Rhizosphere Soil Microbial Properties on Tetraena mongolica in the Arid and Semi-Arid Regions, China
Abstract
:1. Introduction
2. Materials and Methods
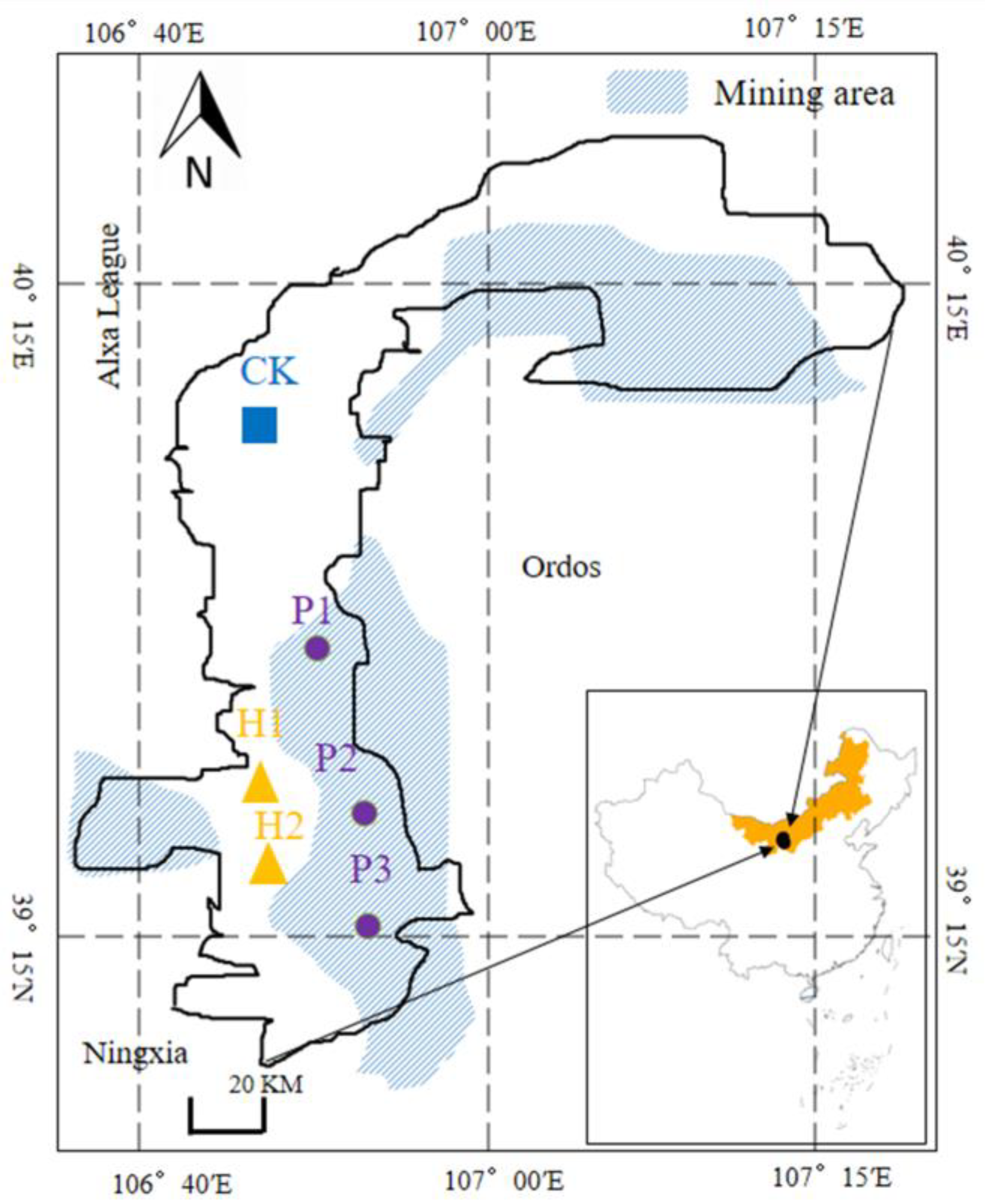
2.1. Study Area
2.2. Analysis of the Properties of Soil Samples
2.3. Soil Microbial Community Analysis
2.4. Data Analysis
3. Results
3.1. Effect of Open Pit Mining on Plant Community Structure
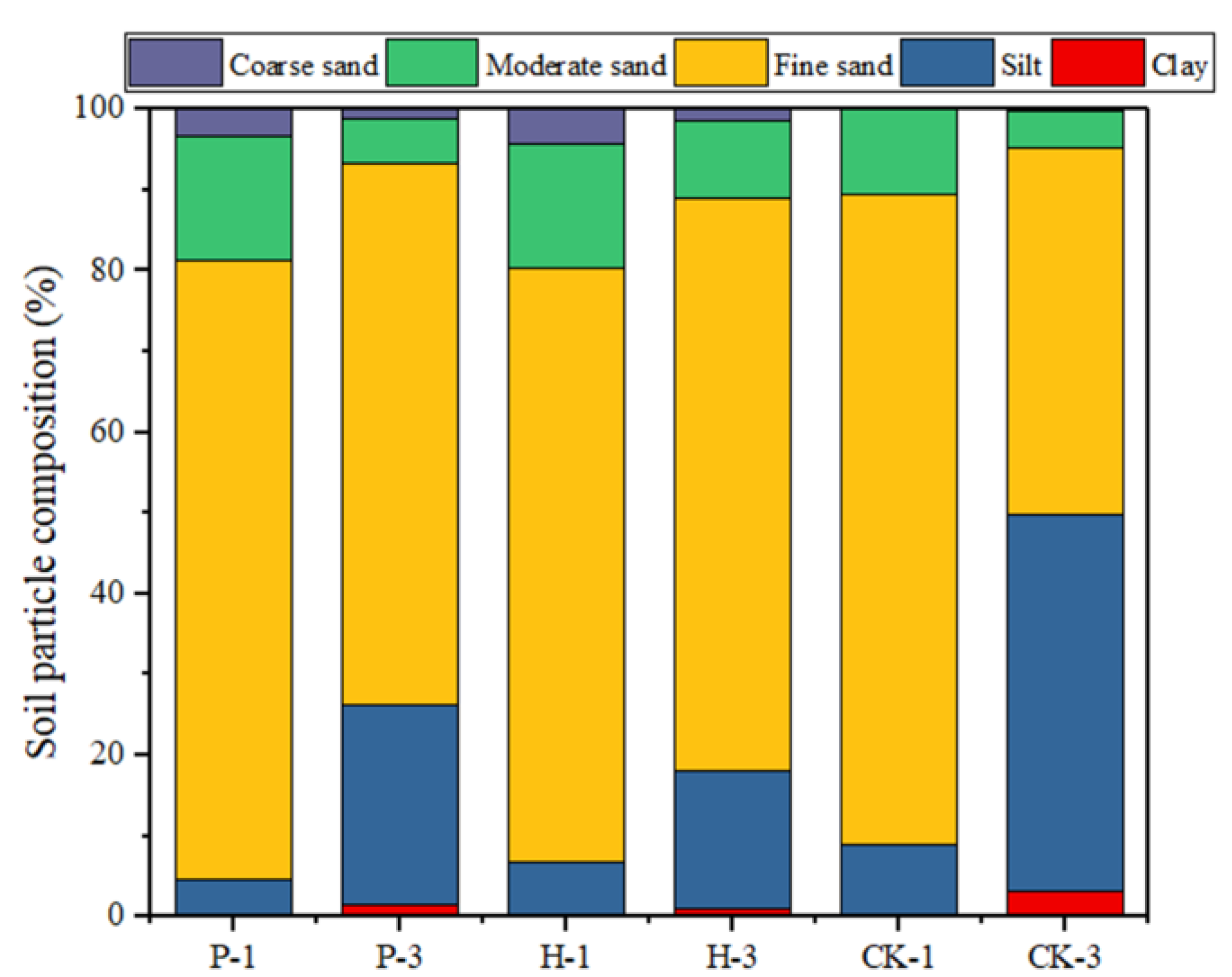
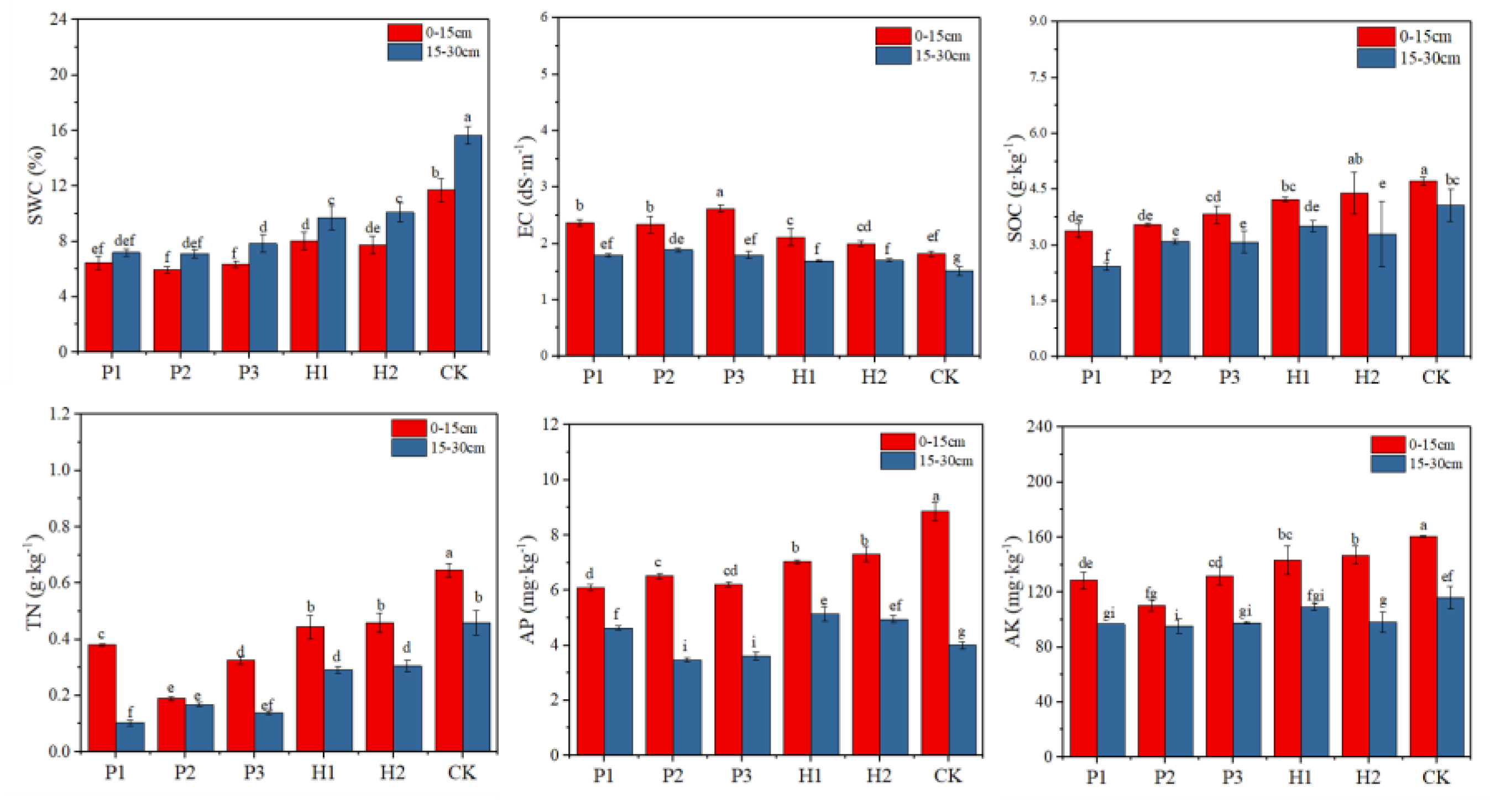
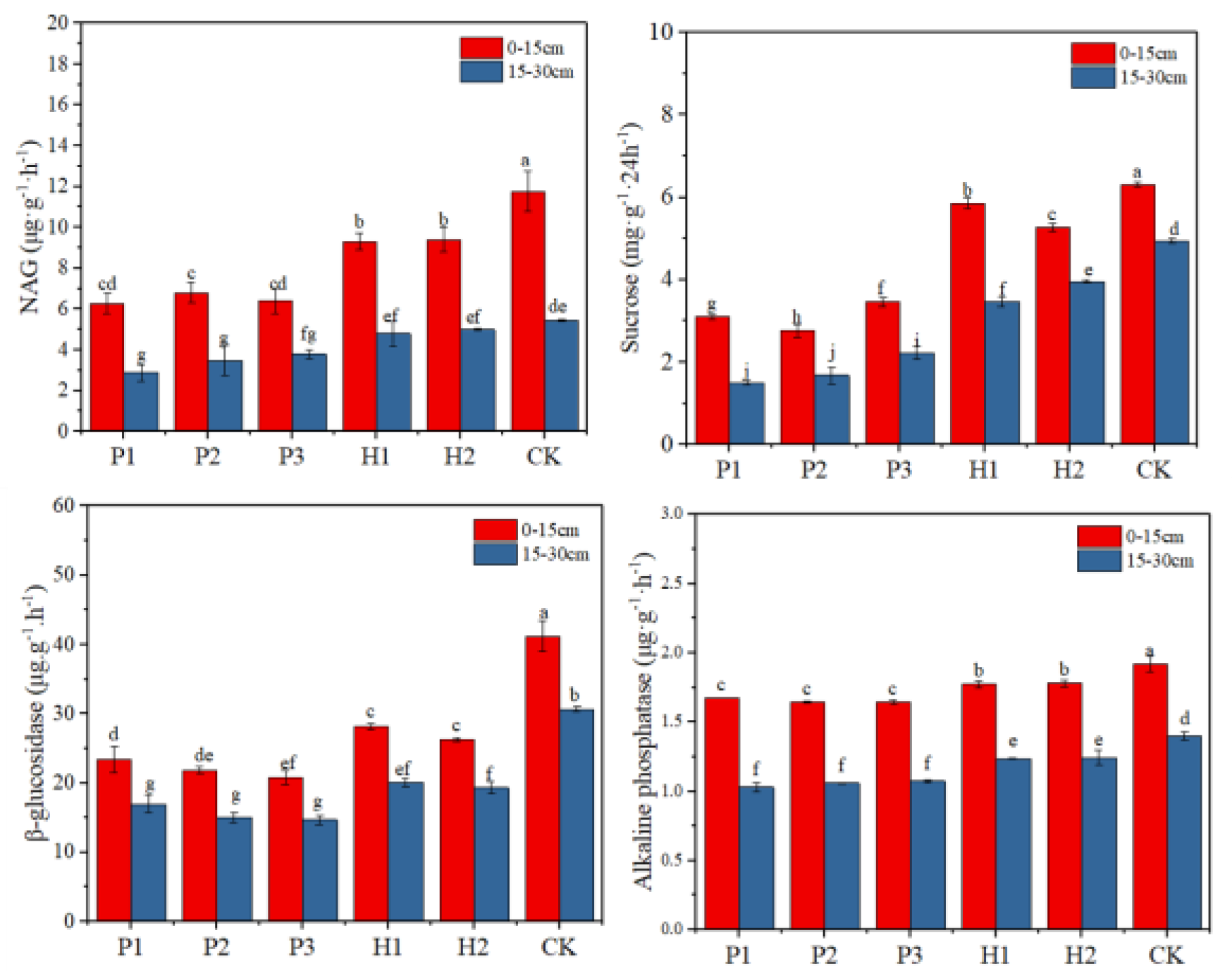
3.2. Effect of Open Pit Mining on Soil Quality
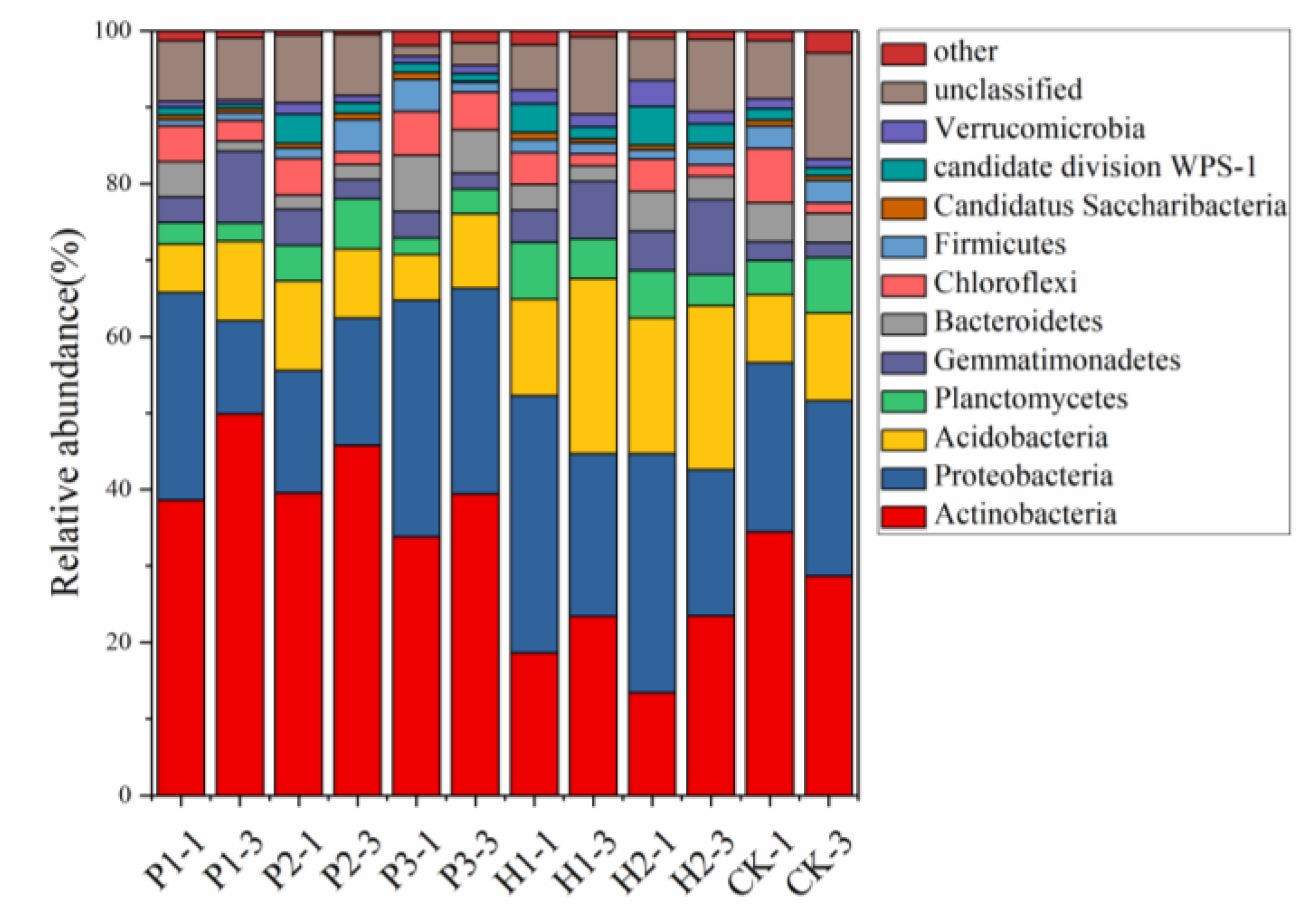
3.3. Effect of Open Pit Mining on Bacterial Community Structure and Composition
3.4. Response of Bacterial Community to Soil Environmental Factors
4. Discussion
4.1. Open Pit Mining Reduced Soil Nutrient and Enzyme Activity
4.2. The Soil Bacterial Community Shift due to Open Pit Mining
4.3. Response of the Soil Bacterial Community to Changes in Soil Properties
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ma, X.; Chang, J.; Li, Z.; Zhai, W.; Yu, X.; Feng, Y. The complete chloroplast genome of Tetraena mongolica (Zygophyllaceae), an endangered shrub endemic to China. Mitochondrial DNA Part B 2019, 4, 1030–1031. [Google Scholar] [CrossRef]
- Zhen, J.H.; Yu, S.; Zhao, M.; Liu, G.H. Habitat suitability assessment of endangered plant Tetraena mongolica Maxim. Sci. Cold Arid Reg. 2011, 3, 149–160. [Google Scholar] [CrossRef]
- Shan, Q. The impact of anthropogenic disturbance on landscape of Tetraena mongolica shrub in the Wuhai City. Acta Ecol. Sin. 2014, 34, 6346–6354. [Google Scholar] [CrossRef]
- Wang, W.F.; Hao, W.D.; Bian, Z.F.; Lei, S.G.; Wang, X.S.; Sang, S.X.; Xu, S.C. Effect of coal mining activities on the environment of Tetraena mongolica in Wuhai, Inner Mongolia, China—A geochemical perspective. Int. J. Coal Geol. 2014, 132, 94–102. [Google Scholar] [CrossRef]
