Cross-Cultural Adaptation and Validation of the Arabic Version of Musculoskeletal Health Questionnaire (MSK-HQ-Ar)
Abstract
:1. Introduction
2. Methods
2.1. Study Design
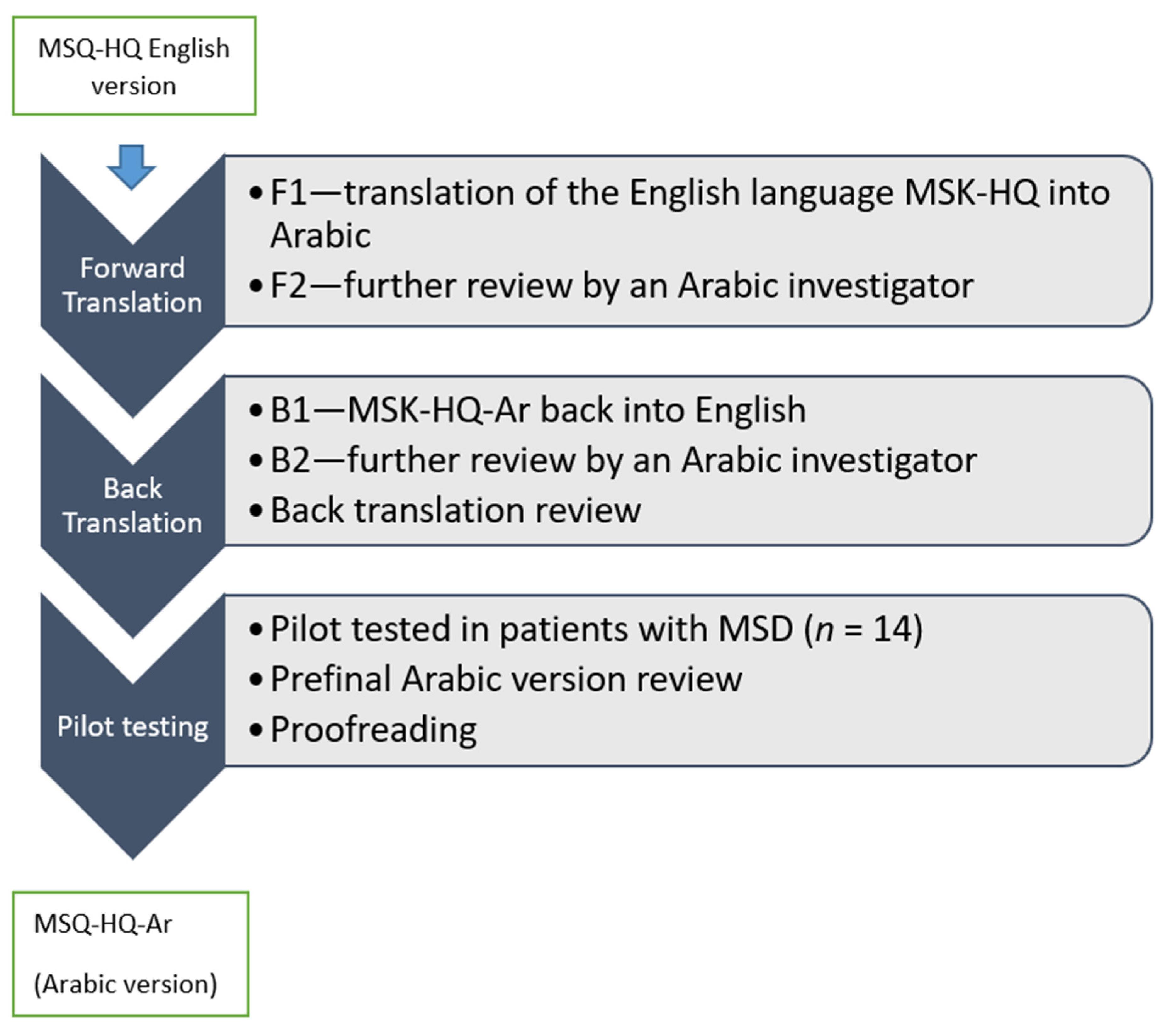
2.1.1. Phase 1: The Translation and Cross-Cultural Adaptation of the MSK-HQ-Ar
2.1.2. Pilot Testing
2.1.3. Phase 2: Testing the Psychometric Properties of the MSK-HQ-Ar
2.1.4. Data Collection
3. Instruments
4. Statistical Analysis
5. Results
5.1. Demographic Characteristics
5.2. MSK-HQ-Ar Scores
5.2.1. Floor and Ceiling Effect
5.2.2. Internal Consistency and Test-Retest Reliability
5.3. Construct Validity
6. Discussion
Study Limitations
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Woolf, A.D.; Erwin, J.; March, L. The need to address the burden of musculoskeletal conditions. Best Pract. Res. Clin. Rheumatol. 2012, 26, 183–224. [Google Scholar] [CrossRef] [PubMed]
- Osborne, A.; Blake, C.; McNamara, J.; Meredith, D.; Phelan, J.; Cunningham, C. Musculoskeletal disorders among Irish farmers. Occup Med. 2010, 60, 598–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menzel, N.N. Psychosocial factors in musculoskeletal disorders. Crit. Care Nurs. Clin. N. Am. 2007, 19, 145–153. [Google Scholar] [CrossRef]
- Hayes, M.; Cockrell, D.; Smith, D. A systematic review of musculoskeletal disorders among dental professionals. Int. J. Dent. Hyg. 2009, 7, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Björklund, L. The Bone and Joint Decade 2000-2010. Inaugural meeting 17 and 18 April 1998, Lund, Sweden. Acta Orthop. Scand. Suppl. 1998, 281, 67–80. [Google Scholar] [PubMed]
- Bevan, S. Economic impact of musculoskeletal disorders (MSDs) on work in Europe. Best Pr. Res. Clin. Rheumatol. 2015, 29, 356–373. [Google Scholar] [CrossRef] [PubMed]
- Steenstra, I.A.; Anema, J.R.; Bongers, P.M.; de Vet, H.C.; van Mechelen, W. Cost effectiveness of a multi-stage return to work program for workers on sick leave due to low back pain, design of a population based controlled trial [ISRCTN60233560]. BMC Musculoskelet. Disord. 2003, 4, 26. [Google Scholar] [CrossRef] [Green Version]
- Badley, E.M.; Rasooly, I.; Webster, G.K. Relative importance of musculoskeletal disorders as a cause of chronic health problems, disability, and health care utilization: Findings from the 1990 Ontario Health Survey. Rheumatology 1994, 21, 505–514. [Google Scholar]
- Abduljabbar, T.A. Musculoskeletal disorders among dentists in Saudi Arabia. PODJ 2008, 28, 135–144. [Google Scholar]
- Abdulmonem, A.; Hanan, A.; Elaf, A.; Haneen, T.; Jenan, A. The prevalence of musculoskeletal pain & its associated factors among female Saudi school teachers. PJMS 2014, 30, 1191. [Google Scholar]
