A Review on the Use of Impedimetric Sensors for the Inspection of Food Quality
Abstract
:1. Introduction
2. Type of Impedimetric Sensors Utilized for the Inspection of Food Quality
2.1. Impedimetric Sensors Based on Graphene and Other Nanomaterials
2.1.1. Graphene-Based Impedimetric Sensors
2.1.2. Other Nanoparticle-Based Impedimetric Sensors
2.2. Impedimetric Sensors Based on Electronic Nose and Other Smart Sensing Circuits
2.2.1. Electronic Noses
2.2.2. Impedimetric Sensors Based on Smart-Sensing Circuits
3. Current Challenges and Future Opportunities
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sze, S.M. Semiconductor Sensors; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Mamishev, A.V.; Sundara-Rajan, K.; Yang, F.; Du, Y.; Zahn, M. Interdigital sensors and transducers. Proc. IEEE 2004, 92, 808–845. [Google Scholar] [CrossRef] [Green Version]
- Pawase, R.; Futane, N. MEMS Seismic Sensor with FPAA-Based Interface Circuit for Frequency-Drift Compensation Using ANN. Int. J. Smart Sens. Intell. Syst. 2018, 11, 1–7. [Google Scholar]
- Errachid, A.; Ivorra, A.; Aguilo, J.; Villa, R.; Zine, N.; Bausells, J. New technology for multi-sensor silicon needles for biomedical applications. Sens. Actuators B Chem. 2001, 78, 279–284. [Google Scholar] [CrossRef]
- Katragadda, R.B.; Xu, Y. A novel intelligent textile technology based on silicon flexible skins. Sens. Actuators A Phys. 2008, 143, 169–174. [Google Scholar] [CrossRef]
- Harraz, F.A. Porous silicon chemical sensors and biosensors: A review. Sens. Actuators B Chem. 2014, 202, 897–912. [Google Scholar] [CrossRef]
- Nag, A.; Zia, A.I.; Li, X.; Mukhopadhyay, S.C.; Kosel, J. Novel Sensing Approach for LPG Leakage Detection: Part I—Operating Mechanism and Preliminary Results. IEEE Sens. J. 2016, 16, 996–1003. [Google Scholar] [CrossRef] [Green Version]
- Han, T.; Nag, A.; Mukhopadhyay, S.C.; Xu, Y. Carbon Nanotubes and its gas-sensing applications: A Review. Sens. Actuators A Phys. 2019, 291, 107–143. [Google Scholar] [CrossRef]
- Russo, S.; Ranzani, T.; Liu, H.; Nefti-Meziani, S.; Althoefer, K.; Menciassi, A. Soft and stretchable sensor using biocompatible electrodes and liquid for medical applications. Soft Robot. 2015, 2, 146–154. [Google Scholar] [CrossRef] [Green Version]
- Nag, A.; Mukhopadhyay, S.C.; Kosel, J. Sensing System for Salinity Testing Using Laser-induced Graphene Sensors. Sens. Actuators A Phys. 2017, 264, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Alahi, M.E.E.; Xie, L.; Mukhopadhyay, S.; Burkitt, L. A temperature compensated smart nitrate-sensor for agricultural industry. IEEE Trans. Ind. Electron. 2017, 64, 7333–7341. [Google Scholar] [CrossRef]
- Hannon, A.; Lu, Y.; Li, J.; Meyyappan, M. A sensor array for the detection and discrimination of methane and other environmental pollutant gases. Sensors 2016, 16, 1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passian, A.; Siopsis, G. Strong quantum squeezing near the pull-in instability of a nonlinear beam. Phys. Rev. A 2016, 94, 023812. [Google Scholar] [CrossRef] [Green Version]
- Passian, A.; Siopsis, G. Quantum state atomic force microscopy. Phys. Rev. A 2017, 95, 043812. [Google Scholar] [CrossRef] [Green Version]
- Disadvantages of Silicon Sensors. Available online: https://www.printedelectronicsworld.com/articles/52/problems-with-silicon-chips (accessed on 17 July 2020).
- Islam, T.; Mukhopadhyay, S. Linearization of the sensors characteristics: A review. Int. J. Smart Sens. Intell. Syst. 2019, 12, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; He, K.; Zhang, Z.; Zhou, A.; Duan, H. Real-time electrochemical detection of hydrogen peroxide secretion in live cells by Pt nanoparticles decorated graphene–carbon nanotube hybrid paper electrode. Biosens. Bioelectron. 2015, 68, 358–364. [Google Scholar] [CrossRef]
- Neitzert, H.C.; Vertuccio, L.; Sorrentino, A. Epoxy/MWCNT composite as temperature sensor and electrical heating element. IEEE Trans. Nanotechnol. 2010, 10, 688–693. [Google Scholar] [CrossRef]
- Nag, A.; Feng, S.; Mukhopadhyay, S.; Kosel, J.; Inglis, D. 3D printed mould-based graphite/PDMS sensor for low-force applications. Sens. Actuators A Phys. 2018, 280, 525–534. [Google Scholar] [CrossRef]
- Faridbod, F.; Gupta, V.K.; Zamani, H.A. Electrochemical sensors and biosensors. Int. J. Electrochem. 2011, 2011. [Google Scholar] [CrossRef] [Green Version]
- The Pros and Cons of Electrochemical Sensors. Available online: https://www.safetyandhealthmagazine.com/articles/the-pros-and-cons-of-electrochemical-sensors-2 (accessed on 5 July 2020).
- Zia, A.I.; Rahman, M.S.A.; Mukhopadhyay, S.C.; Yu, P.-L.; Al-Bahadly, I.; Gooneratne, C.P.; Kosel, J.; Liao, T.-S. Technique for rapid detection of phthalates in water and beverages. J. Food Eng. 2013, 116, 515–523. [Google Scholar] [CrossRef]
- Rahman, M.S.A.; Mukhopadhyay, S.C.; Yu, P.-L.; Goicoechea, J.; Matias, I.R.; Gooneratne, C.P.; Kosel, J. Detection of bacterial endotoxin in food: New planar interdigital sensors based approach. J. Food Eng. 2013, 114, 346–360. [Google Scholar] [CrossRef]
- Farahi, R.H.; Passian, A.; Tetard, L.; Thundat, T. Critical issues in sensor science to aid food and water safety. ACS Nano 2012, 6, 4548–4556. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Soper, S.A.; Pingle, M.R.; Barany, F.; Davis, L.M. Ligase detection reaction generation of reverse molecular beacons for near real-time analysis of bacterial pathogens using single-pair fluorescence resonance energy transfer and a cyclic olefin copolymer microfluidic chip. Anal. Chem. 2010, 82, 9727–9735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, N.; Yang, X.; Xie, D.; Wu, Y.; Wen, W. A disposable organophosphorus pesticides enzyme biosensor based on magnetic composite nano-particles modified screen printed carbon electrode. Sensors 2010, 10, 625–638. [Google Scholar] [CrossRef] [Green Version]
- Nag, A.; Mukhopadhyay, S.C. Fabrication and implementation of printed sensors for taste sensing applications. Sens. Actuators A Phys. 2018, 269, 53–61. [Google Scholar] [CrossRef]
- Prodhan, M.; Alam, S.; Uddin, M.J. Analytical methods in measuring pesticides in foods. In Pesticide Residue in Foods; Springer: Cham, Switzerland, 2017; pp. 135–145. [Google Scholar]
- Mohd, S.A.; Mukhopadhyay, S.; Yu, P. Modelling and fabrication of optimum structure of novel interdigital sensors for food inspection. Int. J. Numer. Model. Electron. Netw. Devices Fields 2012, 25, 64–81. [Google Scholar] [CrossRef]
- Rahman, M.S.A.; Mukhopadhyay, S.C.; Yu, P.-L. Novel Sensors for Food Inspection: Modelling, Fabrication and Experimentation; Springer: Basel, Switzerland, 2014. [Google Scholar]
- Vanegas, D.; Patiño, L.; Mendez, C.; Oliveira, D.; Torres, A.; Gomes, C.; McLamore, E. Laser Scribed Graphene Biosensor for Detection of Biogenic Amines in Food Samples Using Locally Sourced Materials. Biosensors 2018, 8, 42. [Google Scholar] [CrossRef] [Green Version]
- Bunney, J.; Williamson, S.; Atkin, D.; Jeanneret, M.; Cozzolino, D.; Chapman, J. The use of electrochemical biosensors in food analysis. Curr. Res. Nutr. Food Sci. J. 2017, 5, 183–195. [Google Scholar] [CrossRef]
- Gliszczyńska-Świgło, A.; Chmielewski, J. Electronic nose as a tool for monitoring the authenticity of food. A review. Food Anal. Methods 2017, 10, 1800–1816. [Google Scholar] [CrossRef] [Green Version]
- Abbas, O.; Zadravec, M.; Baeten, V.; Mikuš, T.; Lešić, T.; Vulić, A.; Prpić, J.; Jemeršić, L.; Pleadin, J. Analytical methods used for the authentication of food of animal origin. Food Chem. 2018, 246, 6–17. [Google Scholar] [CrossRef]
- Wright, C. Analytical methods for monitoring contaminants in food—An industrial perspective. J. Chromatogr. A 2009, 1216, 316–319. [Google Scholar] [CrossRef]
