Spatio-Temporal Dynamics of Tick-Borne Diseases in North-Central Wisconsin from 2000–2016
Abstract
:1. Introduction
2. Materials and Methods
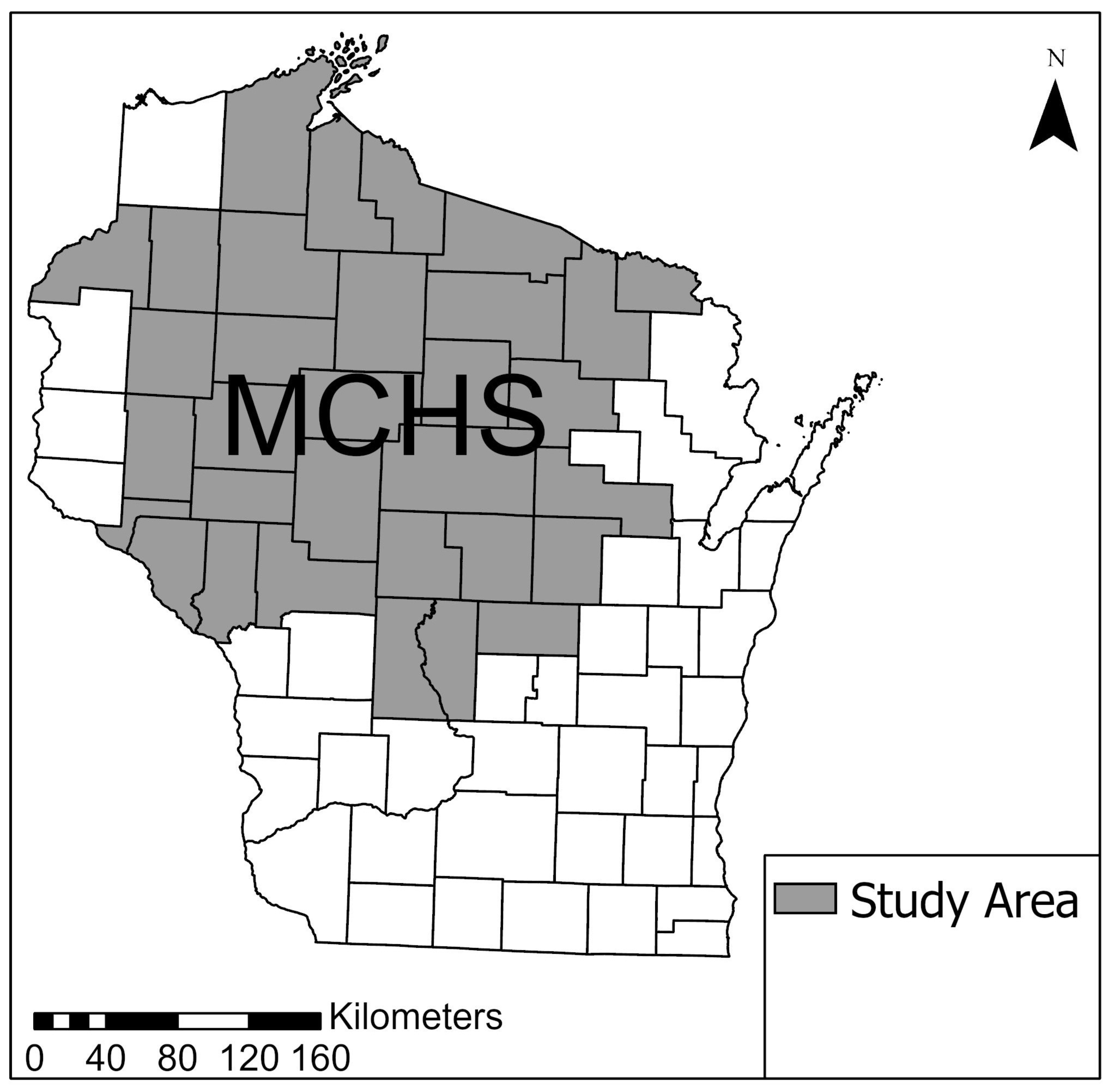
2.1. Study Population
2.2. Data Confidentiality
2.3. Data Inclusion
2.3.1. Case Identification