- Alghadir, A.; Anwer, S. Prevalence of musculoskeletal pain in construction workers in Saudi Arabia. Sci. World J. 2015, 2015, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Memish, Z.A.; Jaber, S.; Mokdad, A.H.; AlMazroa, M.A.; Murray, C.J.; Al Rabeeah, A.A.; Collaborators, T.S.B.O.D. Burden of disease, injuries, and risk factors in the Kingdom of Saudi Arabia, 1990–2010. Prev. Chronic Dis. 2014, 11, 11. [Google Scholar] [CrossRef] [Green Version]
- Shariat, A.; Cleland, J.A.; Danaee, M.; Kargarfard, M.; Sangelaji, B.; Tamrin, S.B.M. Effects of stretching exercise training and ergonomic modifications on musculoskeletal discomforts of office workers: A randomized controlled trial. Braz. J. Phys. Ther. 2018, 22, 144–153. [Google Scholar] [CrossRef]
- Jette, A.M.; Delitto, A. Physical therapy treatment choices for musculoskeletal impairments. Phys. Ther. 1997, 77, 145–154. [Google Scholar] [CrossRef]
- Stokes, E.K.; O’Neill, D. Use of outcome measures in physiotherapy practice in Ireland from 1998 to 2003 and comparison to Canadian trends. Physiother. Can. 2008, 60, 109–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, S.; Haywood, K.; Fitzpatrick, R. Impact of patient-reported outcome measures on routine practice: A structured review. J. Evaluation Clin. Pr. 2006, 12, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.C.; Kang, S.; Benedetto, E.; Myers, H.; Blackburn, S.; Smith, S.; Dunn, K.M.; Hay, E.; Rees, J.; Beard, D.; et al. Development and initial cohort validation of the Arthritis Research UK Musculoskeletal Health Questionnaire (MSK-HQ) for use across musculoskeletal care pathways. BMJ Open 2016, 6, e012331. [Google Scholar] [CrossRef] [PubMed]
- Serra-Sutton, V.; Herdman, M.; Rajmil, L.; Santed, R.; Ferrer, M.; Siméoni, M.C.; Auquier, P. Cross-cultural adaptation to Spanish of the Vécu et Santé Perçue de l’Adolescent (VSP-A): A generic measure of the quality of life of adolescents. Rev. Esp. Salud Publica 2002, 76, 701–712. [Google Scholar] [CrossRef] [Green Version]
- Beaton, D.E.; Bombardier, C.; Guillemin, F.; Ferraz, M.B. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine 2000, 25, 3186–3191. [Google Scholar] [CrossRef] [Green Version]
- Wild, D.; Grove, A.; Martin, M.; Eremenco, S.; McElroy, S.; Verjee-Lorenz, A.; Erikson, P. Principles of Good Practice for the Translation and Cultural Adaptation Process for Patient-Reported Outcomes (PRO) Measures: Report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health 2005, 8, 94–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoukri, M.M.; Asyali, M.; Donner, A. Sample size requirements for the design of reliability study: Review and new results. Stat. Methods Med. Res. 2004, 13, 251–271. [Google Scholar] [CrossRef]
