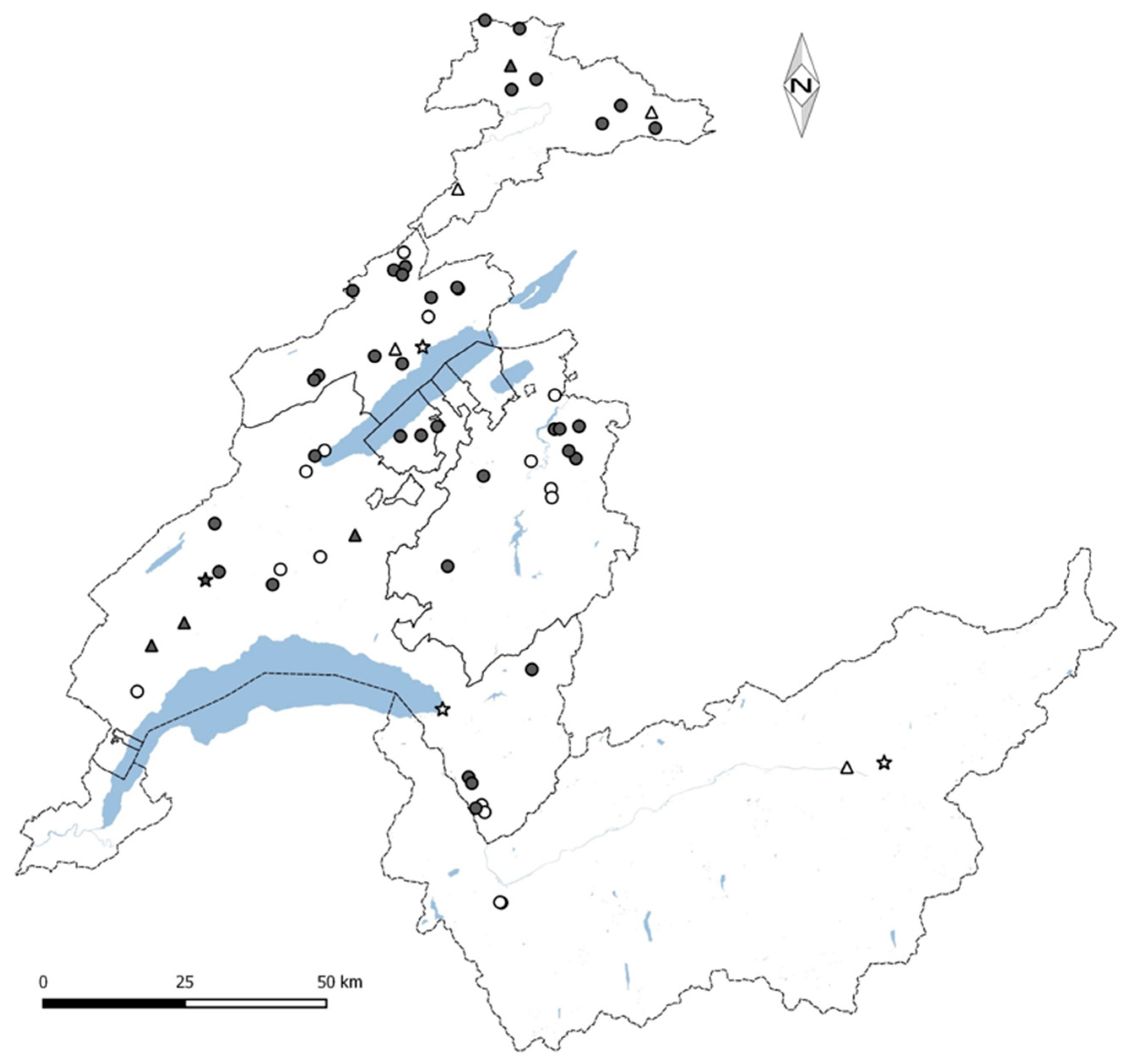
2.1. Building Selection
2.2. Study Design
2.3. Sampling
2.4. Culture
2.5. Identification of Fungi
2.6. Statistical Analysis
3. Results
3.1. Sources of Fungal Particles
3.2. Cultivable Fungi in Settled-Dust
3.3. Surface Mould Contamination in Dwellings
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Energy Agency. Global Status Report. 2018. Available online: https://www.worldgbc.org/sites/default/files/2018%20GlobalABC%20Global%20Status%20Report.pdf (accessed on 3 March 2020).