- Rai, A.K.; Paul, B.; Singh, G. Assessment of Top Soil Quality in The Vicinity of Subsided Area in the South Eastern Part of Jharia Coalfield, Jharkhand, India. Rep. Opin. 2009, 12, 18. [Google Scholar] [CrossRef]
- Pandey, B.; Mukherjee, A.; Agrawal, M.; Singh, S. Assessment of Seasonal and Site-Specific Variations in Soil Physical, Chemical and Biological Properties around Opencast Coal Mines. Pedosphere 2019, 29, 642–655. [Google Scholar] [CrossRef]
- Li, P.F.; Zhang, X.C.; Hao, M.D.; Cui, Y.X.; Zhu, S.L.; Zhang, Y.J. Effects of Vegetation Restoration on Soil Bacterial Communities, Enzyme Activities, and Nutrients of Reconstructed Soil in a Mining Area on the Loess Plateau, China. Sustainability 2019, 11, 2295. [Google Scholar] [CrossRef] [Green Version]
- Lehman, R.M.; Acosta-Martinez, V.; Buyer, J.S.; Cambardella, C.A.; Collins, H.P. Soil biology for resilient, healthy soil. J. Soil Water Conserv. 2015, 70, 1–11. [Google Scholar] [CrossRef]
- Bhandari, K.B.; Longing, S.D.; West, C.P. iSoil Microbial Communities in Corn Fields Treated with Atoxigenic Aspergillus flavus. Soil Syst. 2020, 4, 35. [Google Scholar] [CrossRef]
- Dai, H.C.; Chen, Y.Q.; Yang, X.L.; Cui, J.X.; Sui, P. The effect of different organic materials amendment on soil bacteria communities in barren sandy loam soil. Environ. Sci. Pollut. Res. 2017, 24, 24019–24028. [Google Scholar] [CrossRef]
- Chen, C.R.; Condron, L.M.; Davis, M.R.; Sherlocka, R.R. Phosphorus dynamics in the rhizosphere of perennial ryegrass (Lolium perenne L.) and radiata pine (Pinus radiata D. Don.). Soil Biol. Biochem. 2002, 34, 487–499. [Google Scholar] [CrossRef]
- Beier, B.A.; Chase, M.W.; Thulin, M. Phylogenetic relationships and taxonomy of subfamily Zygophylloideae (Zygophyllaceae) based on molecular and morphological data. Plant Syst. Evol. 2003, 240, 11–39. [Google Scholar] [CrossRef]