- Gerlinger, C.; Bamber, L.; Leverkus, F.; Schwenke, C.; Haberland, C.; Schmidt, G.; Endrikat, J. Comparing the EQ-5D-5L utility index based on value sets of different countries: Impact on the interpretation of clinical study results. BMC Res. Notes 2019, 12, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villarrasa-Sapiña, I.; García-Massó, X.; Serra-Añó, P.; Garcia-Lucerga, C.; Gonzalez, L.-M.; Lurbe, E. Differences in intermittent postural control between normal-weight and obese children. Gait Posture 2016, 49, 1–6. [Google Scholar] [CrossRef]
- Bekairy, A.M.; Bustami, R.T.; Almotairi, M.; Jarab, A.; Katheri, A.M.; Aldebasi, T.M.; AbuRuz, S. Validity and reliability of the Arabic version of the the EuroQOL (EQ-5D). A study from Saudi Arabia. Int. J. Health Sci. 2018, 12, 16–20. [Google Scholar]
- Kamper, S.J.; Maher, C.G.; Mackay, G. Global Rating of Change Scales: A Review of Strengths and Weaknesses and Considerations for Design. J. Man. Manip. Ther. 2009, 17, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Galeoto, G.; Piepoli, V.; Ciccone, E.; Mollica, R.; Federici, C.; Magnifica, F.; Servadio, A. Musculoskeletal Health Questionnaire: Translation, cultural adaptation and validation of the Italian version (MSK-HQ-I). Muscle Ligaments Tendons J. 2019, 9, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Terwee, C.B.; Bot, S.D.; de Boer, M.R.; van der Windt, D.A.; Knol, D.L.; Dekker, J.; Bouter, L.M.; De Vet, H.C.W. Quality criteria were proposed for measurement properties of health status questionnaires. J. Clin. Epidemiology 2007, 60, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Norton, S.; Ellis, B.; Santana Suárez, B.; Schwank, S.; Fitzpatrick, R.; Price, A.; Galloway, J. Validation of the Musculoskeletal Health Questionnaire in inflammatory arthritis: A psychometric evaluation. Rheumatology 2019, 58, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Alnahdi, A.H.; Alrashid, G.I.; Alkhaldi, H.A.; Aldali, A.Z. Cross-cultural adaptation, validity and reliability of the Arabic version of the Lower Extremity Functional Scale. Disabil. Rehabil. 2015, 38, 897–904. [Google Scholar] [CrossRef]
| Age (Year) | n (%) |
|---|---|
| 18–29 | 29 (19.5) |
| 30–39 | 29 (19.5) |
| 40–49 | 42 (28.1) |
| 50–59 | 30 (20.1) |
| 60–65 | 19 (12.8) |
| Mean (SD) | 43.63 (13.69) |
| Gender | |
| Male | 84 (56.4) |
| Female | 64 (43.6) |
| Level of Education | |
| Primary | 26 (17.4) |
| Intermediate | 11 (7.4) |
| Secondary | 37 (24.8) |
| University graduate | 61 (40.9) |
| Postgraduate | 8 (5.4) |
| Employment Status | |
| Student | 19 (12.8) |
| Employee | 60 (40.3) |
| Not working | 54 (36.2) |
| Retired | 16 (10.7) |
| Marital Status | |
| Married | 109 (77.2) |
| Single | 34 (22.8) |
| BMI | |
| Below 18.5 | 8 (5.3) |
| 18.6–24.9 | 29 (19.4) |
| 25–29.9 | 54 (36.3) |
| ≤30 | 58 (39) |
| Mean (SD) | 28.59 (5.601) |
| MSD Region | n (%) |
|---|---|
| Spine | 54 (36.2) |
| Upper extremity | 27 (18.2) |