- International Energy Agency. Energy Efficiency Requirements in Building Codes, Energy Efficiency Policies for New Buildings. 2008. Available online: https://webstore.iea.org/energy-efficiency-requirements-in-building-codes-policies-for-new-buildings (accessed on 3 March 2020).
- United Nations Economic Commission for Europe. Study on Mapping of Existing Energy Efficiency Standards and Technologies in Buildings in the UNECE Region. 2018. Available online: https://www.unece.org/fileadmin/DAM/hlm/Meetings/2018/09_05-07_St._Petersburg/EE_Standards_in_Buildings_full_version.ENG.pdf (accessed on 3 March 2020).
- European Union. Directive (EU) 2018/844 of the European Parliament and of the Council of 30 May 2018. Off. J. Eur. Union 2018, 156, 75–91. [Google Scholar]
- European Comission. Support for Innovation and Market Uptake Under Horizon 2020 Energy Efficiency; High Energy Performing Buildings; 2018; Available online: https://op.europa.eu/en/publication-detail/-/publication/d8e3702d-c782-11e8-9424-01aa75ed71a1 (accessed on 3 March 2020).
- European Comission. Commission Recommendation (EU) 2019/786 of 8 May 2019 on Building Renovation. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32019H0786 (accessed on 3 March 2020).
- Schweiz, M. Available online: https://www.minergie.ch/fr/ (accessed on 3 March 2020).
- Swiss Confederation. Le Programme Bâtiments. Available online: https://www.leprogrammebatiments.ch/fr/ (accessed on 3 March 2020).
- Foldvary, V.; Beko, G.; Langer, S.; Arrhenius, K.; Petras, D. Effect of energy renovation on indoor air quality in multifamily residential buildings in Slovakia. Build. Environ. 2017, 122, 363–372. [Google Scholar] [CrossRef]
- Missia, D.A.; Demetriou, E.; Michael, N.; Tolis, E.I.; Bartzis, J.G. Indoor exposure from building materials: A field study. Atmos. Environ. 2010, 44, 4388–4395. [Google Scholar] [CrossRef]
- Zhang, J.F.; Smith, K.R. Indoor air pollution: A global health concern. Br. Med. Bull. 2003, 68, 209–225. [Google Scholar] [CrossRef]
- Cho, S.H.; Reponen, T.; LeMasters, G.; Levin, L.; Huang, J.; Meklin, T.; Ryan, P.; Villareal, M.; Bernstein, D. Mold damage in homes and wheezing in infants. Ann. Allergy Asthma Immunol. 2006, 97, 539–545. [Google Scholar] [CrossRef]
- Sharpe, R.A.; Thornton, C.R.; Nikolaou, V.; Osborne, N.J. Higher energy efficient homes are associated with increased risk of doctor diagnosed asthma in a UK subpopulation. Environ. Int. 2015, 75, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Moularat, S.; Hulin, M.; Robine, E.; Annesi-Maesano, I.; Caillaud, D. Airborne fungal volatile organic compounds in rural and urban dwellings: Detection of mould contamination in 94 homes determined by visual inspection and airborne fungal volatile organic compounds method. Sci. Total Environ. 2011, 409, 2005–2009. [Google Scholar] [CrossRef]
- Baxi, S.N.; Portnoy, J.M.; Larenas-Linnemann, D.; Phipatanakul, W.; Environmental Allergens, W. Exposure and Health Effects of Fungi on Humans. J. Allergy Clin. Immunol. Pract. 2016, 4, 396–404. [Google Scholar] [CrossRef]
- Nevalainen, A.; Taubel, M.; Hyvarinen, A. Indoor fungi: Companions and contaminants. Indoor Air 2015, 25, 125–156. [Google Scholar] [CrossRef]