- Ge, X.J.; Yu, Y.; Zhao, N.X.; Chen, H.S.; Qi, W.Q. Genetic variation in the endangered Inner Mongolia endemic shrub Tetraena mongolica Maxim. (Zygophyllaceae). Biol. Conserv. 2003, 111, 427–434. [Google Scholar] [CrossRef]
- Wang, G. The Western Ordos Plateau as a Biodiversity Center of Relic Shrubs in Arid Areas of China. Biodivers. Conserv. 2005, 14, 3187–3200. [Google Scholar] [CrossRef]
- Garcia, C.; Roldan, A.; Hernandez, T. Ability of Different Plant Species to Promote Microbiological Processes in Semiarid Soil. Geoderma 2005, 124, 193–202. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Zhang, C.; Niu, S.; Yang, H.; Yu, G. Contrasting responses of phosphatase kinetic parameters to nitrogen and phosphorus additions in forest soils. Funct. Ecol. 2018, 32, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Bao, S. Soil and Agricultural Chemistry Analysis. In Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000; pp. 70–111. [Google Scholar]
- Bowman, R.A.; Cole, C.V. An Exploratory Method for Fractionation of Organic Phosphorus From Grassland Soils. Soil Sci. 1978, 125, 95–101. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of pnitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Yang, D.H.; Zhang, Y.Y.; Du, P.C.; Li, X.; Wang, H.Y.; Han, N.; Chen, C.; Gao, Z.C. Rapid Identification of Bacterial Species Associated with Bronchiectasis via Metagenomic Approach. Biomed. Environ. Sci. 2014, 27, 898–901. [Google Scholar] [CrossRef]
- Duyusen, E.G.; Gorkem, A. Effect of sediment size on bioleaching of heavy metals from contaminated sediments of Izmir Inner Bay. J. Environ. Sci. 2013, 25, 1784–1794. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Maah M., J.; Yusoff, I. Chemical Speciation and Potential Mobility of Heavy Metals in the Soil of Former Tin Mining Catchment. Sci. World J. 2012, 2012, 125608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acosta-Martinez, V.; Amanda, C.; Jane, J. Simultaneous determination of multiple soil enzyme activities for soil health-biogeochemical indices. Appl. Soil Ecol. 2018, 126, 121–128. [Google Scholar] [CrossRef]