2.3.2. Geocoding Patients
2.4. Statistical Analysis
2.4.1. Descriptive Statistics
2.4.2. SatScan Analysis
2.4.3. Spatial Cluster Analysis
2.4.4. Temporal and Seasonal Cluster Analysis
3. Results
3.1. Descriptive Statistics
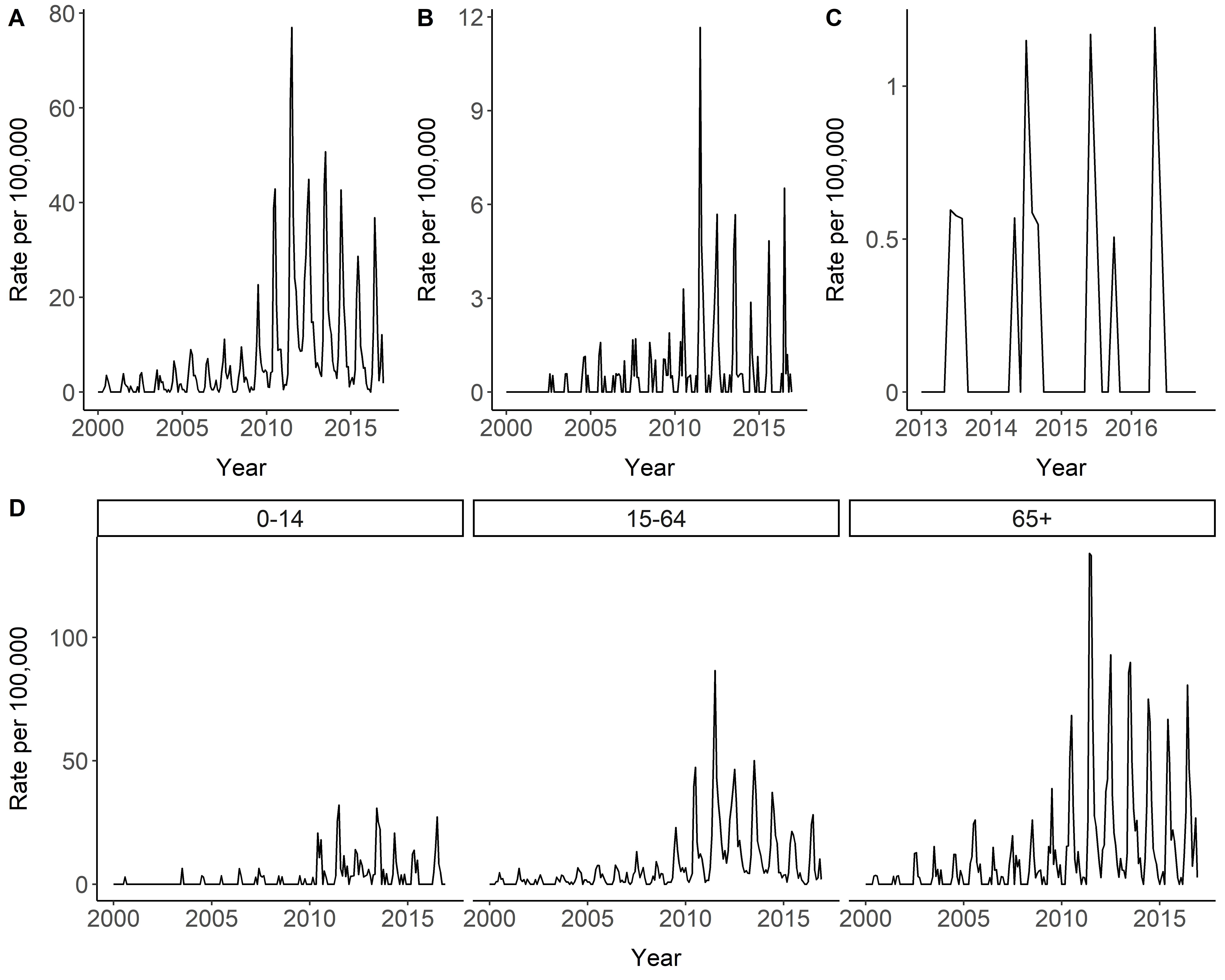
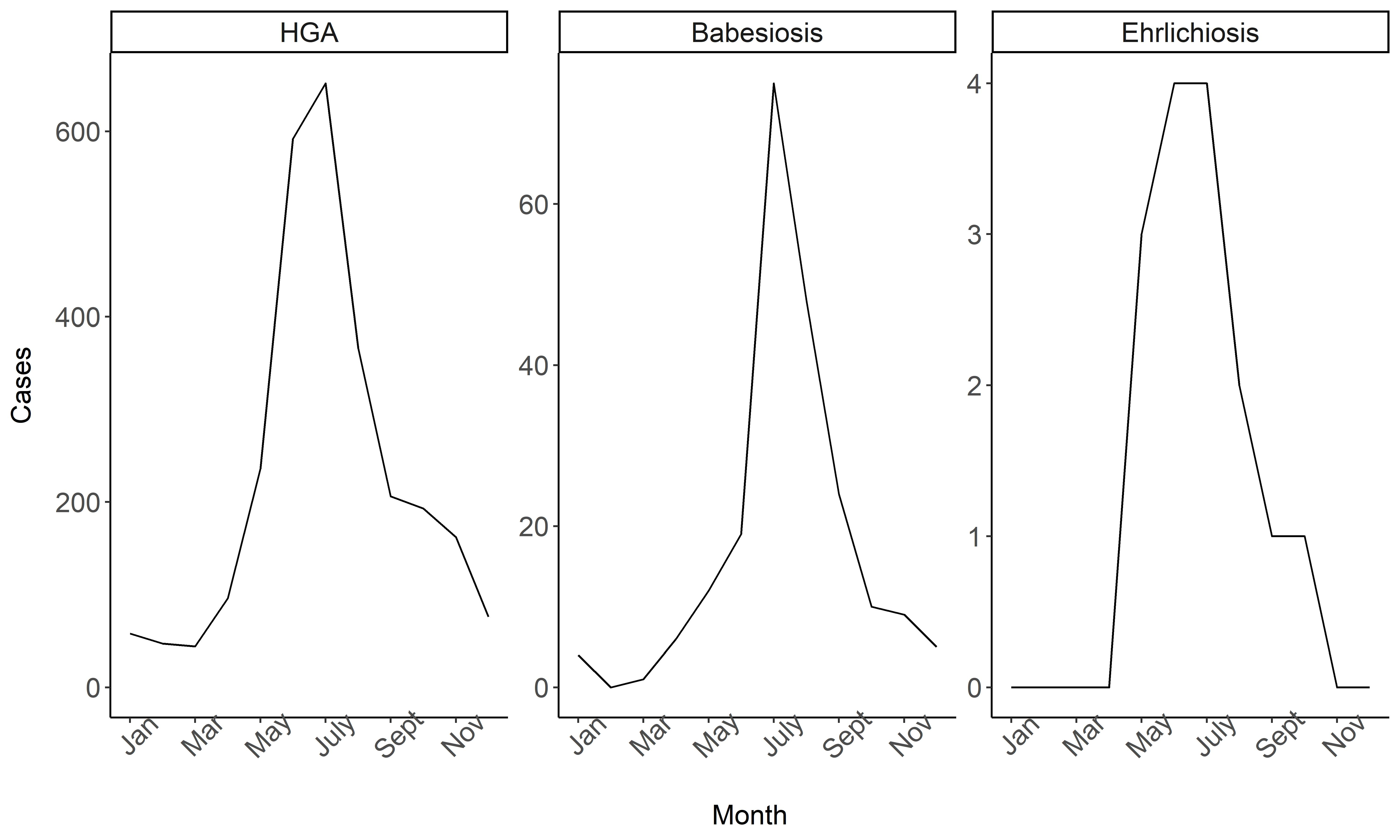