- Sharpe, R.; Thornton, C.R.; Osborne, N.J. Modifiable factors governing indoor fungal diversity and risk of asthma. Clin. Exp. Allergy 2014, 44, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Bragoszewska, E. The Dose of Fungal Aerosol Inhaled by Workers in a Waste-Sorting Plant in Poland: A Case Study. Int. J. Environ. Res. Public Health 2020, 17, 177. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, D.M. Exposure Dose of Bacteria and Fungi in a Public Primary School in Beni Suef, Upper Egypt. J. Adv. Biol. 2019, 12, 2331–2340. [Google Scholar] [CrossRef]
- Mudarri, D.; Fisk, W.J. Public health and economic impact of dampness and mold. Indoor Air 2007, 17, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Tischer, C.G.; Hohmann, C.; Thiering, E.; Herbarth, O.; Muller, A.; Henderson, J.; Granell, R.; Fantini, M.P.; Luciano, L.; Bergstrom, A.; et al. Meta-analysis of mould and dampness exposure on asthma and allergy in eight European birth cohorts: An ENRIECO initiative. Allergy 2011, 66, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
- Hagerhed-Engman, L.; Sigsgaard, T.; Samuelson, I.; Sundell, J.; Janson, S.; Bornehag, C.G. Low home ventilation rate in combination with moldy odor from the building structure increase the risk for allergic symptoms in children. Indoor Air 2009, 19, 184–192. [Google Scholar] [CrossRef]
- Oluwole, O.; Kirychuk, S.P.; Lawson, J.A.; Karunanayake, C.; Cockcroft, D.W.; Willson, P.J.; Senthilselvan, A.; Rennie, D.C. Indoor mold levels and current asthma among school-aged children in Saskatchewan, Canada. Indoor Air 2017, 27, 311–319. [Google Scholar] [CrossRef]
- Norback, D.; Zock, J.P.; Plana, E.; Heinrich, J.; Tischer, C.; Bertelsen, R.J.; Sunyer, J.; Kunzli, N.; Villani, S.; Olivieri, M.; et al. Building dampness and mold in European homes in relation to climate, building characteristics and socio-economic status: The European Community Respiratory Health Survey ECRHS II. Indoor Air 2017, 27, 921–932. [Google Scholar] [CrossRef]
- Moses, L.; Morrissey, K.; Sharpe, R.A.; Taylor, T. Exposure to Indoor Mouldy Odour Increases the Risk of Asthma in Older Adults Living in Social Housing. Int. J. Environ. Res. Public Health 2019, 16, 2600. [Google Scholar] [CrossRef]
- Karvonen, A.M.; Hyvarinen, A.; Korppi, M.; Haverinen-Shaughnessy, U.; Renz, H.; Pfefferle, P.I.; Remes, S.; Genuneit, J.; Pekkanen, J. Moisture damage and asthma: A birth cohort study. Pediatrics 2015, 135, e598–e606. [Google Scholar] [CrossRef]
- Karvonen, A.M.; Hyvarinen, A.; Roponen, M.; Hoffmann, M.; Korppi, M.; Remes, S.; von Mutius, E.; Nevalainen, A.; Pekkanen, J. Confirmed moisture damage at home, respiratory symptoms and atopy in early life: A birth-cohort study. Pediatrics 2009, 124, E329–E338. [Google Scholar] [CrossRef]
- Pekkanen, J.; Hyvarinen, A.; Haverinen-Shaughnessy, U.; Korppi, M.; Putus, T.; Nevalainen, A. Moisture damage and childhood asthma: A population-based incident case-control study. Eur. Respir. J. 2007, 29, 509–515. [Google Scholar] [CrossRef]
- Andersen, B.; Frisvad, J.C.; Sondergaard, I.; Rasmussen, I.S.; Larsen, L.S. Associations between Fungal Species and Water-Damaged Building Materials. Appl. Environ. Microbiol. 2011, 77, 4180–4188. [Google Scholar] [CrossRef]
- Flannigan, B.; Samson, R.A.; Miller, J.D. Microbial growth in indoor environments. In Microorganisms in Home and Indoor Work Environments: Diversity, Health Impacts, Investigation and Control, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 57–144. ISBN 978--1-4200--9334-6. [Google Scholar]