- Bhandari, K.B.; West, C.P.; Acosta-Martinez, V.; Cotton, J.; Cano, A. Soil health indicators as affected by diverse forage species and mixtures in semi-arid pastures. Appl. Soil Ecol. 2018, 132, 179–186. [Google Scholar] [CrossRef]
- Kang, Y.H.; Liu, S.H.; Wan, S.Q.; Wang, R.S. Assessment of soil enzyme activities of saline–sodic soil under drip irrigation in the Songnen plain. Paddy Water Environ. 2013, 11, 87–95. [Google Scholar] [CrossRef]
- Yang, F.; Yang, X.F.; Li, Z.; Du, C.Y.; Wang, J.F.; Li, S. Overexpression and characterization of a glucose-tolerant β-glucosidase from T. aotearoense with high specific activity for cellobiose. Appl. Microbiol. Biotechnol. 2015, 99, 8903–8915. [Google Scholar] [CrossRef]
- García-Ruiz, R.; Ochoa, V.; Belén Hinojosa, M.; Carreira, J.A. Suitability of enzyme activities for the monitoring of soil quality improvement in organic agricultural systems. Soil Biol. Biochem. 2008, 40, 2137–2145. [Google Scholar] [CrossRef]
- Wan, Z.M.; Wu, J.G. Study progress on factors affecting soil enzyme activity. J. Northwest Sci-Tech Univ. Agric. For. 2005, 33, 87–91. [Google Scholar] [CrossRef]
- Shu, Y.Y.; Huang, J.S.; Zhao, G.J.; Bao, W.K.; Li, G.Q.; Pang, X.Y. Effects of afforestation with different tree species on soil enzyme activities and nutrient content in eastern Qinghai-Tibetan Plateau, China. Acta Ecol. Sin. 2016, 36, 394–402. [Google Scholar] [CrossRef]
- Xu, H.S.; Ren, Y.F.; Zhao, T.Q.; He, Y.X. Research on the Woodland Soil Enzyme Activities in Coal Mining Subsidence Area. In Proceedings of the International Conference on Bioinformatics & Biomedical Engineering, Shanghai, China, 16–18 May 2008. [Google Scholar] [CrossRef]