- Dhand, V.; Rhee, K.Y.; Ju Kim, H.; Ho Jung, D. A comprehensive review of graphene nanocomposites: Research status and trends. J. Nanomater. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, J.; Rodriquez, D.; Urbina, A.D.; Cardenas, A.M.; Lipomi, D.J. Combining High Sensitivity and Dynamic Range: Wearable Thin-Film Composite Strain Sensors of Graphene, Ultrathin Palladium, and PEDOT: PSS. ACS Appl. Nano Mater. 2019, 2, 2222–2229. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofacial Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malhotra, B.D.; Ali, M.A. Nanomaterials for Biosensors: Fundamentals and Applications. Nanomater. Biosens. 2018, 1–74. [Google Scholar] [CrossRef]
- Wang, C.; Tan, R.; Li, J.; Zhang, Z. Exonuclease I-assisted fluorescent method for ochratoxin A detection using iron-doped porous carbon, nitrogen-doped graphene quantum dots, and double magnetic separation. Anal. Bioanal. Chem. 2019, 411, 2405–2414. [Google Scholar] [CrossRef]
- Linting, Z.; Ruiyi, L.; Zaijun, L.; Qianfang, X.; Yinjun, F.; Junkang, L. An immunosensor for ultrasensitive detection of aflatoxin B1 with an enhanced electrochemical performance based on graphene/conducting polymer/gold nanoparticles/the ionic liquid composite film on modified gold electrode with electrodeposition. Sens. Actuators B Chem. 2012, 174, 359–365. [Google Scholar] [CrossRef]
- Dong, B.; Li, H.; Mari, G.M.; Yu, X.; Yu, W.; Wen, K.; Ke, Y.; Shen, J.; Wang, Z. Fluorescence immunoassay based on the inner-filter effect of carbon dots for highly sensitive amantadine detection in foodstuffs. Food Chem. 2019, 294, 347–354. [Google Scholar] [CrossRef]
- Li, H.; Yan, X.; Lu, G.; Su, X. Carbon dot-based bioplatform for dual colorimetric and fluorometric sensing of organophosphate pesticides. Sens. Actuators B Chem. 2018, 260, 563–570. [Google Scholar] [CrossRef]
- Cinti, S.; Volpe, G.; Piermarini, S.; Delibato, E.; Palleschi, G. Electrochemical biosensors for rapid detection of foodborne Salmonella: A critical overview. Sensors 2017, 17, 1910. [Google Scholar] [CrossRef]
- Nasir, S.; Hussein, M.Z.; Zainal, Z.; Yusof, N.A. Carbon-based nanomaterials/allotropes: A glimpse of their synthesis, properties and some applications. Materials 2018, 11, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, M.; Yin, Z.; Liu, K.; Du, X.; Liu, H.; Wang, S. Carbon-Based Nanomaterials in Sensors for Food Safety. Nanomaterials 2019, 9, 1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, N.-R.; Jung, I.-P.; La, I.-J.; Jung, H.-S.; Yoon, M.-Y. Ultra-sensitive detection of kanamycin for food safety using a reduced graphene oxide-based fluorescent aptasensor. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.; Liu, S.; Zhang, C.; Zeng, G.; Huang, D.; Qin, L.; Liu, X.; Yi, H.; Wang, R.; Huang, F. Electrochemical aptasensor based on sulfur–nitrogen codoped ordered mesoporous carbon and thymine–Hg2+–thymine mismatch structure for Hg2+ detection. ACS Sens. 2018, 3, 2566–2573. [Google Scholar] [CrossRef] [PubMed]
- Govindasamy, M.; Chen, S.-M.; Mani, V.; Devasenathipathy, R.; Umamaheswari, R.; Santhanaraj, K.J.; Sathiyan, A. Molybdenum disulfide nanosheets coated multiwalled carbon nanotubes composite for highly sensitive determination of chloramphenicol in food samples milk, honey and powdered milk. J. Colloid Interface Sci. 2017, 485, 129–136. [Google Scholar] [CrossRef]
- Pan, M.; Fang, G.; Duan, Z.; Kong, L.; Wang, S. Electrochemical sensor using methimazole imprinted polymer sensitized with MWCNTs and Salen-Co (III) as recognition element. Biosens. Bioelectron. 2012, 31, 11–16. [Google Scholar] [CrossRef]
- Roushani, M.; Abdi, Z. Novel electrochemical sensor based on graphene quantum dots/riboflavin nanocomposite for the detection of persulfate. Sens. Actuators B Chem. 2014, 201, 503–510. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Wu, K.; Chen, J.; Zhou, Y. Electrochemical sensor for hazardous food colourant quinoline yellow based on carbon nanotube-modified electrode. Food Chem. 2011, 128, 569–572. [Google Scholar] [CrossRef]
- Shan, J.; Li, J.; Chu, X.; Xu, M.; Jin, F.; Wang, X.; Ma, L.; Fang, X.; Wei, Z.; Wang, X. High sensitivity glucose detection at extremely low concentrations using a MoS 2-based field-effect transistor. RSC Adv. 2018, 8, 7942–7948. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Yao, D.; Xie, C.; Liu, D. Development of an aptasensor for electrochemical detection of tetracycline. Food Control 2014, 42, 109–115. [Google Scholar] [CrossRef]
- Que, X.; Chen, X.; Fu, L.; Lai, W.; Zhuang, J.; Chen, G.; Tang, D. Platinum-catalyzed hydrogen evolution reaction for sensitive electrochemical immunoassay of tetracycline residues. J. Electroanal. Chem. 2013, 704, 111–117. [Google Scholar] [CrossRef]
- Que, X.; Liu, B.; Fu, L.; Zhuang, J.; Chen, G.; Tang, D. Molecular imprint for electrochemical detection of streptomycin residues using enzyme signal amplification. Electroanalysis 2013, 25, 531–537. [Google Scholar] [CrossRef]
- Syahir, A.; Usui, K.; Tomizaki, K.-Y.; Kajikawa, K.; Mihara, H. Label and label-free detection techniques for protein microarrays. Microarrays 2015, 4, 228–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dincer, C.; Bruch, R.; Costa-Rama, E.; Fernández-Abedul, M.T.; Merkoçi, A.; Manz, A.; Urban, G.A.; Güder, F. Disposable sensors in diagnostics, food, and environmental monitoring. Adv. Mater. 2019, 31, 1806739. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Navaraj, W.T.; Lorenzelli, L.; Dahiya, R. Ultra-thin chips for high-performance flexible electronics. NPJ Flex. Electron. 2018, 2, 8. [Google Scholar] [CrossRef]
- Rim, Y.S.; Bae, S.H.; Chen, H.; De Marco, N.; Yang, Y. Recent progress in materials and devices toward printable and flexible sensors. Adv. Mater. 2016, 28, 4415–4440. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, L.S.; Cheung, K.M.; Kaappa, S.; Cao, H.H.; Yang, Q.; Young, T.D.; Serino, A.C.; Malola, S.; Olson, J.M.; Link, S. Patterning of supported gold monolayers via chemical lift-off lithography. Beilstein J. Nanotechnol. 2017, 8, 2648–2661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamedi, M.M.; Ainla, A.; Güder, F.; Christodouleas, D.C.; Fernández-Abedul, M.T.; Whitesides, G.M. Integrating electronics and microfluidics on paper. Adv. Mater. 2016, 28, 5054–5063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Noviana, E.; Nguyen, M.P.; Geiss, B.J.; Dandy, D.S.; Henry, C.S. based microfluidic devices: Emerging themes and applications. Anal. Chem. 2017, 89, 71–91. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, E.F.; Garcia, P.T.; Cardoso, T.M.; Lopes, F.M.; Martins, F.T.; Coltro, W.K. Highly sensitive colorimetric detection of glucose and uric acid in biological fluids using chitosan-modified paper microfluidic devices. Analyst 2016, 141, 4749–4756. [Google Scholar] [CrossRef]
- Golmohammadi, H.; Morales-Narvaez, E.; Naghdi, T.; Merkoci, A. Nanocellulose in sensing and biosensing. Chem. Mater. 2017, 29, 5426–5446. [Google Scholar] [CrossRef]
- Song, J.; Chen, C.; Wang, C.; Kuang, Y.; Li, Y.; Jiang, F.; Li, Y.; Hitz, E.; Zhang, Y.; Liu, B. Superflexible wood. ACS Appl. Mater. Interfaces 2017, 9, 23520–23527. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.L.; Ng, W.N.; Shao, R.; Pereles, B.D.; Ong, K.G. A wireless, passive sensor for quantifying packaged food quality. Sensors 2007, 7, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, V.S.; Adhikari, B.; Chen, A. Nanomaterial based electrochemical sensors for the safety and quality control of food and beverages. Analyst 2018, 143, 4537–4554. [Google Scholar] [CrossRef]
- Rotariu, L.; Lagarde, F.; Jaffrezic-Renault, N.; Bala, C. Electrochemical biosensors for fast detection of food contaminants–trends and perspective. Trac Trends Anal. Chem. 2016, 79, 80–87. [Google Scholar] [CrossRef]