3.2. Temporal and Seasonal Trends
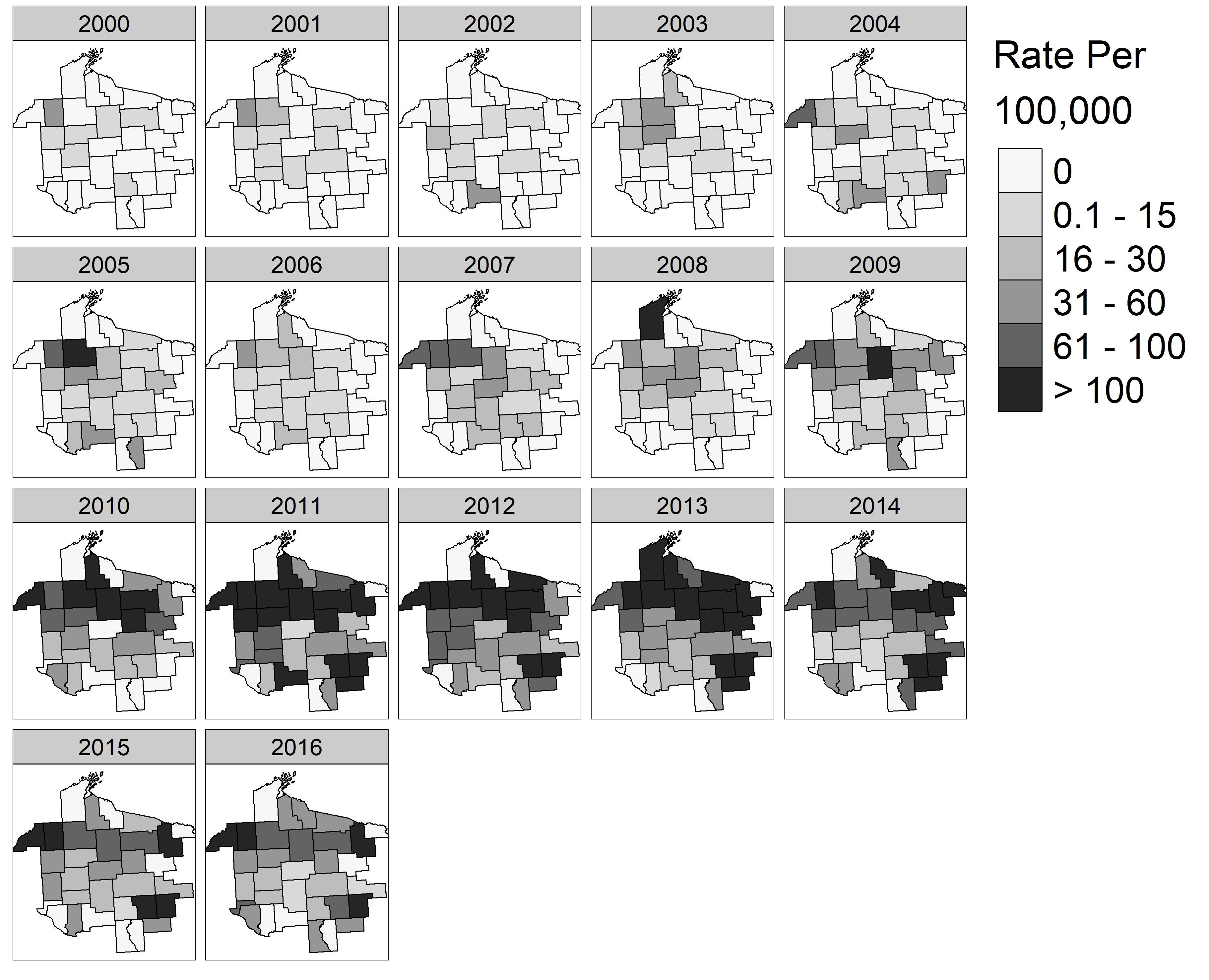
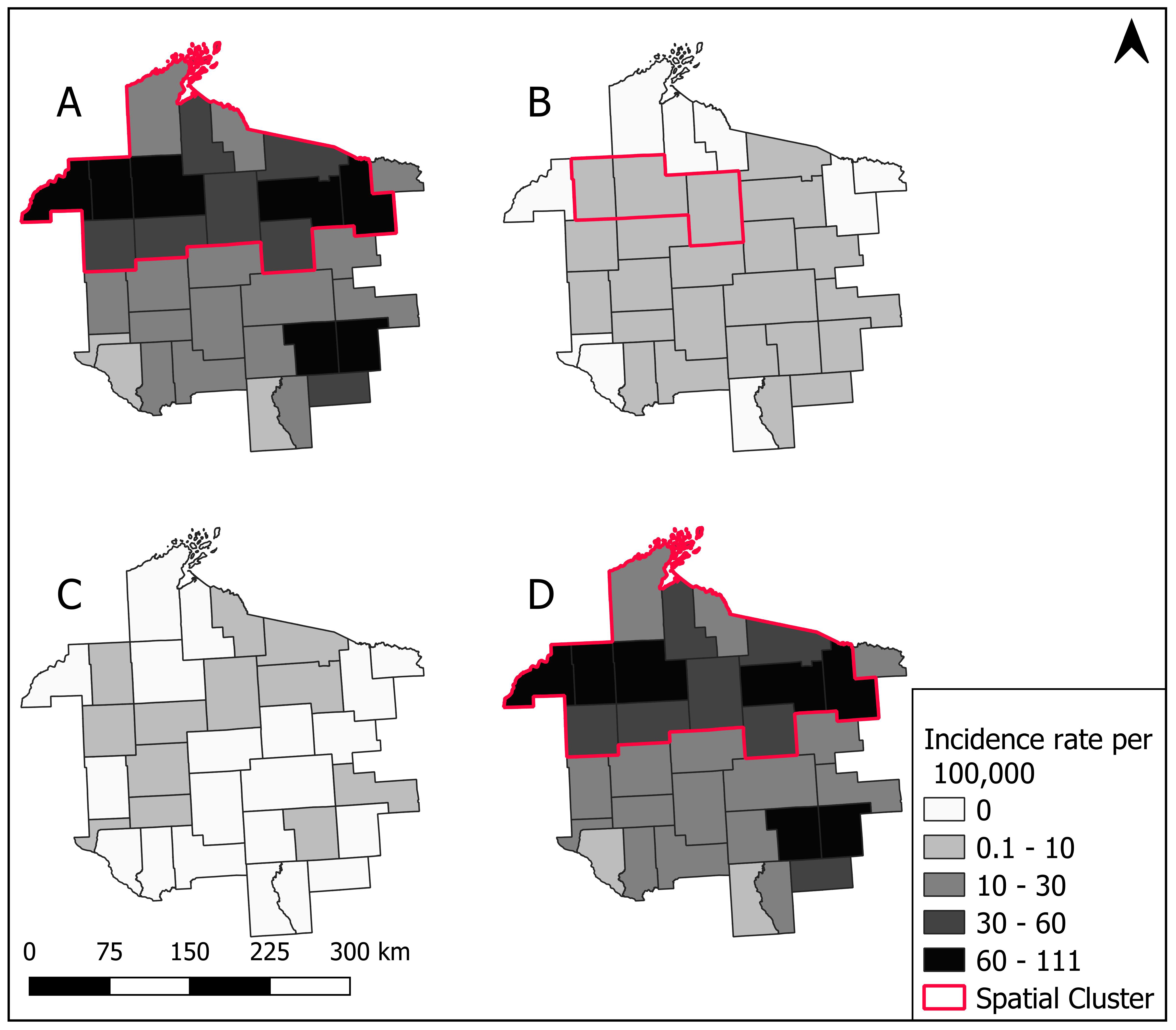
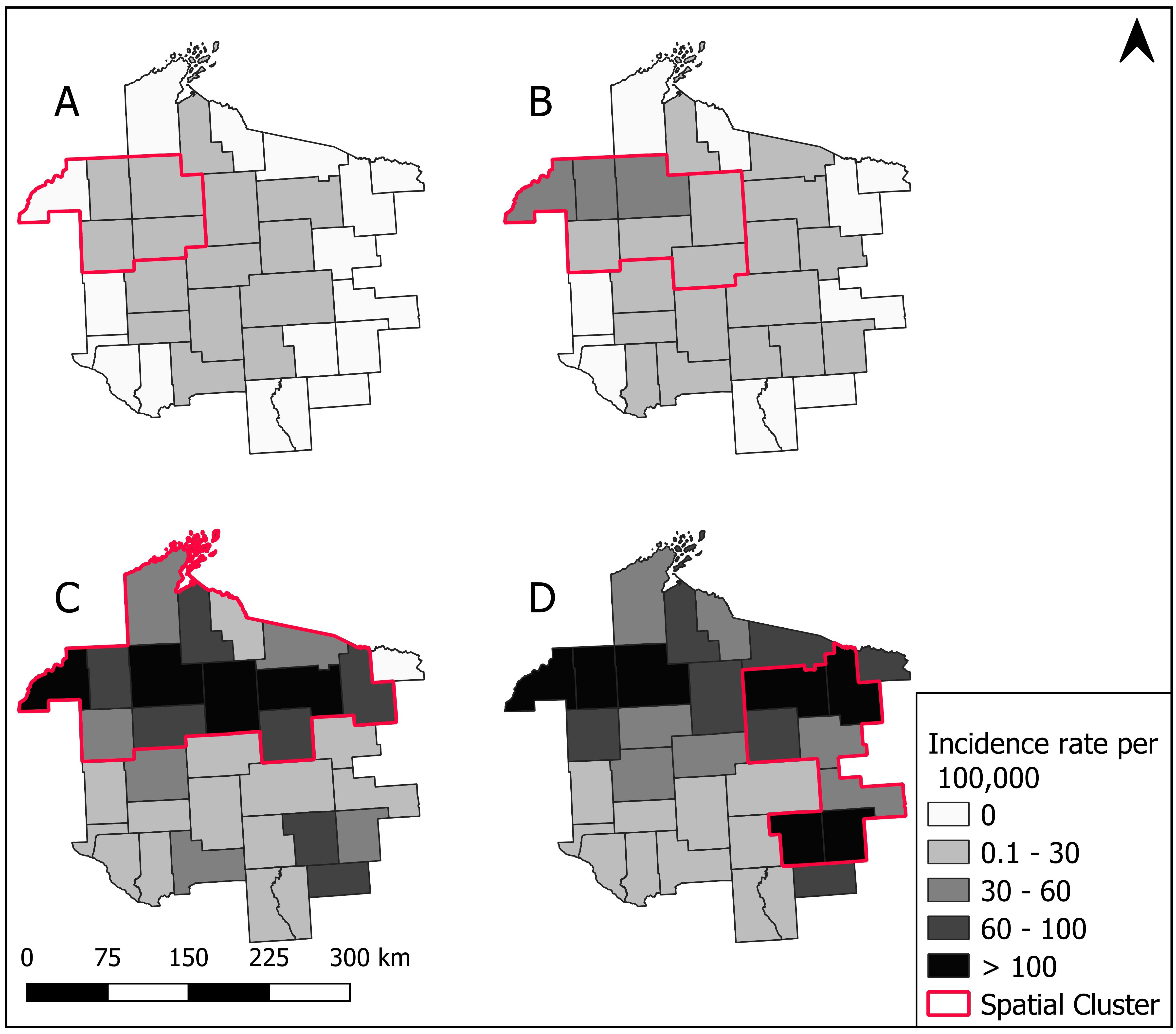
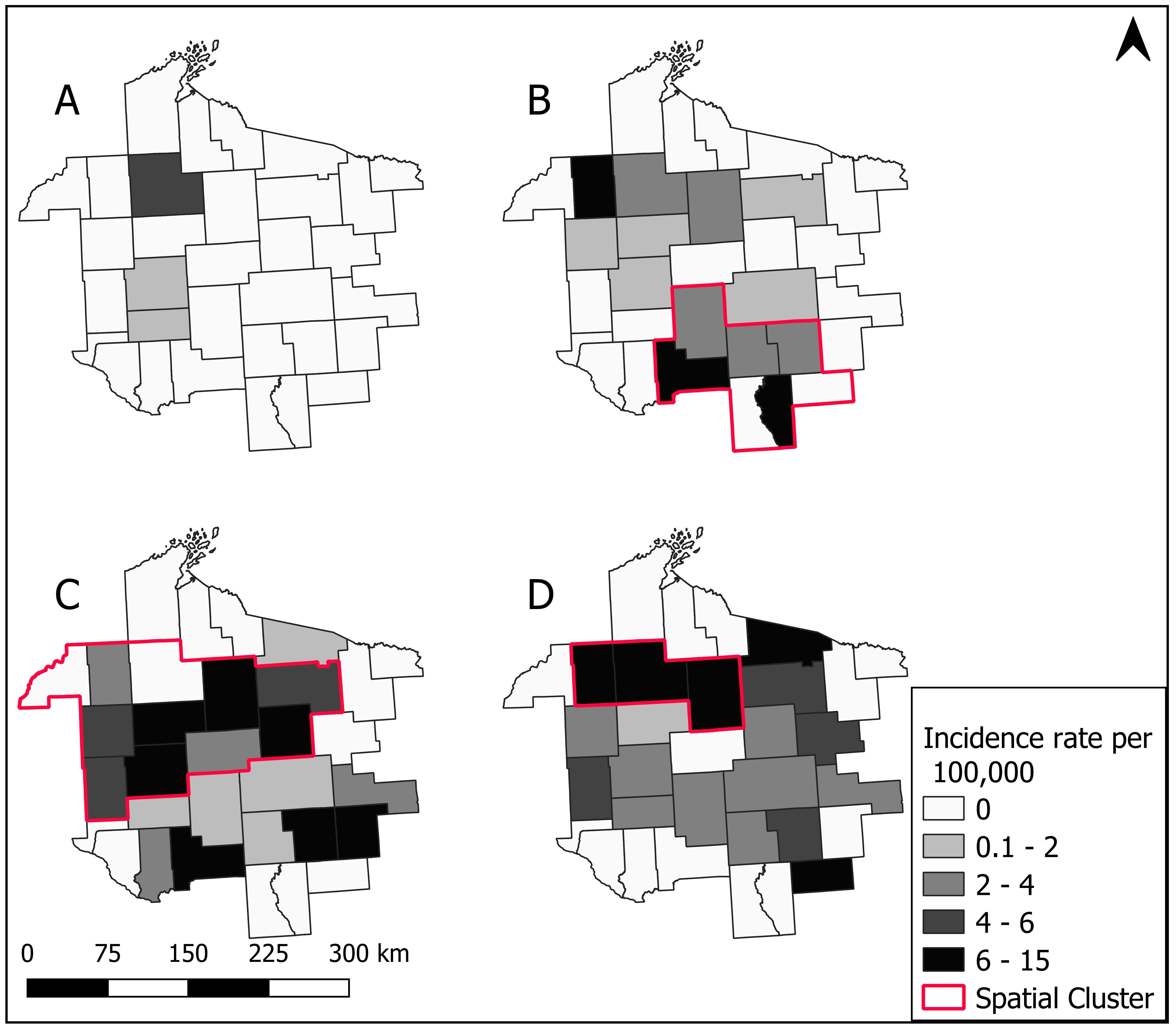
3.3. Spatial Patterns and Trends Over Time
3.4. Spatial Cluster Analysis
3.5. Sensitivity of Spatial Clusters
3.6. Temporal and Seasonal Cluster Analysis
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Tick-Borne Disease Surveillance Data Summary. 2019. Available online: https://www.cdc.gov/ticks/data-summary/index.html (accessed on 5 April 2020).