- Janssen, P.H. Identifying the dominant soil bacterial taxa in libraries of 16S rRNA and 16S rRNA genes. Appl. Environ. Microbiol. 2006, 72, 1719–1728. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.Y.; Chen, L.Q.; Wen, H.Y. Changes in the composition and diversity of bacterial communities 13 years after soil reclamation of abandoned mine land in eastern China. Ecol. Res. 2014, 30, 357–366. [Google Scholar] [CrossRef]
- Liu, J.; Lin, H.; Dong, Y.B.; Li, B. Elucidating the biodegradation mechanism of tributyl phosphate (TBP) by Sphingomonas sp. isolated from TBP-contaminated mine tailings. Environ. Pollut. 2019, 250, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, F.; Huang, Y.L.; Zhou, M.; Gao, J.L.; Yan, T.Z.; Sheng, H.M.; An, L.Z. Sphingomonas sp. Cra20 Increases Plant Growth Rate and Alters Rhizosphere Microbial Community Structure of Arabidopsis thaliana Under Drought Stress. Front. Microbiol. 2019, 10, 1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.J.; Zheng, S.X.; Cao, C.G.; Li, C.F. Tillage practices and straw-returning methods affect topsoil bacterial community and organic C under a rice-wheat cropping system in central China. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Wang, Y.H.; Yu, Z.H.; Li, Y.S.; Wang, G.H.; Liu, J.J.; Liu, J.D.; Liu, X.B.; Jin, J. Microbial association with the dynamics of particulate organic carbon in response to the amendment of elevated CO2-derived wheat residue into a Mollisol. Sci. Total Environ. 2017, 607, 972–981. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, J.; Peng, C. Shift of Soil Polycyclic Aromatic Hydrocarbons (PAHs) Dissipation Pattern and Microbial Community Composition due to Rhamnolipid Supplementation. Water Air Soil Pollut. 2019, 230, 107. [Google Scholar] [CrossRef]