| Lower extremity | 68 (45.6) |
| Pain Site | |
| Cervical | 8 (5.4) |
| Lumber | 45 (30.2) |
| Shoulder | 25 (16.8) |
| Knee | 62 (41.6) |
| Other (i.e., elbow, wrist, hip and ankle) | 9 (6) |
| Clinical Variables | Mean (±SD) |
| NRS | 5.52 (2.37) |
| MSK-HQ-Ar total score | 32.29 (10.42) |
| EQ-5D-5L utility score | 0.21 (0.22) |
| Descriptive Statistics of the MSK-HQ-Ar Scores | Statistics | SE | |
|---|---|---|---|
| Mean | 32.29 | 0.854 | |
| Median | 33.00 | ||
| Standard Deviation | 10.428 | ||
| Range | 48 | ||
| Skewness | −0.122 | 0.199 | |
| Kurtosis | −0.189 | 0.395 | |
| Normality test | df | Sig. | |
| Shapiro-Wilk | 0.998 | 149 | 0.226 |
| Item | Mean | SD (±) | Corrected Item to Total Correlation | Cronbach’s Alpha if Item Deleted |
|---|---|---|---|---|
| 1. Pain/stiffness during the day | 2.17 | 0.98 | 0.53 | 0.87 |
| 2. Pain/stiffness during the night | 2.15 | 1.16 | 0.45 | 0.87 |
| 3. Walking | 2.43 | 1.23 | 0.53 | 0.87 |
| 4. Washing/Dressing | 3.07 | 1.25 | 0.50 | 0.87 |
| 5. Physical activity levels. | 2.14 | 1.35 | 0.57 | 0.87 |
| 6. Work/daily routine | 2.01 | 1.07 | 0.67 | 0.86 |
| 7. Social activities and hobbies | 2.28 | 1.23 | 0.63 | 0.86 |
| 8. Needing help | 2.42 | 1.19 | 0.55 | 0.87 |
| 9. Sleep | 1.99 | 1.23 | 0.62 | 0.86 |
| 10. Fatigue or low energy | 2.28 | 1.01 | 0.62 | 0.87 |
| 11. Emotional well-being | 2.46 | 1.21 | 0.53 | 0.87 |
| 12. Understanding of your condition and any current treatment | 2.50 | 1.33 | 0.41 | 0.88 |
| 13. Confidence in being able to manage your symptoms | 2.46 | 1.23 | 0.45 | 0.87 |
| 14. Overall impact | 1.87 | 1.06 | 0.64 | 0.86 |
| Mean (SD) | ICC (95%CI) | SEM | MDC95 | ||
|---|---|---|---|---|---|
| Test Scores | Retest Scores | ||||
| MSK-HQ-Ar total scores | 32.29 (10.4) | 35.5 (10.4) | 0.94 (0.92–0.95) | 2.46 | 6.83 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algarni, F.S.; Alotaibi, A.N.; Altowaijri, A.M.; Al-Sobayel, H. Cross-Cultural Adaptation and Validation of the Arabic Version of Musculoskeletal Health Questionnaire (MSK-HQ-Ar). Int. J. Environ. Res. Public Health 2020, 17, 5168. https://doi.org/10.3390/ijerph17145168
Algarni FS, Alotaibi AN, Altowaijri AM, Al-Sobayel H. Cross-Cultural Adaptation and Validation of the Arabic Version of Musculoskeletal Health Questionnaire (MSK-HQ-Ar). International Journal of Environmental Research and Public Health. 2020; 17(14):5168. https://doi.org/10.3390/ijerph17145168
Chicago/Turabian StyleAlgarni, Fahad Saad, Abdulmajeed Nasser Alotaibi, Abdulrahman Mohammed Altowaijri, and Hana Al-Sobayel. 2020. "Cross-Cultural Adaptation and Validation of the Arabic Version of Musculoskeletal Health Questionnaire (MSK-HQ-Ar)" International Journal of Environmental Research and Public Health 17, no. 14: 5168. https://doi.org/10.3390/ijerph17145168