- Bakken, J.S.; Dumler, J.S. Human Granulocytic HGA. Infect. Dis. Clin. N. Am. 2015, 29, 341–355. [Google Scholar] [CrossRef] [Green Version]
- Bloch, E.M.; Kumar, S.; Krause, P.J. Persistence of Babesia microti Infection in Humans. Pathogens 2019, 8, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberg, R.; Lindsey, N.P.; Fischer, M.; Gregory, C.J.; Hinckley, A.F.; Mead, P.S.; Paz-Bailey, G.; Waterman, S.H.; Drexler, N.A.; Kersh, G.J.; et al. Vital Signs: Trends in Reported Vectorborne Disease Cases—United States and Territories, 2004–2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 496–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- French, J.B.; Schell, W.L.; Kazmierczak, J.J.; Davis, J.P. Changes in Population Density and Distribution of Ixodes dammini (Acari: Ixodidae) in Wisconsin During the 1980s. J. Med. Entomol. 1992, 29, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Lee, X.; Hardy, K.; Johnson, D.H.; Paskewitz, S.M. Hunter-killed deer surveillance to assess changes in the prevalence and distribution of Ixodes scapularis (Acari: Ixodidae) in Wisconsin. J. Med. Entomol. 2013, 50, 632–639. [Google Scholar] [CrossRef]
- Riehle, M.; Paskewitz, S.M. Ixodes scapularis (Acari: Ixodidae): Status and Changes in Prevalence and Distribution in Wisconsin Between 1981 and 1994 Measured by Deer Surveillance. J. Med. Entomol. 1996, 33, 933–938. [Google Scholar] [CrossRef]
- Michalski, M.; Rosenfield, C.; Erickson, M.; Selle, R.; Bates, K.; Essar, D.; Massung, R. Anaplasma phagocytophilum in central and western Wisconsin: A molecular survey. Parasitol. Res. 2006, 99, 694–699. [Google Scholar] [CrossRef]
- Murphy, D.S.; Lee, X.; Larson, S.R.; Johnson, D.K.H.; Loo, T.; Paskewitz, S.M. Prevalence and Distribution of Human and Tick Infections with the Ehrlichia muris -Like Agent and Anaplasma phagocytophilum in Wisconsin, 2009–2015. Vector-Borne Zoonotic Dis. 2017, 17, 229–236. [Google Scholar] [CrossRef]
- Paskewitz, S.M.; Vandermause, M.; Belongia, E.A.; Kazmierczak, J.J. Ixodes scapularis (Acari: Ixodidae): Abundance and Rate of Infection with Borrelia burgdorferi in Four State Parks in Wisconsin. J. Med. Entomol. 2001, 38, 33–38. [Google Scholar] [CrossRef]
- Diuk-Wasser, M.; Hoen, A.G.; Cislo, P.; Brinkerhoff, R.; Hamer, S.A.; Rowland, M.; Cortinas, R.; Vourc’H, G.; Melton, F.; Hickling, G.J.; et al. Human Risk of Infection with Borrelia burgdorferi, the Lyme Disease Agent, in Eastern United States. Am. J. Trop. Med. Hyg. 2012, 86, 320–327. [Google Scholar] [CrossRef]
- Eisen, R.J.; Eisen, L.; Beard, C.B. County-Scale Distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the Continental United States. J. Med. Entomol. 2016, 53, 349–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, T.; Bjork, J.K.H.; Neitzel, D.F.; Dorr, F.M.; Schiffman, E.K.; Eisen, R.J. Habitat Suitability Model for the Distribution of Ixodes scapularis (Acari: Ixodidae) in Minnesota. J. Med. Entomol. 2016, 53, 598–606. [Google Scholar] [CrossRef] [Green Version]
- Johnson, T.; Graham, C.B.; Maes, S.E.; Hojgaard, A.; Fleshman, A.; Boegler, K.A.; DeLory, M.J.; Slater, K.S.; Karpathy, S.E.; Bjork, J.K.; et al. Prevalence and distribution of seven human pathogens in host-seeking Ixodes scapularis (Acari: Ixodidae) nymphs in Minnesota, USA. Ticks Tick-Borne Dis. 2018, 9, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.J.; Neitzel, D.F.; Moen, R.A.; Craft, M.E.; Hamilton, K.E.; Johnson, L.B.; Mulla, D.J.; Munderloh, U.G.; Redig, P.T.; Smith, K.E.; et al. Disease risk in a dynamic environment: The spread of tick-borne pathogens in Minnesota, USA. EcoHealth 2015, 12, 152–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisen, R.J.; Kugeler, K.J.; Eisen, L.; Beard, C.B.; Paddock, C.D. Tick-Borne Zoonoses in the United States: Persistent and Emerging Threats to Human Health. ILAR J. 2017, 58, 319–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pritt, B.S.; Sloan, L.M.; Johnson, D.K.H.; Munderloh, U.G.; Paskewitz, S.M.; McElroy, K.M.; McFadden, J.D.; Binnicker, M.J.; Neitzel, D.F.; Liu, G.; et al. Emergence of a New Pathogenic Ehrlichia Species, Wisconsin and Minnesota, 2009. N. Engl. J. Med. 2011, 365, 422–429. [Google Scholar] [CrossRef] [Green Version]
- Wisconsin DHS. Tickborne Diseases Risk in Wisconsin. 2019. Available online: https://www.dhs.wisconsin.gov/publications/p01751.pdf (accessed on 5 May 2020).