- Juárez-Ramírez, C.; Galíndez-Mayer, J.; Ruiz-Ordaz, N.; Ramos-Monroy, O.; Santoyo-Tepole, F.; Poggi-Varaldo, H. Steady-state inhibition model for the biodegradation of sulfonated amines in a packed bed reactor. New Biotechnol. 2015, 32, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Takagi, K.; Kataoka, R.; Kiyota, H.; Iwasaki, A. Dissipation, dehalogenation, and denitration of chloroaromatic compounds by Nocardioides sp. strain PD653: Characterization of the substrate specificity. J. Pestic. Sci. 2019, 44, 171–176. [Google Scholar] [CrossRef]
- Sul, W.J.; Asuming-Brempong, S.; Wang, Q.; Tourlousse, D.M.; Penton, C.R. Tropical agricultural land management influences on soil microbial communities through its effect on soil organic carbon. Soil Biol. Biochem. 2013, 65, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Schumann, P.; De-Chao, Z.; Franca, L. Marmoricola silvestris sp. nov., a novel actinobacterium isolated from alpine forest soil. Int. J. Syst. Evol. Microbiol. 2018, 68, 1313–1318. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Barrett, A.J. Families of cysteine peptidases. Methods Enzym. 1994, 244, 461–486. [Google Scholar] [CrossRef]
- Hartman, W.H.; Ye, R.Z.; Horwath, W.R.; Tringe, S.G. A genomic perspective on stoichiometric regulation of soil carbon cycling. ISME J. 2017, 11, 2652–2665. [Google Scholar] [CrossRef]
- Hou, D.D.; Wang, K.; Liu, T.; Wang, H.X.; Lin, Z.; Qian, J.; Lu, L.L.; Tian, S.K. Unique Rhizosphere Micro-characteristics Facilitate Phytoextraction of Multiple Metals in Soil by the Hyperaccumulating Plant Sedum alfredii. Environ. Sci. Technol. 2017, 51, 5675–5684. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, K.B.; West, C.P.; Acosta-Martinez, V. Assessing the role of interseeding alfalfa into grass on improving pasture soil health in semi-arid Texas High Plains. Appl. Soil Ecol. 2020, 147, 103399. [Google Scholar] [CrossRef]
- Zeng, Q.C.; Dong, Y.H.; An, S.S. Bacterial Community Responses to Soils along a Latitudinal and Vegetation Gradient on the Loess Plateau, China. PLoS ONE 2016, 11, e0152894. [Google Scholar] [CrossRef]