- Gebicki, J. Application of electrochemical sensors and sensor matrixes for measurement of odorous chemical compounds. Trac Trends Anal. Chem. 2016, 77, 1–13. [Google Scholar] [CrossRef]
- Kannan, P.; Guo, L. Nanosensors for food safety. In Nanosensors for Smart Cities; Elsevier: Amsterdam, The Netherlands, 2020; pp. 339–354. [Google Scholar]
- Jafarizadeh-Malmiri, H.; Sayyar, Z.; Anarjan, N.; Berenjian, A. Nano-sensors in Food Nanobiotechnology. In Nanobiotechnology in Food: Concepts, Applications and Perspectives; Springer: Berlin/Heidelberg, Germany, 2019; pp. 81–94. [Google Scholar]
- Park, H.; Lee, E.; Chung, Y.; Lee, S.; Ahn, H.; Kim, D.-J. VOC gas sensors fabricated with graphene oxide composites for food safety and quality. ECS Trans. 2015, 69, 41–45. [Google Scholar] [CrossRef]
- Sundramoorthy, A.K.; Kumar, T.H.V.; Gunasekaran, S. Graphene-Based Nanosensors and Smart Food Packaging Systems for Food Safety and Quality Monitoring. In Graphene Bioelectronics; Elsevier: Amsterdam, The Netherlands, 2018; pp. 267–306. [Google Scholar]
- Chen, H.; Zhou, K.; Zhao, G. Gold nanoparticles: From synthesis, properties to their potential application as colorimetric sensors in food safety screening. Trends Food Sci. Technol. 2018, 78, 83–94. [Google Scholar] [CrossRef]
- Guo, L.; Wu, X.; Liu, L.; Kuang, H.; Xu, C. Gold Nanoparticle-Based Paper Sensor for Simultaneous Detection of 11 Benzimidazoles by One Monoclonal Antibody. Small 2018, 14, 1701782. [Google Scholar] [CrossRef]
- Llorens, A.; Lloret, E.; Picouet, P.A.; Trbojevich, R.; Fernandez, A. Metallic-based micro and nanocomposites in food contact materials and active food packaging. Trends Food Sci. Technol. 2012, 24, 19–29. [Google Scholar] [CrossRef]
- Beigmohammadi, F.; Peighambardoust, S.H.; Hesari, J.; Azadmard-Damirchi, S.; Peighambardoust, S.J.; Khosrowshahi, N.K. Antibacterial properties of LDPE nanocomposite films in packaging of UF cheese. Lwt-Food Sci. Technol. 2016, 65, 106–111. [Google Scholar] [CrossRef]
- Avramescu, A.; Andreescu, S.; Noguer, T.; Bala, C.; Andreescu, D.; Marty, J.-L. Biosensors designed for environmental and food quality control based on screen-printed graphite electrodes with different configurations. Anal. Bioanal. Chem. 2002, 374, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Sundramoorthy, A.K.; Gunasekaran, S. Applications of graphene in quality assurance and safety of food. Trac Trends Anal. Chem. 2014, 60, 36–53. [Google Scholar] [CrossRef]
- Bobrinetskiy, I.I.; Knezevic, N.Z. Graphene-based biosensors for on-site detection of contaminants in food. Anal. Methods 2018, 10, 5061–5070. [Google Scholar] [CrossRef]
- Chen, X.; Cai, Z.; Huang, Z.; Oyama, M.; Jiang, Y.; Chen, X. Non-enzymatic oxalic acid sensor using platinum nanoparticles modified on graphene nanosheets. Nanoscale 2013, 5, 5779–5783. [Google Scholar] [CrossRef]
- Chen, X.-M.; Cai, Z.-X.; Huang, Z.-Y.; Oyama, M.; Jiang, Y.-Q.; Chen, X. Ultrafine palladium nanoparticles grown on graphene nanosheets for enhanced electrochemical sensing of hydrogen peroxide. Electrochim. Acta 2013, 97, 398–403. [Google Scholar] [CrossRef]
- Gan, T.; Sun, J.; He, M.; Wang, L. Highly sensitive electrochemical sensor for Sudan I based on graphene decorated with mesoporous TiO2. Ionics 2014, 20, 89–95. [Google Scholar] [CrossRef]
- Gan, T.; Sun, J.; Lin, Z.; Li, Y. Highly sensitive determination of Orange II based on the dual amplified electrochemical signal of graphene and mesoporous TiO2. Anal. Methods 2013, 5, 2964–2970. [Google Scholar] [CrossRef]
- Gan, T.; Sun, J.; Meng, W.; Song, L.; Zhang, Y. Electrochemical sensor based on graphene and mesoporous TiO2 for the simultaneous determination of trace colourants in food. Food Chem. 2013, 141, 3731–3737. [Google Scholar] [CrossRef]
- Lim, H.; Nurzulaikha, R.; Harrison, I.; Lim, S.; Tan, W.; Yeo, M.; Yarmo, M.A.; Huang, N. Preparation and characterization of tin oxide, SnO2 nanoparticles decorated graphene. Ceram. Int. 2012, 38, 4209–4216. [Google Scholar] [CrossRef]
- Hou, J.; Bei, F.; Wang, M.; Ai, S. Electrochemical determination of malachite green at graphene quantum dots–gold nanoparticles multilayers–modified glassy carbon electrode. J. Appl. Electrochem. 2013, 43, 689–696. [Google Scholar] [CrossRef]
- Li, Z.; Hou, M.; Bai, S.; Wang, C.; Wang, Z. Extraction of imide fungicides in water and juice samples using magnetic graphene nanoparticles as adsorbent followed by their determination with gas chromatography and electron capture detection. Anal. Sci. 2013, 29, 325–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, G.; Song, S.; Wang, C.; Wu, Q.; Wang, Z. Determination of triazine herbicides in environmental water samples by high-performance liquid chromatography using graphene-coated magnetic nanoparticles as adsorbent. Anal. Chim. Acta 2011, 708, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, S.; Du, D.; Shao, Y.; Li, Z.; Wang, J.; Engelhard, M.H.; Li, J.; Lin, Y. Self assembly of acetylcholinesterase on a gold nanoparticles–graphene nanosheet hybrid for organophosphate pesticide detection using polyelectrolyte as a linker. J. Mater. Chem. 2011, 21, 5319–5325. [Google Scholar] [CrossRef]
- Yang, Y.; Asiri, A.M.; Du, D.; Lin, Y. Acetylcholinesterase biosensor based on a gold nanoparticle–polypyrrole–reduced graphene oxide nanocomposite modified electrode for the amperometric detection of organophosphorus pesticides. Analyst 2014, 139, 3055–3060. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Han, G.; Xiao, Y.; Li, M.; Zhou, W. An acetylcholinesterase biosensor based on graphene/polyaniline composite film for detection of pesticides. Chin. J. Chem. 2016, 34, 82–88. [Google Scholar] [CrossRef]
- Cao, T.T.; Nguyen, H.B.; Bui, H.T.; Vu, T.T.; Phan, N.H.; Phan, B.T.; Hoang, L.; Bayle, M.; Paillet, M.; Sauvajol, J.L. Fabrication of few-layer graphene film based field effect transistor and its application for trace-detection of herbicide atrazine. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 035007. [Google Scholar] [CrossRef]
- Liu, T.; Su, H.; Qu, X.; Ju, P.; Cui, L.; Ai, S. Acetylcholinesterase biosensor based on 3-carboxyphenylboronic acid/reduced graphene oxide–gold nanocomposites modified electrode for amperometric detection of organophosphorus and carbamate pesticides. Sens. Actuators B Chem. 2011, 160, 1255–1261. [Google Scholar] [CrossRef]
- Tian, J.; Xu, J.; Zhu, F.; Lu, T.; Su, C.; Ouyang, G. Application of nanomaterials in sample preparation. J. Chromatogr. A 2013, 1300, 2–16. [Google Scholar] [CrossRef]
- Zhang, B.-T.; Zheng, X.; Li, H.-F.; Lin, J.-M. Application of carbon-based nanomaterials in sample preparation: A review. Anal. Chim. Acta 2013, 784, 1–17. [Google Scholar] [CrossRef]
- Migliorini, F.L.; Sanfelice, R.C.; Mercante, L.A.; Facure, M.H.; Correa, D.S. Electrochemical sensor based on polyamide 6/polypyrrole electrospun nanofibers coated with reduced graphene oxide for malathion pesticide detection. Mater. Res. Express 2019, 7, 015601. [Google Scholar] [CrossRef]
- Zhang, B.; Tang, D.; Liu, B.; Chen, H.; Cui, Y.; Chen, G. GoldMag nanocomposite-functionalized graphene sensing platform for one-step electrochemical immunoassay of alpha-fetoprotein. Biosens. Bioelectron. 2011, 28, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Tang, D.; Li, Q.; Su, B.; Qiu, B.; Chen, G. Sensitive electrochemical immunoassay of carcinoembryonic antigen with signal dual-amplification using glucose oxidase and an artificial catalase. Anal. Chim. Acta 2011, 697, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tang, D.; Liu, B.; Cui, Y.; Chen, H.; Chen, G. Nanogold-functionalized magnetic beads with redox activity for sensitive electrochemical immunoassay of thyroid-stimulating hormone. Anal. Chim. Acta 2012, 711, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Almeida, A.J.; Vale, N. Combination of cell-penetrating peptides with nanoparticles for therapeutic application: A review. Biomolecules 2019, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Zhao, Y.; Du, B.; Wu, D.; Li, H.; Yang, M. Ultrasensitive detection of kanamycin in animal derived foods by label-free electrochemical immunosensor. Food Chem. 2012, 134, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Zhang, B.; Liu, M.; Hu, X.; Fang, G.; Wang, S. Molecularly imprinted electrochemical sensor based on polypyrrole/dopamine@graphene incorporated with surface molecularly imprinted polymers thin film for recognition of olaquindox. Bioelectrochemistry 2020, 132, 107398. [Google Scholar] [CrossRef]