- Kieke, A.L.; A Kieke, B.; Kopitzke, S.L.; McClure, D.L.; A Belongia, E.; VanWormer, J.J.; Greenlee, R.T. Validation of Health Event Capture in the Marshfield Epidemiologic Study Area. Clin. Med. Res. 2015, 13, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention. Anplasmosis. 2019. Available online: https://www.cdc.gov/anaplasmosis/healthcare-providers/index.html (accessed on 8 July 2020).
- Centers for Disease Control and Prevention. Parasites—Babesiosis. 2019. Available online: https://www.cdc.gov/parasites/babesiosis/health_professionals/index.html (accessed on 8 July 2020).
- Centers for Disease Control and Prevention. Ehrlichiosis. 2019. Available online: https://www.cdc.gov/ehrlichiosis/healthcare-providers/index.html (accessed on 8 July 2020).
- Centers for Disease Control and Prevention. Ehrlichiosis and HGA 2008 Case Definition. 2008. Available online: https://wwwn.cdc.gov/nndss/conditions/anaplasma-phagocytophilum-infection/case-definition/2008 (accessed on 5 April 2020).
- Hoang Johnson, D.K.; Schiffman, E.K.; Davis, J.P.; Neitzel, D.F.; Sloan, L.M.; Nicholson, W.L.; Fritsche, T.R.; Steward, C.R.; Ray, J.A.; Miller, T.K.; et al. Human Infection with Ehrlichia muris–like Pathogen, United States, 2007–2013. Emerg. Infect. Dis. 2015, 21, 1794. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Babesiosis 2011 Case Definition. 2011. Available online: https://wwwn.cdc.gov/nndss/conditions/babesiosis/case-definition/2011/ (accessed on 5 April 2020).
- Aguero-Rosenfeld, M.E.; Donnarumma, L.; Zentmaier, L.; Jacob, J.; Frey, M.; Noto, R.; Carbonaro, C.A.; Wormser, G.P. Seroprevalence of Antibodies That React with Anaplasma phagocytophila, the Agent of Human Granulocytic Ehrlichiosis, in Different Populations in Westchester County, New York. J. Clin. Microbiol. 2002, 40, 2612–2615. [Google Scholar] [CrossRef] [Green Version]
- Ruebush, T.K.; Chisholm, E.S.; Sulzer, A.J.; Healy, G.R. Development and Persistence of Antibody in Persons Infected with Babesia Microti. Am. J. Trop. Med. Hyg. 1981, 30, 291–292. [Google Scholar] [CrossRef] [PubMed]
- ESRI. ArcGIS Pro: Release 2.3.0.; Environmental Systems Research Institute: Redlands, CA, USA, 2018. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. In R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2019. [Google Scholar]
- Kulldorff, M.; Information Management Services, Inc. SaTScan™ Software for the Spatial and Space-Time Scan Statistics (Version 9.6) [Software]; Information Management Services Inc.: Calverton, MD, USA, 2018; Available online: www.satscan.org (accessed on 30 December 2019).
- Kulldorf, M. Bernoulli, Discrete Poisson and Continuous Poisson Models: A spatial scan statistic. Commun. Stat. Theory Methods 1997, 26, 1481–1496. [Google Scholar] [CrossRef]
- Ahmadkhani, M.; Alesheikh, A.A.; Khakifirouz, S.; Vaziri, M.S. Space-time epidemiology of Crimean-Congo hemorrhagic fever (CCHF) in Iran. Ticks Tick-Borne Dis. 2018, 9, 207–216. [Google Scholar] [CrossRef]
- Lantos, P.M.; Tsao, J.; Nigrovic, L.E.; Auwaerter, P.G.; Fowler, V.G.; Ruffin, F.; Foster, E.; Hickling, G. Geographic Expansion of Lyme Disease in Michigan, 2000-2014. Open Forum Infect. Dis. 2017, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Kolivras, K.N.; Hong, Y.; Duan, Y.; Seukep, S.E.; Prisley, S.P.; Campbell, J.B.; Gaines, D.N. Spatial and Temporal Emergence Pattern of Lyme Disease in Virginia. Am. J. Trop. Med. Hyg. 2014, 91, 1166–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mollalo, A.; Blackburn, J.K.; Morris, L.R.; Glass, G.E. A twenty-four-year exploratory spatial data analysis of Lyme disease incidence rate in Connecticut, USA. Geospat. Health 2017, 12. [Google Scholar] [CrossRef]
- Omodior, O.; Kianersi, S.; Luetke, M. Spatial Clusters and Non-spatial Predictors of Tick-Borne Disease Diagnosis in Indiana. J. Community Heal. 2019, 44, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Adjemian, J.Z.; Krebs, J.; McQuiston, J.; Mandel, E. Spatial clustering by disease severity among reported Rocky Mountain spotted fever cases in the United States, 2001-2005. Am. J. Trop. Med. Hyg. 2009, 80, 72–77. [Google Scholar] [CrossRef] [Green Version]
- Kulldorf, M. SatScan User Guide. 2018. Available online: https://www.satscan.org/cgi-bin/satscan/register.pl/SaTScan_Users_Guide.pdf?todo=process_userguide_download (accessed on 6 April 2020).
- QGIS Development Team. QGIS Geographic Information System. In Open Source Geospatial Foundation Project; 2019. Available online: http://qgis.osgeo.org (accessed on 30 December 2019).