- Chew, G.L.; Rogers, C.; Burge, H.A.; Muilenberg, M.L.; Gold, D.R. Dustborne and airborne fungal propagules represent a different spectrum of fungi with differing relations to home characteristics. Allergy 2003, 58, 13–20. [Google Scholar] [CrossRef]
- Dharmage, S.; Bailey, M.; Raven, J.; Mitakakis, T.; Thien, F.; Forbes, A.; Guest, D.; Abramson, M.; Walters, E.H. Prevalence and residential determinants of fungi within homes in Melbourne, Australia. Clin. Exp. Allergy 1999, 29, 1481–1489. [Google Scholar] [CrossRef]
- Fairs, A.; Wardlaw, A.J.; Thompson, J.R.; Pashley, C.H. Guidelines on Ambient Intramural Airborne Fungal Spores. J. Investig. Allergol. Clin. Immunol. 2010, 20, 490–498. [Google Scholar] [PubMed]
- Reponen, T.; Levin, L.; Zheng, S.; Vesper, S.; Ryan, P.; Grinshpun, S.A.; LeMasters, G. Family and home characteristics correlate with mold in homes. Environ. Res. 2013, 124, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Zock, J.P.; Jarvis, D.; Luczynska, C.; Sunyer, J.; Burney, P.; Surv, E.C.R.H. Housing characteristics, reported mold exposure, and asthma in the European Community Respiratory Health Survey. J. Allergy Clin. Immunol. 2002, 110, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Semple, S.; Garden, C.; Coggins, M.; Galea, K.S.; Whelan, P.; Cowie, H.; Sanchez-Jimenez, A.; Thorne, P.S.; Hurley, J.F.; Ayres, J.G. Contribution of solid fuel, gas combustion, or tobacco smoke to indoor air pollutant concentrations in Irish and Scottish homes. Indoor Air 2012, 22, 212–223. [Google Scholar] [CrossRef]
- Howden-Chapman, P.; Saville-Smith, K.; Crane, J.; Wilson, N. Risk factors for mold in housing: A national survey. Indoor Air 2005, 15, 469–476. [Google Scholar] [CrossRef]
- Gent, J.F.; Ren, P.; Belanger, K.; Triche, E.; Bracken, M.B.; Holford, T.R.; Leaderer, B.P. Levels of household mold associated with respiratory symptoms in the first year of life in a cohort at risk for asthma. Environ. Health Perspect. 2002, 110, A781–A786. [Google Scholar] [CrossRef]
- Sharpe, R.A.; Le Cocq, K.; Nikolaou, V.; Osborne, N.J.; Thornton, C.R. Identifying risk factors for exposure to culturable allergenic moulds in energy efficient homes by using highly specific monoclonal antibodies. Environ. Res. 2016, 144, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Goyette Pernot, J.; Hager Jörin, C.; Niculita-Hirzel, H.; Perret, V.; Licina, D. Radon Investigation in 650 Energy Efficient Dwellings in Western Switzerland: Impact of Energy Renovation and Building Characteristics. Atmosphere 2019, 10, 777. [Google Scholar] [CrossRef]
- Yang, S.; Goyette Pernot, J.; Hager Jörin, C.; Niculita-Hirzel, H.; Perret, V.; Licina, D. Energy, indoor air quality, occupant behavior, self-reported symptoms and satisfaction in energy-efficient dwellings in Switzerland. Build. Environ. 2020, 171, 106618. [Google Scholar] [CrossRef]
- Yang, S.; Perret, V.; Niculita-Hirzel, H.; Hager Jörin, C.; Goyette Pernot, J.; Licina, D. Volatile organic compounds in 169 energy-efficient dwellings in Switzerland. Indoor Air 2020, 30, 481–491. [Google Scholar] [CrossRef]
- Swiss Federal Statistics Office. Available online: https://www.bfs.admin.ch/bfs/fr/home/bases-statistiques/niveaux-geographiques.html (accessed on 3 March 2020).