- Mao, H.; Liang, J.; Zhang, H.; Pei, Q.; Liu, D.; Wu, S.; Zhang, Y.; Song, X.-M. Poly (ionic liquids) functionalized polypyrrole/graphene oxide nanosheets for electrochemical sensor to detect dopamine in the presence of ascorbic acid. Biosens. Bioelectron. 2015, 70, 289–298. [Google Scholar] [CrossRef]
- Yang, J.; Strickler, J.R.; Gunasekaran, S. Indium tin oxide-coated glass modified with reduced graphene oxide sheets and gold nanoparticles as disposable working electrodes for dopamine sensing in meat samples. Nanoscale 2012, 4, 4594–4602. [Google Scholar] [CrossRef]
- Zhou, X.; Ma, P.; Wang, A.; Yu, C.; Qian, T.; Wu, S.; Shen, J. Dopamine fluorescent sensors based on polypyrrole/graphene quantum dots core/shell hybrids. Biosens. Bioelectron. 2015, 64, 404–410. [Google Scholar] [CrossRef]
- Yu, S.; Wei, Q.; Du, B.; Wu, D.; Li, H.; Yan, L.; Ma, H.; Zhang, Y. Label-free immunosensor for the detection of kanamycin using Ag@ Fe3O4 nanoparticles and thionine mixed graphene sheet. Biosens. Bioelectron. 2013, 48, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wei, Q.; Xu, C.; Li, H.; Wu, D.; Cai, Y.; Mao, K.; Cui, Z.; Du, B. Label-free electrochemical immunosensor for sensitive detection of kanamycin. Sens. Actuators B Chem. 2011, 155, 618–625. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, C.; Lu, L.; Su, X. A novel aptamer-mediated CuInS 2 quantum dots@ graphene oxide nanocomposites-based fluorescence “turn off–on” nanosensor for highly sensitive and selective detection of kanamycin. RSC Adv. 2016, 6, 10205–10214. [Google Scholar] [CrossRef]
- Wu, C.; Sun, D.; Li, Q.; Wu, K. Electrochemical sensor for toxic ractopamine and clenbuterol based on the enhancement effect of graphene oxide. Sens. Actuators B Chem. 2012, 168, 178–184. [Google Scholar] [CrossRef]
- Talib, N.A.A.; Salam, F.; Sulaiman, Y. Development of Highly Sensitive Immunosensor for Clenbuterol Detection by Using Poly (3, 4-ethylenedioxythiophene)/Graphene Oxide Modified Screen-Printed Carbon Electrode. Sensors 2018, 18, 4324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthumariyappan, A.; Rajaji, U.; Chen, S.-M.; Baskaran, N.; Chen, T.-W.; Ramalingam, R.J. Sonochemical synthesis of perovskite-type barium titanate nanoparticles decorated on reduced graphene oxide nanosheets as an effective electrode material for the rapid determination of ractopamine in meat samples. Ultrason. Sonochemistry 2019, 56, 318–326. [Google Scholar] [CrossRef]
- Poo-arporn, Y.; Pakapongpan, S.; Chanlek, N.; Poo-arporn, R.P. The development of disposable electrochemical sensor based on Fe3O4-doped reduced graphene oxide modified magnetic screen-printed electrode for ractopamine determination in pork sample. Sens. Actuators B Chem. 2019, 284, 164–171. [Google Scholar] [CrossRef]
- Chyan, Y.; Ye, R.; Li, Y.; Singh, S.P.; Arnusch, C.J.; Tour, J.M. Laser-induced graphene by multiple lasing: Toward electronics on cloth, paper, and food. ACS Nano 2018, 12, 2176–2183. [Google Scholar] [CrossRef]
- Cardoso, A.R.; Marques, A.C.; Santos, L.; Carvalho, A.F.; Costa, F.M.; Martins, R.; Sales, M.G.F.; Fortunato, E. Molecularly-imprinted chloramphenicol sensor with laser-induced graphene electrodes. Biosens. Bioelectron. 2019, 124, 167–175. [Google Scholar] [CrossRef]
- Kumar, V.; Guleria, P.; Mehta, S.K. Nanosensors for food quality and safety assessment. Environ. Chem. Lett. 2017, 15, 165–177. [Google Scholar] [CrossRef]
- Farah, A.; Sukor, R.; Fatimah, A.; Jinap, S. Application of nanomaterials in the development of biosensors for food safety and quality control. Int. Food Res. J. 2016, 23, 1849. [Google Scholar]
- Zappa, D. Low-Power Detection of Food Preservatives by a Novel Nanowire-Based Sensor Array. Foods 2019, 8, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Campbell, A.S.; Wang, J. Wearable non-invasive epidermal glucose sensors: A review. Talanta 2018, 177, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Iwu, K.O.; Lombardo, A.; Sanz, R.; Scirè, S.; Mirabella, S. Facile synthesis of Ni nanofoam for flexible and low-cost non-enzymatic glucose sensing. Sens. Actuators B Chem. 2016, 224, 764–771. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, Y.; Yang, X.; Chen, W.; Zhou, Y.; Li, C. Flexible 3D porous CuO nanowire arrays for enzymeless glucose sensing: In situ engineered versus ex situ piled. Nanoscale 2015, 7, 559–569. [Google Scholar] [CrossRef]
- Bell, C.; Nammari, A.; Uttamchandani, P.; Rai, A.; Shah, P.; Moore, A.L. Flexible electronics-compatible non-enzymatic glucose sensing via transparent CuO nanowire networks on PET films. Nanotechnology 2017, 28, 245502. [Google Scholar] [CrossRef]
- Solanki, P.R.; Kaushik, A.; Ansari, A.A.; Tiwari, A.; Malhotra, B. Multi-walled carbon nanotubes/sol–gel-derived silica/chitosan nanobiocomposite for total cholesterol sensor. Sens. Actuators B Chem. 2009, 137, 727–735. [Google Scholar] [CrossRef]
- Švorc, Ľ.; Haššo, M.; Sarakhman, O.; Kianičkova, K.; Stanković, D.M.; Otřísal, P. A progressive electrochemical sensor for food quality control: Reliable determination of theobromine in chocolate products using a miniaturized boron-doped diamond electrode. Microchem. J. 2018, 142, 297–304. [Google Scholar] [CrossRef]
- Parra, V.; Hernando, T.; Rodríguez-Méndez, M.L.; de Saja, J.A. Electrochemical sensor array made from bisphthalocyanine modified carbon paste electrodes for discrimination of red wines. Electrochim. Acta 2004, 49, 5177–5185. [Google Scholar] [CrossRef]
- Cinti, S.; Basso, M.; Moscone, D.; Arduini, F. A paper-based nanomodified electrochemical biosensor for ethanol detection in beers. Anal. Chim. Acta 2017, 960, 123–130. [Google Scholar] [CrossRef]
- Cinti, S.; Moscone, D.; Arduini, F. Preparation of paper-based devices for reagentless electrochemical (bio) sensor strips. Nat. Protoc. 2019, 14, 2437–2451. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Hubble, L.J.; Martín, A.; Kumar, R.; Barfidokht, A.; Kim, J.; Musameh, M.M.; Kyratzis, I.L.; Wang, J. Wearable flexible and stretchable glove biosensor for on-site detection of organophosphorus chemical threats. ACS Sens. 2017, 2, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-D.; Deb, S.; Seo, Y.-S.; Rao, S.; Chiao, M.; Chiao, J. A passive radio-frequency pH-sensing tag for wireless food-quality monitoring. IEEE Sens. J. 2011, 12, 487–495. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, Z.; Wu, X.; Jiang, H. Immune-biosensor for aflatoxin B1 based bio-electrocatalytic reaction on micro-comb electrode. Biochem. Eng. J. 2006, 32, 211–217. [Google Scholar] [CrossRef]
- Khan, R.; Dhayal, M. Nanocrystalline bioactive TiO2–chitosan impedimetric immunosensor for ochratoxin-A. Electrochem. Commun. 2008, 10, 492–495. [Google Scholar] [CrossRef]
- Gomathi, P.; Ragupathy, D.; Choi, J.H.; Yeum, J.H.; Lee, S.C.; Kim, J.C.; Lee, S.H.; Do Ghim, H. Fabrication of novel chitosan nanofiber/gold nanoparticles composite towards improved performance for a cholesterol sensor. Sens. Actuators B Chem. 2011, 153, 44–49. [Google Scholar] [CrossRef]