- Schotthoefer, A.M.; Meece, J.K.; Ivacic, L.C.; Bertz, P.D.; Zhang, K.; Weiler, T.; Uphoff, T.S.; Fritsche, T.R. Comparison of a Real-Time PCR Method with Serology and Blood Smear Analysis for Diagnosis of Human Anaplasmosis: Importance of Infection Time Course for Optimal Test Utilization. J. Clin. Microbiol. 2013, 51, 2147–2153. [Google Scholar] [CrossRef] [Green Version]
- Steketee, R.W.; Eckman, M.R.; Burgess, E.C.; Kuritsky, J.N.; Dickerson, J.; Schell, W.L.; Godsey, M.S.; Davis, J.P. Babesiosis in Wisconsin. A new focus of disease transmission. J. Am. Med. Assoc. 1985, 253, 2675–2678. [Google Scholar] [CrossRef]
- Stein, E.; Elbadawi, L.I.; Kazmierczak, J.; Davis, J.P. Babesiosis Surveillance—Wisconsin, 2001–2015. Morb. Mortal. Wkly. Rep. 2017, 66, 687. [Google Scholar] [CrossRef] [Green Version]
- Dahlgren, F.S.; Mandel, E.J.; Krebs, J.W.; Massung, R.F.; McQuiston, J.H. Increasing Incidence of Ehrlichia chaffeensis and Anaplasma phagocytophilum in the United States, 2000–2007. Am. J. Trop. Med. Hyg. 2011, 85, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.B.; Herwaldt, B.L. Babesiosis Surveillance—United States, 2011–2015. Morb. Mortal. Wkly. Rep. 2019, 68, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Stromdahl, E.; Hamer, S.; Jenkins, S.; Sloan, L.; Williamson, P.; Foster, E.; Nadolny, R.; Elkins, C.; Vince, M.; Pritt, B. Comparison of phenology and pathogen prevalence, including infection with the Ehrlichia muris-like (EML) agent, of Ixodes scapularis removed from soldiers in the midwestern and the northeastern United States over a 15 year period (1997–2012). Parasites Vectors 2014, 7, 553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, N.; Bloch, K.C.; McBride, J.W. Human Ehrlichiosis and HGA. Clin. Lab. Med. 2010, 30, 261–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massung, R.F.; Levin, M.L.; Munderloh, U.G.; Silverman, D.J.; Lynch, M.J.; Gaywee, J.K.; Kurtti, T.J. Isolation and Propagation of the Ap-Variant 1 Strain of Anaplasma phagocytophilum in a Tick Cell Line. J. Clin. Microbiol. 2007, 45, 2138–2143. [Google Scholar] [CrossRef] [Green Version]
- Massung, R.F.; Priestley, R.A.; Miller, N.J.; Mather, T.N.; Levin, M.L. Inability of a Variant Strain of Anaplasma phagocytophilum to Infect Mice. J. Infect. Dis. 2003, 188, 1757–1763. [Google Scholar] [CrossRef] [Green Version]
- Curtis, T.R.; Shi, M.; Qiao, X. Patience is not always a virtue: Effects of terrain complexity on the host-seeking behaviour of adult blacklegged ticks, Ixodes scapularis, in the presence of a stationary host. Med. Veter. Entomol. 2020. [Google Scholar] [CrossRef]
- Oliver, J.; Bennett, S.W.; Beati, L.; Bartholomay, L.C. Range Expansion and Increasing Borrelia burgdorferi Infection of the Tick Ixodes scapularis (Acari: Ixodidae) in Iowa, 1990–2013. J. Med. Entomol. 2017, 54, 1727–1734. [Google Scholar] [CrossRef]
- Kitron, U.K.; Kazmierczak, J.J. Spatial Analysis of the Distribution of Lyme Disease in Wisconsin. Am. J. Epidemiol. 1997, 145, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Mead, P.S. Epidemiology of Lyme Disease. Infect. Dis. Clin. 2015, 29, 187–210. [Google Scholar] [CrossRef]
- Butler, S.; Lee, S.; Fernandez, M.P.; Bron, G.M.; Kache, P.A.; Larson, S.R.; Maus, A.; Jr, D.G.; Tsao, J.I.; Bartholomay, L.C.; et al. Usability and Feasibility of a Smartphone App to Assess Human Behavioral Factors Associated with Tick Exposure (The Tick App): Quantitative and Qualitative Study. JMIR MHealth UHealth 2019, 7, e14769. [Google Scholar] [CrossRef]
- Bayles, B.R.; Allan, B.F. Social-ecological factors determine spatial variation in human incidence of tick-borne ehrlichiosis. Epidemiol. Infect. 2014, 142, 1911–1924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raghavan, R.K.; Goodin, U.G.; Neises, D.; Anderson, G.A.; Ganta, R.R. Hierarchical Bayesian Spatio–Temporal Analysis of Climatic and Socio–Economic Determinants of Rocky Mountain Spotted Fever. PLoS ONE 2016, 11, e0150180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raghavan, R.K.; Neises, D.; Goodin, D.G.; Andresen, D.A.; Ganta, R.R. Bayesian Spatio-Temporal Analysis and Geospatial Risk Factors of Human Monocytic Ehrlichiosis. PLoS ONE 2014, 9, e100850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Springer, Y.P.; Johnson, P.T.J. Large-scale health disparities associated with Lyme disease and human monocytic ehrlichiosis in the United States, 2007–2013. PLoS ONE 2018, 13, e0204609. [Google Scholar] [CrossRef] [PubMed]
- Vannier, E.; Krause, P.J. Human Babesiosis. N. Engl. J. Med. 2012, 366, 2397–2407. [Google Scholar] [CrossRef] [Green Version]
- Nelder, M.P.; Russell, C.B.; Lindsay, L.R.; Dibernardo, A.; Brandon, N.C.; Pritchard, J.; Johnson, S.; Cronin, K.; Patel, S.N. Recent Emergence of Anaplasma phagocytophilum in Ontario, Canada: Early Serological and Entomological Indicators. Am. J. Trop. Med. Hyg. 2019, 101, 1249–1258. [Google Scholar] [CrossRef] [Green Version]
- Schotthoefer, A.M.; Hall, M.C.; Vittala, S.; Bajwa, R.; Frost, H.M. Clinical Presentation and Outcomes of Children with Human Granulocytic Anaplasmosis. J. Pediatr. Infect. Dis. Soc. 2018, 7, e9–e15. [Google Scholar] [CrossRef] [Green Version]
- Nelson, C.A.; Saha, S.; Kugeler, K.J.; DeLorey, M.J.; Shankar, M.B.; Hinckley, A.F.; Mead, P.S. Incidence of Clinician-Diagnosed Lyme Disease, United States, 2005–2010. Emerg. Infect. Dis. 2015, 21, 1625–1631. [Google Scholar] [CrossRef]
- Naleway, A.L.; Belongia, E.A.; Kazmierczak, J.J.; Greenlee, R.T.; Davis, J.P. Lyme disease incidence in Wisconsin: A comparison of state-reported rates and rates from a population-based cohort. Am. J. Epidemiol. 2002, 155, 1120–1127. [Google Scholar] [CrossRef] [Green Version]
- Wisconsin DHS. Wisconsin Electronic Disease Surveillance System (WEDSS). 2020. Available online: https://www.dhs.wisconsin.gov/wiphin/wedss.htm (accessed on 4 May 2020).