- Adams, R.I.; Bateman, A.C.; Bik, H.M.; Meadow, J.F. Microbiota of the indoor environment: A meta-analysis. Microbiome 2015, 3, 49. [Google Scholar] [CrossRef] [PubMed]
- Noss, I.; Wouters, I.M.; Visser, M.; Heederik, D.J.; Thorne, P.S.; Brunekreef, B.; Doekes, G. Evaluation of a low-cost electrostatic dust fall collector for indoor air endotoxin exposure assessment. Appl. Environ. Microbiol. 2008, 74, 5621–5627. [Google Scholar] [CrossRef]
- Frankel, M.; Timm, M.; Hansen, E.W.; Madsen, A.M. Comparison of sampling methods for the assessment of indoor microbial exposure. Indoor Air 2012, 22, 405–414. [Google Scholar] [CrossRef]
- Scherer, E.; Rocchi, S.; Reboux, G.; Vandentorren, S.; Roussel, S.; Vacheyrou, M.; Raherison, C.; Millon, L. qPCR standard operating procedure for measuring microorganisms in dust from dwellings in large cohort studies. Sci. Total Environ. 2014, 466–467, 716–724. [Google Scholar] [CrossRef]
- de Hoog, G.S.; Guarro, J.; Géné, J.; Figueras, M.J. Atlas of Clinical Fungi: The Ultimate Benchtool for Diagnostic, USB Version 4.1. ed.; Stichting Atlas of Clinical Fungi: Utrecht, The Netherlands, 2016. [Google Scholar]
- Pitt, J. The Genus Penicillium and Its Teleomorphic States Eupenicillium and Talaromyces; Academic Press Inc. Ltd.: London, UK, 1979; p. 634. [Google Scholar]
- Samson, R.A.; Houdbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi, 2nd ed.; CBS Laboratory Manual Series: Utrecht, The Netherlands, 2019; p. 481. ISBN 9789491751189. [Google Scholar]
- Samson, R.; Pitt, J. Advances in Penicillium and Aspergillus Systematics; Plenum Press: New York, NY, USA, 1986; p. 483. [Google Scholar]
- Amend, A.S.; Seifert, K.A.; Samson, R.; Bruns, T.D. Indoor fungal composition is geographically patterned and more diverse in temperate zones than in the tropics. Proc. Natl. Acad. Sci. USA 2010, 107, 13748–13753. [Google Scholar] [CrossRef]
- Salonen, H.; Duchaine, C.; Mazaheri, M.; Clifford, S.; Lappalainen, S.; Reijula, K.; Morawska, L. Airborne viable fungi in school environments in different climatic regions—A review. Atmos. Environ. 2015, 104, 186–194. [Google Scholar] [CrossRef]
- Ege, M.J.; Mayer, M.; Normand, A.C.; Genuneit, J.; Cookson, W.O.C.M.; Braun-Fahrlander, C.; Heederik, D.; Piarroux, R.; von Mutius, E.; Grp, G.T.S. Exposure to Environmental Microorganisms and Childhood Asthma. N. Engl. J. Med. 2011, 364, 701–709. [Google Scholar] [CrossRef]
- Allen, R.W.; Adar, S.D.; Avol, E.; Cohen, M.; Curl, C.L.; Larson, T.; Liu, L.J.; Sheppard, L.; Kaufman, J.D. Modeling the residential infiltration of outdoor PM(2.5) in the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air). Environ. Health Perspect. 2012, 120, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Reponen, T.; Hyvarinen, A.; Ruuskanen, J.; Raunemaa, T.; Nevalainen, A. Comparison of Concentrations and Size Distributions of Fungal Spores in Buildings with and without Mold Problems. J. Aerosol Sci. 1994, 25, 1595–1603. [Google Scholar] [CrossRef]