- Gao, Y.C.; Wang, J.N.; Guo, S.H.; Hu, Y.L.; Li, T.T. Effects of salinization and crude oil contamination on soil bacterial community structure in the Yellow River Delta region, China. Appl. Soil Ecol. 2015, 86, 165–173. [Google Scholar] [CrossRef]
- Taylor, J.P.; Wilson, B.; Mills, M.S.; Burns, R.G. Comparison of microbial numbers and enzymatic activities in surface soils and subsoils using various techniques. Soil Biol. Biochem. 2002, 34, 387–401. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Dowd, S.E.; Sun, Y.; Wester, D.; Allen, V. Pyrosequencing analysis for characterization of soil bacterial populations as affected by an integrated livestock-cotton production system. Appl. Soil Ecol. 2010, 45, 13–25. [Google Scholar] [CrossRef]



| Sample Code | Vegetation Coverage (%) | Shannon- Index | Pielou’s Index | Simpson Index | Main Companion Species |
|---|---|---|---|---|---|
| P1 | 23.33 | 1.05 | 0.71 | 0.87 | Achnatherum splendens |
| P2 | 28.49 | 1.23 | 0.72 | 0.89 | Reaumuria soongarica |
| P3 | 21.29 | 1.18 | 0.69 | 0.90 | Achnatherum splendens |
| H1 | 46.79 | 1.35 | 0.67 | 0.86 | Zygophyllum xanthoxylon |
| H2 | 51.31 | 1.47 | 0.64 | 0.82 | Zygophyllum xanthoxylon |
| CK | 72.34 | 1.53 | 0.68 | 0.72 | Phragmites australis |
| Sample Code | Shannon | ACE | Chao1 | Coverage | Simpson |
|---|---|---|---|---|---|
| P1-1 | 6.69 | 8139.96 | 7828.36 | 0.95 | 0.06 |
| P1-3 | 5.93 | 7124.62 | 7228.36 | 0.95 | 0.09 |
| P2-1 | 6.87 | 8496.79 | 7847.48 | 0.95 | 0.07 |
| P2-3 | 5.84 | 7051.32 | 7025.39 | 0.95 | 0.10 |
| P3-1 | 6.82 | 8308.25 | 7956.83 | 0.96 | 0.07 |
| P3-3 | 5.75 | 7150.23 | 7081.62 | 0.97 | 0.09 |
| H1-1 | 7.27 | 10,527.34 | 9096.68 | 0.96 | 0.05 |
| H1-3 | 6.36 | 9488.99 | 8049.60 | 0.95 | 0.08 |
| H2-1 | 7.15 | 11,035.31 | 8847.98 | 0.96 | 0.05 |