- Kaushik, A.; Solanki, P.R.; Pandey, M.; Ahmad, S.; Malhotra, B.D. Cerium oxide-chitosan based nanobiocomposite for food borne mycotoxin detection. Appl. Phys. Lett. 2009, 95, 173703. [Google Scholar] [CrossRef] [Green Version]
- Sun, A.-L.; Qi, Q.-A.; Dong, Z.-L.; Liang, K.Z. An electrochemical enzyme immunoassay for aflatoxin B 1 based on bio-electrocatalytic reaction with room-temperature ionic liquid and nanoparticle-modified electrodes. Sens. Instrum. Food Qual. Saf. 2008, 2, 43–50. [Google Scholar] [CrossRef]
- Villamizar, R.A.; Maroto, A.; Rius, F.X.; Inza, I.; Figueras, M.J. Fast detection of Salmonella Infantis with carbon nanotube field effect transistors. Biosens. Bioelectron. 2008, 24, 279–283. [Google Scholar] [CrossRef]
- Çubukçu, M.; Timur, S.; Anik, Ü. Examination of performance of glassy carbon paste electrode modified with gold nanoparticle and xanthine oxidase for xanthine and hypoxanthine detection. Talanta 2007, 74, 434–439. [Google Scholar] [CrossRef]
- Loutfi, A.; Coradeschi, S.; Mani, G.K.; Shankar, P.; Rayappan, J.B.B. Electronic noses for food quality: A review. J. Food Eng. 2015, 144, 103–111. [Google Scholar] [CrossRef]
- Gardner, J.W.; Bartlett, P.N. A brief history of electronic noses. Sens. Actuators B Chem. 1994, 18, 210–211. [Google Scholar] [CrossRef]
- Harper, W.J. The strengths and weaknesses of the electronic nose. In Headspace Analysis of Foods and Flavors; Springer: Boston, MA, USA, 2001; pp. 59–71. [Google Scholar]
- Sanaeifar, A.; ZakiDizaji, H.; Jafari, A.; de la Guardia, M. Early detection of contamination and defect in foodstuffs by electronic nose: A review. Trac Trends Anal. Chem. 2017, 97, 257–271. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, M.; Adhikari, B. Advances of electronic nose and its application in fresh foods: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2700–2710. [Google Scholar] [CrossRef] [PubMed]
- Wojnowski, W.; Majchrzak, T.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Portable electronic nose based on electrochemical sensors for food quality assessment. Sensors 2017, 17, 2715. [Google Scholar] [CrossRef] [Green Version]
- Severini, C.; Derossi, A.; Fiore, A.G.; Ricci, I.; Marone, M. The electronic nose system: Study on the global aromatic profile of espresso coffee prepared with two types of coffee filter holders. Eur. Food Res. Technol. 2016, 242, 2083–2091. [Google Scholar] [CrossRef]
- O’Connell, M.; Valdora, G.; Peltzer, G.; Negri, R.M. A practical approach for fish freshness determinations using a portable electronic nose. Sens. Actuators B Chem. 2001, 80, 149–154. [Google Scholar] [CrossRef]
- Wojnowski, W.; Majchrzak, T.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Electronic noses: Powerful tools in meat quality assessment. Meat Sci. 2017, 131, 119–131. [Google Scholar] [CrossRef]
- Gursoy, O.; Somervuo, P.; Alatossava, T. Preliminary study of ion mobility based electronic nose MGD-1 for discrimination of hard cheeses. J. Food Eng. 2009, 92, 202–207. [Google Scholar] [CrossRef]
- Dymerski, T.; Gębicki, J.; Wardencki, W.; Namieśnik, J. Application of an electronic nose instrument to fast classification of Polish honey types. Sensors 2014, 14, 10709–10724. [Google Scholar] [CrossRef] [Green Version]
- Dymerski, T.; Gębicki, J.; Wardencki, W.; Namieśnik, J. Quality evaluation of agricultural distillates using an electronic nose. Sensors 2013, 13, 15954–15967. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Méndez, M.L.; De Saja, J.A.; González-Antón, R.; García-Hernández, C.; Medina-Plaza, C.; García-Cabezón, C.; Martín-Pedrosa, F. Electronic noses and tongues in wine industry. Front. Bioeng. Biotechnol. 2016, 4, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinheiro, C.; Schäfer, T.; Crespo, J.G. Direct integration of pervaporation as a sample preparation method for a dedicated “electronic nose”. Anal. Chem. 2005, 77, 4927–4935. [Google Scholar] [CrossRef] [PubMed]
- García, M.; Fernández, M.; Fontecha, J.; Lozano, J.; Santos, J.; Aleixandre, M.; Sayago, I.; Gutiérrez, J.; Horrillo, M. Differentiation of red wines using an electronic nose based on surface acoustic wave devices. Talanta 2006, 68, 1162–1165. [Google Scholar] [CrossRef]
- Macías, M.M.; Agudo, J.E.; Manso, A.G.; Orellana, C.J.G.; Velasco, H.M.G.; Caballero, R.G. A compact and low cost electronic nose for aroma detection. Sensors 2013, 13, 5528–5541. [Google Scholar] [CrossRef] [Green Version]
- Falasconi, M.; Concina, I.; Gobbi, E.; Sberveglieri, V.; Pulvirenti, A.; Sberveglieri, G. Electronic nose for microbiological quality control of food products. Int. J. Electrochem. 2012, 2012. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Bai, J.; Plotto, A.; Dea, S. Electronic noses and tongues: Applications for the food and pharmaceutical industries. Sensors 2011, 11, 4744–4766. [Google Scholar] [CrossRef]
- Ong, K.G.; Bitler, J.S.; Grimes, C.A.; Puckett, L.G.; Bachas, L.G. Remote query resonant-circuit sensors for monitoring of bacteria growth: Application to food quality control. Sensors 2002, 2, 219–232. [Google Scholar] [CrossRef]
- Vasilescu, A.; Marty, J.-L. Electrochemical aptasensors for the assessment of food quality and safety. Trac Trends Anal. Chem. 2016, 79, 60–70. [Google Scholar] [CrossRef]
- Hunt, H.K.; Armani, A.M. Label-free biological and chemical sensors. Nanoscale 2010, 2, 1544–1559. [Google Scholar] [CrossRef]
- Label-Free Detection: New Biosensors Facilitate Broader Range of Drug Discovery Applications. Available online: https://www.ddw-online.com/screening/p148356-label-free-detection-new-biosensors-facilitate-broader-range-of-drug-discovery-applications.html (accessed on 5 July 2020).
- Cunningham, B.T.; Laing, L.G. Advantages and application of label-free detection assays in drug screening. Expert Opin. Drug Discov. 2008, 3, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Label-Free: The Way to Be? Available online: http://www.ddn-news.com/index.php?newsarticle=4580%20 (accessed on 5 July 2020).
- Afsarimanesh, N.; Mukhopadhyay, S.C.; Kruger, M. Electrochemical Biosensor: Point-of-Care for Early Detection of Bone Loss; Springer: Berlin, Germany, 2019. [Google Scholar]
- Liao, K.-H.; Lo, C.-Y. Thermoresistive strain sensor and positioning method for roll-to-roll processes. Sensors 2014, 14, 8082–8095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qureshi, Y.; Tarfaoui, M.; Lafdi, K.K.; Lafdi, K. Nanotechnology and development of strain sensor for damage detection. In Advances in Structural Health Monitoring; IntechOpen: London, UK, 2019. [Google Scholar]
- Bahadır, E.B.; Sezgintürk, M.K. Applications of commercial biosensors in clinical, food, environmental, and biothreat/biowarfare analyses. Anal. Biochem. 2015, 478, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Vermeiren, L.; Devlieghere, F.; van Beest, M.; de Kruijf, N.; Debevere, J. Developments in the active packaging of foods. Trends Food Sci. Technol. 1999, 10, 77–86. [Google Scholar] [CrossRef]
- Weber, W.; Luzi, S.; Karlsson, M.; Fussenegger, M. A novel hybrid dual-channel catalytic-biological sensor system for assessment of fruit quality. J. Biotechnol. 2009, 139, 314–317. [Google Scholar] [CrossRef]
- Hosseini, S.; Vázquez-Villegas, P.; Rito-Palomares, M.; Martinez-Chapa, S.O. General Overviews on Applications of ELISA. In Enzyme-Linked Immunosorbent Assay (ELISA); Springer: Singapore, 2018; pp. 19–29. [Google Scholar]
- Biosensors Market by Type (Sensor Patch and Embedded Device), Product (Wearable and Nonwearable), Technology (Electrochemical and Optical), Application (POC, Home Diagnostics, Research Lab, Food & Beverages), and Geography—Global Forecast to 2024. Available online: https://www.marketsandmarkets.com/Market-Reports/biosensors-market-798.html (accessed on 5 July 2020).