- Reboux, G.; Rocchi, S.; Laboissiere, A.; Ammari, H.; Bochaton, M.; Gardin, G.; Rame, J.M.; Millon, L. Survey of 1012 moldy dwellings by culture fungal analysis: Threshold proposal for asthmatic patient management. Indoor Air 2019, 29, 5–16. [Google Scholar] [CrossRef]
- Reponen, T.; Singh, U.; Schaffer, C.; Vesper, S.; Johansson, E.; Adhikari, A.; Grinshpun, S.A.; Indugula, R.; Ryan, P.; Levin, L.; et al. Visually observed mold and moldy odor versus quantitatively measured microbial exposure in homes. Sci. Total Environ. 2010, 408, 5565–5574. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Bibby, K.; Qian, J.; Hospodsky, D.; Rismani-Yazdi, H.; Nazaroff, W.W.; Peccia, J. Particle-size distributions and seasonal diversity of allergenic and pathogenic fungi in outdoor air. Isme J. 2012, 6, 1801–1811. [Google Scholar] [CrossRef] [PubMed]
- Madsen, A.M.; Larsen, S.T.; Koponen, I.K.; Kling, K.I.; Barooni, A.; Karottki, D.G.; Tendal, K.; Wolkoff, P. Generation and Characterization of Indoor Fungal Aerosols for Inhalation Studies. Appl. Environ. Microbiol. 2016, 82, 2479–2493. [Google Scholar] [CrossRef] [PubMed]
- Reboux, G.; Bellanger, A.P.; Roussel, S.; Grenouillet, F.; Sornin, S.; Piarroux, R.; Dalphin, J.C.; Millon, L. Indoor mold concentration in Eastern France. Indoor Air 2009, 19, 446–453. [Google Scholar] [CrossRef]
- Sivasubramani, S.K.; Niemeier, R.T.; Reponen, T.; Grinshpun, S.A. Assessment of the aerosolization potential for fungal spores in moldy homes. Indoor Air 2004, 14, 405–412. [Google Scholar] [CrossRef]
- Kembel, S.W.; Jones, E.; Kline, J.; Northcutt, D.; Stenson, J.; Womack, A.M.; Bohannan, B.J.; Brown, G.Z.; Green, J.L. Architectural design influences the diversity and structure of the built environment microbiome. ISME J. 2012, 6, 1469–1479. [Google Scholar] [CrossRef]
- Lamarche, D.; Johnstone, J.; Zytaruk, N.; Clarke, F.; Hand, L.; Loukov, D.; Szamosi, J.C.; Rossi, L.; Schenck, L.P.; Verschoor, C.P.; et al. Microbial dysbiosis and mortality during mechanical ventilation: A prospective observational study. Respir. Res. 2018, 19, 245. [Google Scholar] [CrossRef] [PubMed]
- Wallner, P.; Munoz, U.; Tappler, P.; Wanka, A.; Kundi, M.; Shelton, J.F.; Hutter, H.P. Indoor Environmental Quality in Mechanically Ventilated, Energy-Efficient Buildings vs. Conventional Buildings. Int. J. Environ. Res. Public Health 2015, 12, 14132–14147. [Google Scholar] [CrossRef] [PubMed]
- Hulin, M.; Moularat, S.; Kirchner, S.; Robine, E.; Mandin, C.; Annesi-Maesano, I. Positive associations between respiratory outcomes and fungal index in rural inhabitants of a representative sample of French dwellings. Int. J. Hyg. Environ. Health 2013, 216, 155–162. [Google Scholar] [CrossRef] [PubMed]



| Type of Energy Efficient Dwellings | p Value | Ventilation Type | p Value | |||
|---|---|---|---|---|---|---|