| H2-3 | 6.56 | 9468.66 | 7766.70 | 0.95 | 0.07 |
| CK-1 | 7.43 | 12,458.37 | 10,510.84 | 0.98 | 0.02 |
| CK-3 | 6.71 | 10,703.70 | 9766.70 | 0.97 | 0.04 |
| Sphingomonas | Gp6 | Gemmatimonas | Nocardioides | Gaiella | Aciditerrimonas | Saccharibacteria | |
|---|---|---|---|---|---|---|---|
| EC | 0.135 | 0.463 | 0.643 * | −0.747 * | 0.365 | −0.201 | −0.764 ** |
| SWC | 0.351 | 0.785 * | 0.722 * | −0.678 * | 0.146 | −0.434 | −0.763 * |
| SOC | 0.576 | 0.414 | 0.347 | −0.820 ** | −0.106 | −0.321 | −0.797 ** |
| TN | 0.253 | 0.239 | 0.292 | −0.839 ** | 0.055 | 0.023 | −0.870 ** |
| AP | 0.602 * | −0.190 | −0.191 | −0.522 | −0.317 | −0.151 | −0.485 |
| AK | 0.526 | −0.112 | −0.054 | −0.581 * | −0.115 | −0.177 | −0.582 |
| Sand | −0.139 | −0.277 | −0.508 | 0.576 | −0.399 | 0.109 | 0.621 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, M.; Zhang, Y.; Chai, T. Rhizosphere Soil Microbial Properties on Tetraena mongolica in the Arid and Semi-Arid Regions, China. Int. J. Environ. Res. Public Health 2020, 17, 5142. https://doi.org/10.3390/ijerph17145142
Ruan M, Zhang Y, Chai T. Rhizosphere Soil Microbial Properties on Tetraena mongolica in the Arid and Semi-Arid Regions, China. International Journal of Environmental Research and Public Health. 2020; 17(14):5142. https://doi.org/10.3390/ijerph17145142
Chicago/Turabian StyleRuan, Mengying, Yuxiu Zhang, and Tuanyao Chai. 2020. "Rhizosphere Soil Microbial Properties on Tetraena mongolica in the Arid and Semi-Arid Regions, China" International Journal of Environmental Research and Public Health 17, no. 14: 5142. https://doi.org/10.3390/ijerph17145142
APA StyleRuan, M., Zhang, Y., & Chai, T. (2020). Rhizosphere Soil Microbial Properties on Tetraena mongolica in the Arid and Semi-Arid Regions, China. International Journal of Environmental Research and Public Health, 17(14), 5142. https://doi.org/10.3390/ijerph17145142