- Wang, X.; Gu, Y.; Xiong, Z.; Cui, Z.; Zhang, T. Silk-molded flexible, ultrasensitive, and highly stable electronic skin for monitoring human physiological signals. Adv. Mater. 2014, 26, 1336–1342. [Google Scholar] [CrossRef]
- Nag, A.; Mukhopadhyay, S.C.; Kosel, J. Flexible carbon nanotube nanocomposite sensor for multiple physiological parameter monitoring. Sens. Actuators A Phys. 2016, 251, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Suryadevara, N.; Mukhopadhyay, S.C.; Wang, R.; Rayudu, R. Forecasting the behavior of an elderly using wireless sensors data in a smart home. Eng. Appl. Artif. Intell. 2013, 26, 2641–2652. [Google Scholar] [CrossRef]
- Nag, A.; Mukhopadhyay, S.C. Occupancy detection at smart home using real-time dynamic thresholding of flexiforce sensor. IEEE Sens. J. 2015, 15, 4457–4463. [Google Scholar] [CrossRef]
- The Internet of Disposable Things Will Be Made of Paper and Plastic Sensors-For disposable sensors, silicon will never be the right fit—But cheaper tech is nearly here. Available online: https://spectrum.ieee.org/semiconductors/materials/the-internet-of-disposable-things-will-be-made-of-paper-and-plastic-sensors (accessed on 5 July 2020).
- Wilson, A.D.; Baietto, M. Applications and advances in electronic-nose technologies. Sensors 2009, 9, 5099–5148. [Google Scholar] [CrossRef]
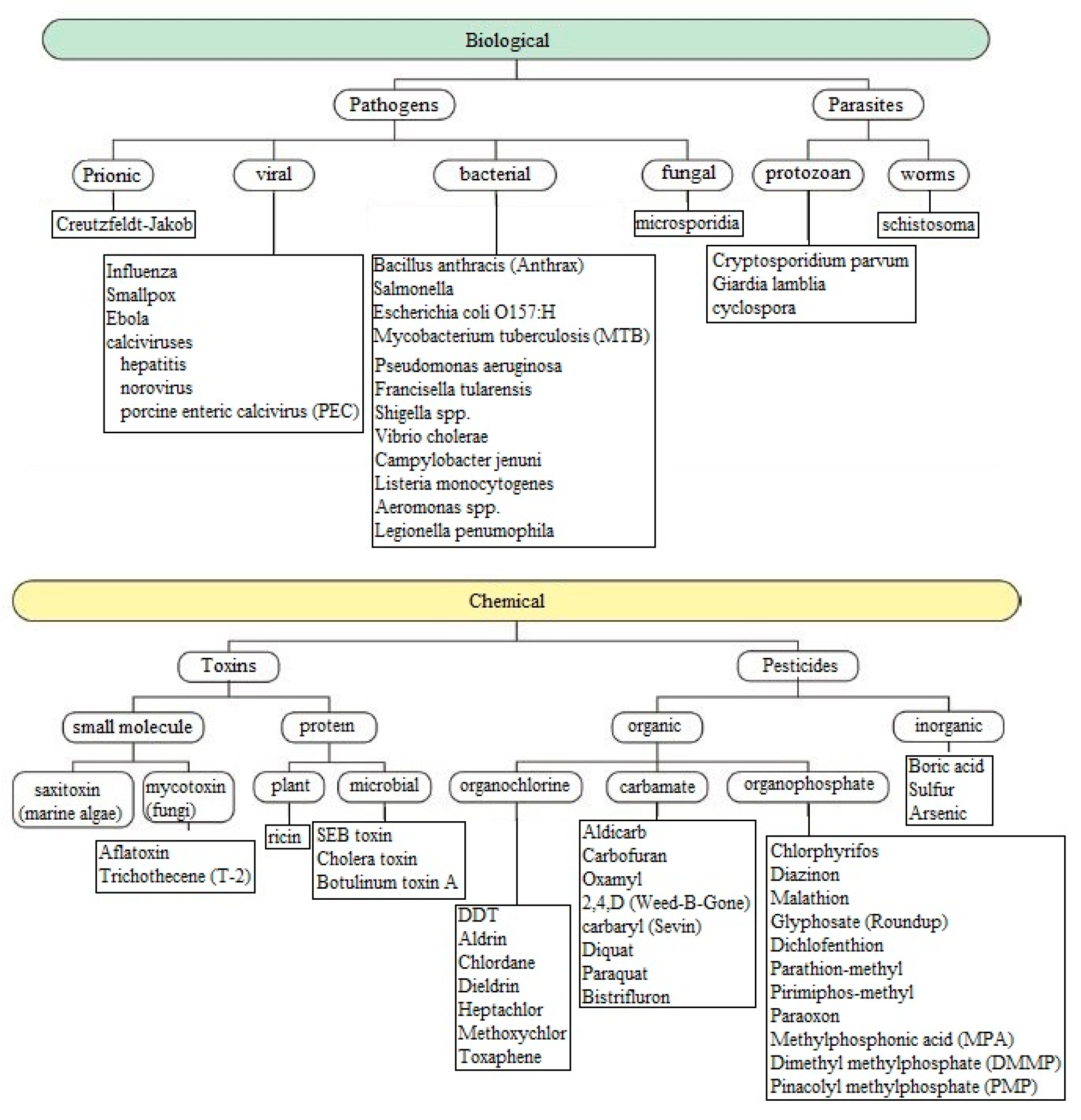
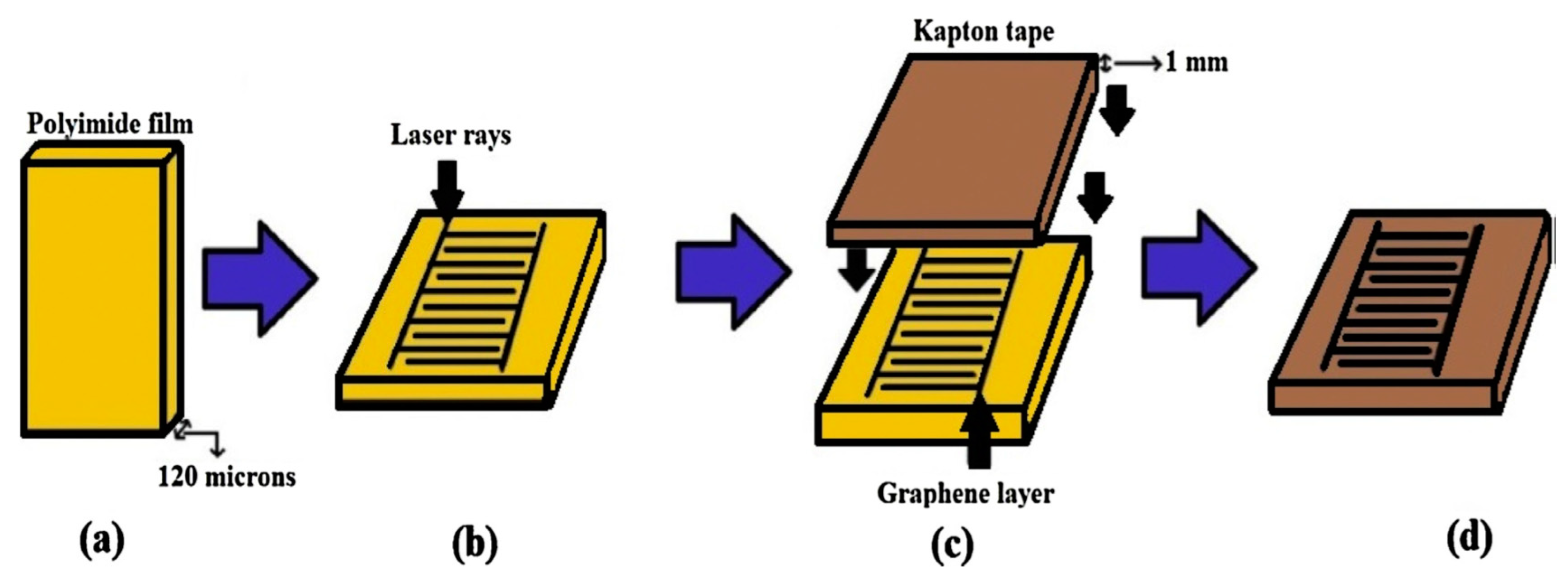



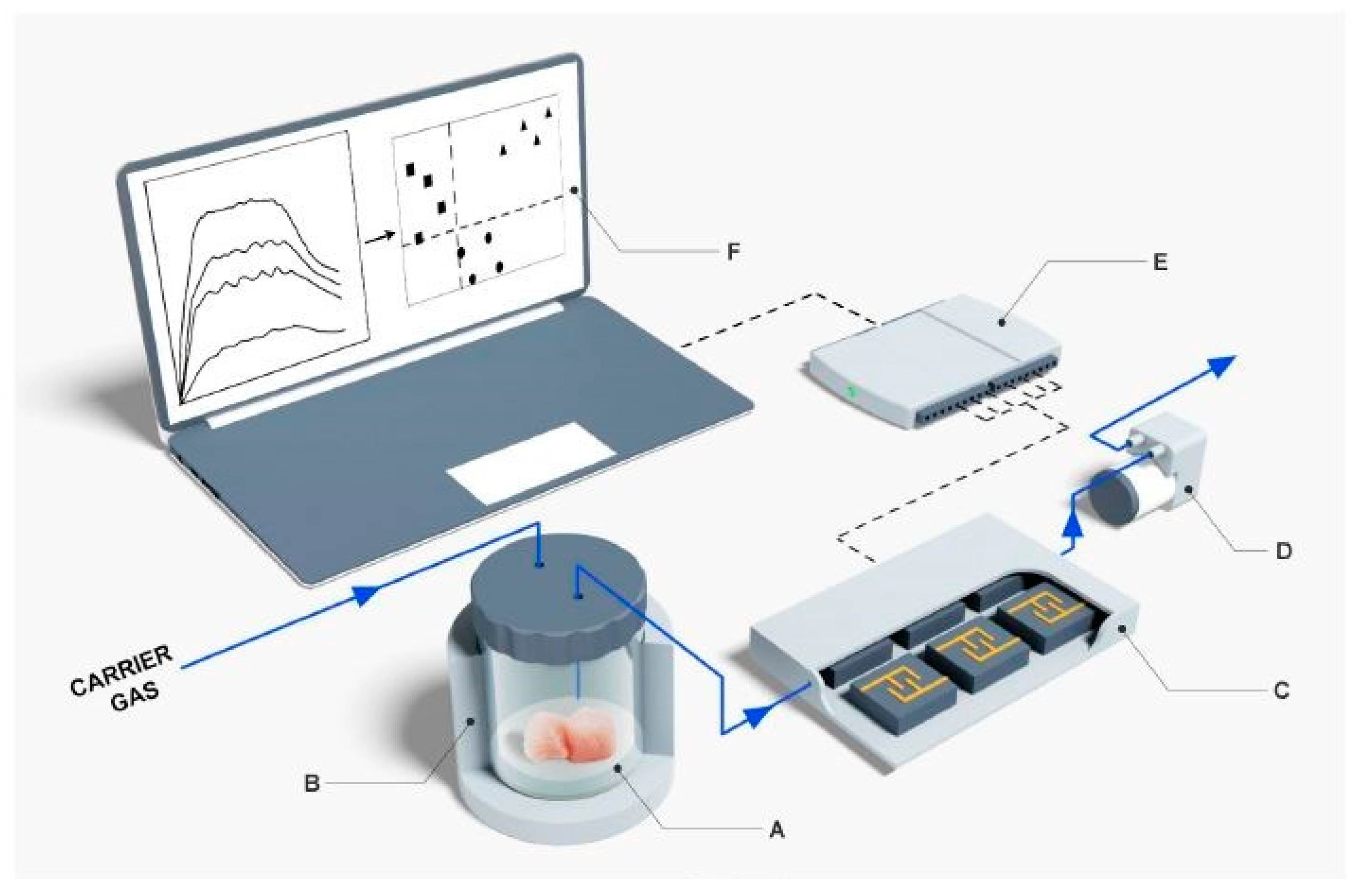
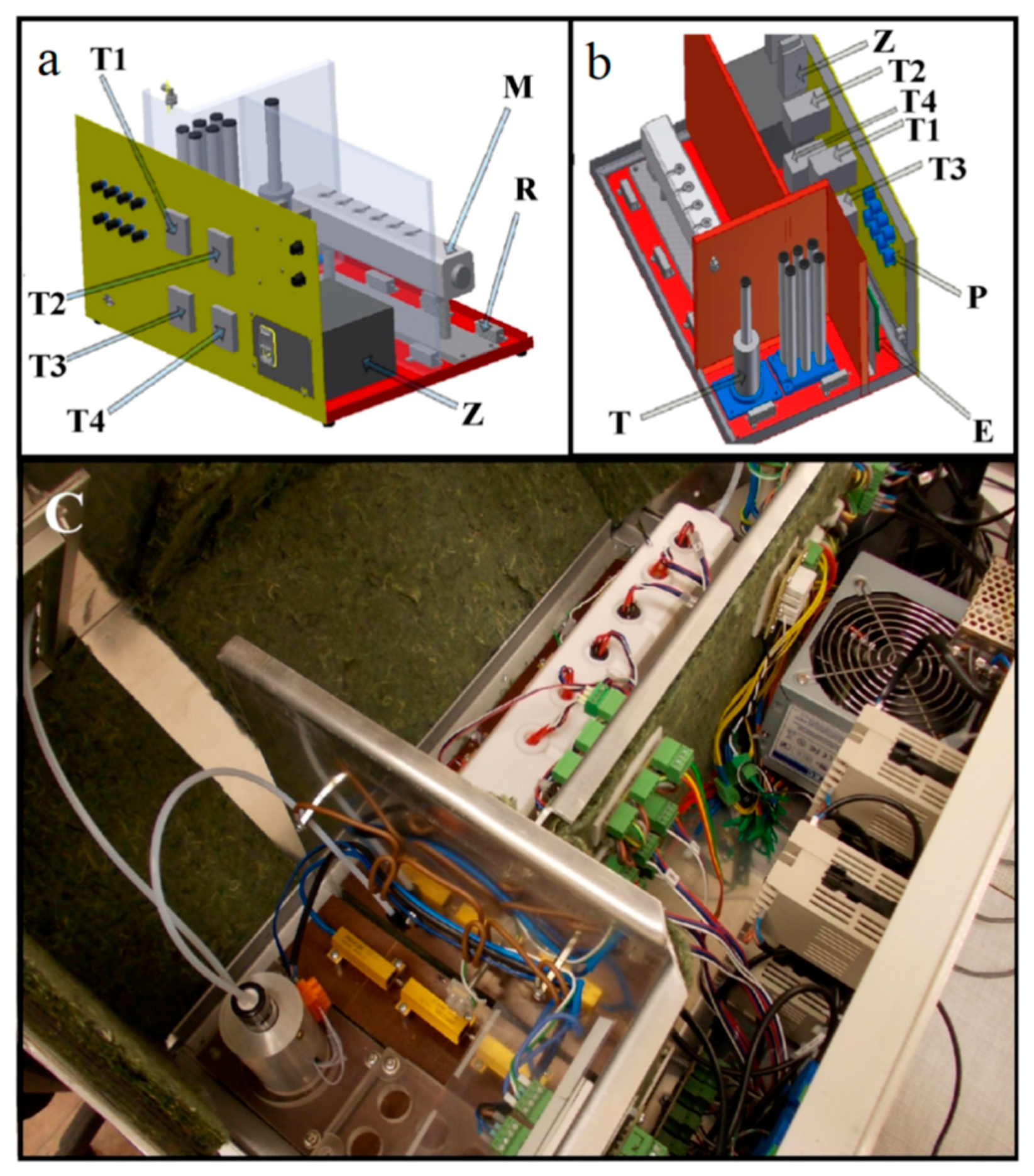
| Types of Sensors | Pros | Cons |
|---|---|---|
| Non-flexible sensors |
|
|
| Flexible sensors |
|
|
| Material | Linear Range | Limit of Detection | Target Product | Reference |
|---|---|---|---|---|
| Sulphur, nitrogen, mesoporous carbon | 0.001–1000 nM | 0.00045 nM | Mercury ion | [49] |
| Multi-Walled Carbon Nanotubes (MWCNTs), Molybdenum disulphide nanosheets (MoS2) | 0.08–1392 μM | 0.015 μM | Chloramphenicol | [50] |
| Multi-Walled Carbon Nanotubes (MWCNTs), Salen, Cobalt (III) | 0.5–6.0 mg L−1 | 0.048 mg L−1 | Methimazole | [51] |
| Reduced graphene oxide (RGO) | 1 pM–20 pM | 1 pM | Kanamycin | [48] |
| Modified glassy carbon electrode, graphene quantum dots, riboflavin | 0.001 μM–1.0 μM | 0.2 μM | Persulfate | [52] |
| Multi-Walled Carbon Nanotubes (MWCNTs) | 0.75–20 mg L−1 | 0.5 mg L−1 | Quinoline yellow | [53] |
| Molybdenum disulphide (MoS2) | 300 nM–30 mM | 300 nM | Glucose | [54] |
| Graphene sheet, Nafion, thionine, platinum nanoparticles | 0.01–12.0 ng/mL | 0.00574 ng/mL | Kanamycin | [55] |
| Graphene nanosheets, platinum-catalysed hydrogen | 0.05–100 ng mL−1 | 0.006 ng mL−1 | Tetracycline | [56] |
| Glucose oxidase, aniline, o-phenylenediamine | 0.01 to 10 ng mL−1 | 0.007 ng mL−1 | Streptomycin | [57] |
| Material Used | Analyte | Linear Range | Limit of Detection | Sample Matrix | Reference |
|---|---|---|---|---|---|
| Graphene, Polypyrrole, glassy carbon electrode | Dopamine | 50–500 nmol/L | 7.5 nmol/L | Fish | [105] |