- Bakken, J.S.; Dumler, J.S. Clinical Diagnosis and Treatment of Human Granulocytotropic Anaplasmosis. Ann. N. Y. Acad. Sci. 2006, 1078, 236–247. [Google Scholar] [CrossRef] [PubMed]
| Disease by Type & Lab Test | Total | Age at Positive Lab Result (Years) | Age Range 2 (Years) | Sex | |
|---|---|---|---|---|---|
| N (% Total) | Mean (SD) | Male (%) | Female (%) | ||
| HGA 3 | 2728 (92.3%) | 54 (20) | 1 to >89 | 1480 (54.3%) | 1248 (45.7%) |
| Confirmed: | 809 | 59 (18) | 2 to >89 | 480 (59.3%) | 329 (40.7%) |
| PCR + | 665 | 60 (18) | 2 to >89 | 390 (58.6%) | 275 (41.4%) |
| IFA 4-fold rise | 144 | 56 (17) | 4 to >89 | 90 (62.5%) | 54 (37.5%) |
| Supportive: | 1919 | 51 (20) | 1 to >89 | 1000 (52.1%) | 919 (47.9%) |
| Smear + | 109 | 62 (17) | 4 to >89 | 66 (60.6%) | 43 (39.4%) |
| IFA + | 1810 | 51 (20) | 1 to >89 | 934 (51.6%) | 876 (48.4%) |
| Babesiosis | 213 (7.2%) | 59 (16) | 7 to >89 | 134 (62.9%) | 79 (37.1%) |
| Confirmed: | 98 | 62 (15) | 18 to >89 | 68 (69.4%) | 30 (30.6%) |
| PCR + | 55 | 60 (15) | 18 to >89 | 36 (65.5%) | 19 (34.5%) |
| Smear + | 26 | 65 (15) | 29 to 86 | 19 (73.1%) | 7 (26.9%) |
| PCR+ & Smear + | 17 | 63 (16) | 28 to 85 | 13 (76.4%) | 4 (23.6%) |
| Supportive: IFA + | 115 | 56 (17) | 7 to >89 | 66 (57.4% | 49 (42.6%) |
| Ehrlichiosis 4 | 15 (<1%) | 69 (8) | 60 to 83 | 6 (40.0%) | 9 (60.0%) |
| All Diseases Combined | 2956 (100%) | 54 (20) | 1 to >89 | 1620 (54.8%) | 1336 (45.2%) |
| Tick-Borne Disease | Years | Relative Risk of Disease 1 | Cluster Size (% Population at Risk) | p-Value | Observed Cases in Cluster | Expected Cases in Cluster |
|---|---|---|---|---|---|---|
| HGA | 2000–2003 | 11.85 | 12.70% | <0.001 | 50 | 10.04 |
| 2004–2007 | 4.10 | 16.70% | <0.001 | 102 | 37.74 | |
| 2008–2011 | 3.30 | 27.68% | <0.001 | 542 | 268.78 | |
| 2012–2016 | 3.88 | 14.41% | <0.001 | 574 | 209.30 | |
| Full Period | 3.00 | 27.54% | <0.001 | 1454 | 751.34 | |
| Babesiosis 2 | 2000–2003 | - | - | - | - | - |
| 2004–2007 | 4.39 | 24.41% | 0.013 | 17 | 7.08 | |
| 2008–2011 | 4.33 | 35.44% | <0.001 | 57 | 28.71 | |
| 2012–2016 | 4.81 | 0.06% | <0.001 | 22 | 5.55 | |
| Full Period | 3.90 | 0.05% | <0.001 | 38 | 11.24 | |
| Combined Disease 3 | Full Period | 2.92 | 27.54% | <0.001 | 1554 | 814.14 |
| Tick-Borne Disease | Timeframe 1 | Scanning Window 2 | Relative Risk 3 | p Value | Observed Cases | Expected Cases |
|---|---|---|---|---|---|---|
| HGA | 05/2011–10/2012 | 10% | 4.85 | 0.001 | 898 | 250.48 |
| 05/2011–08/2014 | 20% | 5.07 | 0.001 | 1512 | 537.41 | |
| 06/2010–09/2014 | 30% | 5.51 | 0.001 | 1799 | 709.29 | |
| 06/2010–08/2016 | 40% | 6.61 | 0.001 | 2163 | 1000.92 | |
| 06/2010–08/2016 | 50% | 6.61 | 0.001 | 2163 | 1000.92 | |
| Babesiosis | 06/2011–08/2012 | 10% | 6.34 | 0.001 | 73 | 16.19 |
| 06/2011–08/2013 | 20% | 5.28 | 0.001 | 96 | 28.67 | |
| 06/2011–08/2013 | 30% | 5.28 | 0.001 | 96 | 28.67 | |
| 06/2011–08/2013 | 40% | 5.28 | 0.001 | 96 | 28.67 | |
| 06/2011–08/2013 | 50% | 5.28 | 0.001 | 96 | 28.67 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rau, A.; Munoz-Zanzi, C.; Schotthoefer, A.M.; Oliver, J.D.; Berman, J.D. Spatio-Temporal Dynamics of Tick-Borne Diseases in North-Central Wisconsin from 2000–2016. Int. J. Environ. Res. Public Health 2020, 17, 5105. https://doi.org/10.3390/ijerph17145105
Rau A, Munoz-Zanzi C, Schotthoefer AM, Oliver JD, Berman JD. Spatio-Temporal Dynamics of Tick-Borne Diseases in North-Central Wisconsin from 2000–2016. International Journal of Environmental Research and Public Health. 2020; 17(14):5105. https://doi.org/10.3390/ijerph17145105
Chicago/Turabian StyleRau, Austin, Claudia Munoz-Zanzi, Anna M. Schotthoefer, Jonathan D. Oliver, and Jesse D. Berman. 2020. "Spatio-Temporal Dynamics of Tick-Borne Diseases in North-Central Wisconsin from 2000–2016" International Journal of Environmental Research and Public Health 17, no. 14: 5105. https://doi.org/10.3390/ijerph17145105