| Minergie (n = 44) | Renovated (n = 105) | Mechanical (n = 49) | Natural (n = 98) | |||
| Cloth sampling | ||||||
| Cladosporium | 57 ± 54 | 54 ± 40 | 0.723 | 59 ± 53 | 54 ± 40 | 0.998 |
| Penicillium | 21 ± 31 | 21 ± 26 | 0.505 | 25 ± 37 | 19 ± 21 | 0.879 |
| Aspergillus | 12 ± 17 | 11 ± 25 | 0.566 | 12 ± 16 | 11 ± 25 | 0.492 |
| EDC * sampling | ||||||
| Cladosporium | 9 ± 10 | 13 ± 14 | 0.065 | 9 ± 10 | 13 ± 14 | 0.053 |
| Penicillium | 19 ± 30 | 27 ± 23 | 0.033 | 21 ± 30 | 27 ± 23 | 0.010 |
| Aspergillus | 5 ± 11 | 8 ± 13 | 0.004 | 6 ± 11 | 8 ± 13 | 0.127 |
| Total CFUs ± SD in Owner Bedroom with | p Value | ||
|---|---|---|---|
| No Fungal Growth | Fungal Growth | ||
| Settled dust sampled with cloth | |||
| All dwellings (n = 27) | 51 ± 37 | 110 ± 67 | 0.007 |
| Recent (n = 6) | 18 ± 10 | 85 ± 42 | 0.054 |
| Renovated (n = 21) | 59 ± 37 | 119 ± 74 | 0.022 |
| Settled dust sampled with EDC | |||
| All dwellings (n = 27) | 47 ± 29 | 45 ± 22 | 0.853 |
| Recent (n = 6) | 33 ± 34 | 57 ± 20 | 0.346 |
| Renovated (n = 21) | 50 ± 29 | 41 ± 22 | 0.423 |
| Total CFUs ± SD in Dwellings with | p Value | ||
|---|---|---|---|
| No Fungal Growth | Fungal Growth | ||
| Settled dust sampled with cloth | |||
| All dwellings (n = 60) | 64 ± 44 | 100 ± 66 | 0.034 |
| New (n = 19) | 25 ± 15 | 101 ± 68 | 0.044 |
| Renovated (n = 41) | 74 ± 44 | 99 ± 65 | 0.191 |
| Settled dust sampled with EDC | |||
| All dwellings (n = 60) | 42 ± 32 | 52 ± 30 | 0.243 |
| New (n = 19) | 53 ± 49 | 52 ± 26 | 0.937 |
| Renovated (n = 41) | 40 ± 28 | 53 ± 32 | 0.194 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niculita-Hirzel, H.; Yang, S.; Hager Jörin, C.; Perret, V.; Licina, D.; Goyette Pernot, J. Fungal Contaminants in Energy Efficient Dwellings: Impact of Ventilation Type and Level of Urbanization. Int. J. Environ. Res. Public Health 2020, 17, 4936. https://doi.org/10.3390/ijerph17144936
Niculita-Hirzel H, Yang S, Hager Jörin C, Perret V, Licina D, Goyette Pernot J. Fungal Contaminants in Energy Efficient Dwellings: Impact of Ventilation Type and Level of Urbanization. International Journal of Environmental Research and Public Health. 2020; 17(14):4936. https://doi.org/10.3390/ijerph17144936
Chicago/Turabian StyleNiculita-Hirzel, Hélène, Shen Yang, Corinne Hager Jörin, Vincent Perret, Dusan Licina, and Joëlle Goyette Pernot. 2020. "Fungal Contaminants in Energy Efficient Dwellings: Impact of Ventilation Type and Level of Urbanization" International Journal of Environmental Research and Public Health 17, no. 14: 4936. https://doi.org/10.3390/ijerph17144936
APA StyleNiculita-Hirzel, H., Yang, S., Hager Jörin, C., Perret, V., Licina, D., & Goyette Pernot, J. (2020). Fungal Contaminants in Energy Efficient Dwellings: Impact of Ventilation Type and Level of Urbanization. International Journal of Environmental Research and Public Health, 17(14), 4936. https://doi.org/10.3390/ijerph17144936