| Poly (ionic liquids) functionalized polypyrrole, graphene oxide nanosheets | Dopamine | 4–18 µM | 0.07 µM | Meat | [106] |
| Reduced Graphene Oxide, gold nanoparticles | Dopamine | 10–1000 µM | 6 × 10−2 µM | Meat | [107] |
| Polypyrrole, graphene quantum dots | Dopamine | 0.005–8 µM | 0.00001 µM | Meat | [108] |
| Graphene sheet, silver hybridized mesoporous ferroferric oxide nanoparticles | Kanamycin | 0.050–16 ng/mL | 0.15 ng/mL | Pork | [109] |
| Graphene, Prussian blue-chitosan, nanoporous Gold, Kanamycin antibody | Kanamycin | 0.02–14 ng/mL | 0.0631 ng/mL | Pork | [110] |
| Graphene, Nafion, thionines, platinum nanoparticles, anti-kanamycin antibody | Kanamycin | 0.01–12 ng/mL | 0.0574 ng/mL | Chicken liver | [104] |
| Copper Indium Sulfide quantum dots, graphene oxide | Kanamycin | 0.03–45 nmol/L | 0.12 nmol/L | Milk | [111] |
| Graphene oxide | Clenbuterol | 0.001–25 µg/L | 15 µg/L | Pork samples | [112] |
| Poly (3,4-ethylenedioxythiophene), graphene oxide | Clenbuterol | 0–250 ng/mL | 0.196 ng/mL | Milk | [113] |
| Perovskite-type barium titanate (BaTiO3) nanoparticles, reduced graphene oxide sheets | Ractopamine | 0.01–527.19 µM | 1.57 nM | Meat | [114] |
| Iron oxide nanoparticles, graphene oxide | Ractopamine | 0.05–100 µM | 0.013 µM | Pork samples | [115] |
| Materials | Detection Technique | Target Molecule | Detection Range | LOD | Reference |
|---|---|---|---|---|---|
| Graphene nano-hybrids, platinum NPs | CV, DPV | Oxalic acid | 0.1–50 mM | 0.1 mM | [83] |
| Graphene nanosheets, palladium NPs | CV, Amperometry | Hydrogen Peroxide | 0.0001–1 mM | 5 × 10−4 mM | [84] |
| Graphene sheets, Titanium oxide, glassy carbon electrode | CV | Sudan I | 3.3 × 10−6–6.6 × 10−4 mM | 6 × 10−5 mM | [85] |
| Graphene quantum dots, gold NPs | CV | Malachite green | 4 × 10−4–0.1 mM | 1 × 10−4 mM | [89] |
| Reduced graphene oxide, Polyamide 6, Polypyrrole | CV, EIS | Malathion | 0.5–20 mM | 8 × 10−4 mM | [99] |
| Laser-induced graphene, Polyimide | EIS | Citric acid, sodium chloride, L-tryptophan, Sucrose, Guanosine monophosphate | 1–1000 ppm | 1 ppm | [27] |
| Nanowires | Nitrogen Dioxide (ng/mL) | Ethanol (ng/mL) | Acetone (ng/mL) | Ozone (ng/mL) |
|---|---|---|---|---|
| Tin oxide | >1000 | 5000 | 15,000 | 40 |
| Tungsten trioxide | 100 | 25,000 | 15,000 | 150 |
| Copper oxide | >1000 | 40,000 | 50,000 | 300 |
| Materials | Detection Technique | Target Molecule | Detection Range | LOD | Reference |
|---|---|---|---|---|---|
| Tin oxide, Copper oxide, Tungsten trioxide | Change in conductance | Nitrogen dioxide, ethanol, oxygen, ozone | Nitrogen dioxide: 0.1–1 ppm, Ethanol: 5–40 ppm, Acetone: 15–50 ppm, Ozone: 0.04–0.3 ppm | Nitrogen dioxide: 0.1 ppm Ethanol: 5 ppm, Acetone: 15 ppm, Ozone: 0.04 ppm | [120] |
| MWCNTs, ITO, Chitosan, Silica | CV | Cholesterol | 100–5000 ppm | 100 ppm | [125] |
| Boron-doped diamond, ceramic | CV, DPV, SWV | Theobromine | 0.99–54.5 µM | 0.99 µM | [126] |
| Carbon Paste | CV, SWV | Letutium, gadolinium and praseodymium bisphthalo-cyaninates | 15,000 ppm | 15,000 ppm | [127] |
| Carbon Black, Prussian blue NPs | CV | Ethanol in beer samples | 0.52–10 mM | 0.52 mM | [128] |
| Carbon black, Prussian blue NPs, Filter paper, Office paper | CV | Phosphate | 10–50 mM | 10 mM | [129] |
| Carbon ink | CV, SWV | Organo-phosphorous | 200 μM | 200 μM | [130] |
| Iridium oxide, silver chloride | CV | pH levels in food | 2–12 | 2 | [131] |
| Electrode | Nanomaterials | Analyte | Limit of Detection | Reference |
|---|---|---|---|---|
| Micro-comb | 2-aminoethane thiol, gold nanoparticles | Aflatoxin B1 | 0.10 ng/mL | [132] |
| Indium-tin-oxide | Chitosan, titanium dioxide nanoparticles | Ochratoxin A | 10 ng/mL | [133] |
| Indium-tin-oxide | Chitosan, gold nanoparticles | Cholesterol | - | [134] |
| Indium-tin-oxide | Chitosan, cerium oxide nanoparticles | Mycotoxin | 0.25 ng/dL | [135] |
| Glassy carbon | Nafion, room temperature ionic liquid, titanium dioxide nanoparticles, gold nanoparticles | Aflatoxin B1 | - | [136] |
| Glassy carbon | Bismuth nano-film | E. coli | 100 cfu/mL | [137] |
| Glassy carbon | Gold nanoparticles | Xanthine and hypoxanthine | - | [138] |
| Types of Sensors | Target Material | Reproducibility | Reference |
|---|---|---|---|
| Screen-printed FR4 sensors | Rapeseed oil | High (100%) | [144] |
| α-FOX sensors | Roasted coffee beans | High | [145] |
| MGD-1 sensor | Emmental cheese | - | [148] |
| FIGARO sensors | Honey: acacia flower, linden flower, rape, buckwheat, honeydew | High (96%) | [149] |
| FIGARO sensors | Triticale, corn, wheat, barley, bread rye, diamond rye and golden rye | High (93%) | [150] |
| TGS Figaro gas sensors | Three types of wines: alc10, alc12, alc14 | High | [154] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Yuan, Y.; Nag, A.; Feng, S.; Afsarimanesh, N.; Han, T.; Mukhopadhyay, S.C.; Organ, D.R. A Review on the Use of Impedimetric Sensors for the Inspection of Food Quality. Int. J. Environ. Res. Public Health 2020, 17, 5220. https://doi.org/10.3390/ijerph17145220
He S, Yuan Y, Nag A, Feng S, Afsarimanesh N, Han T, Mukhopadhyay SC, Organ DR. A Review on the Use of Impedimetric Sensors for the Inspection of Food Quality. International Journal of Environmental Research and Public Health. 2020; 17(14):5220. https://doi.org/10.3390/ijerph17145220
Chicago/Turabian StyleHe, Shan, Yang Yuan, Anindya Nag, Shilun Feng, Nasrin Afsarimanesh, Tao Han, Subhas Chandra Mukhopadhyay, and Dominic Rowan Organ. 2020. "A Review on the Use of Impedimetric Sensors for the Inspection of Food Quality" International Journal of Environmental Research and Public Health 17, no. 14: 5220. https://doi.org/10.3390/ijerph17145220
APA StyleHe, S., Yuan, Y., Nag, A., Feng, S., Afsarimanesh, N., Han, T., Mukhopadhyay, S. C., & Organ, D. R. (2020). A Review on the Use of Impedimetric Sensors for the Inspection of Food Quality. International Journal of Environmental Research and Public Health, 17(14), 5220. https://doi.org/10.3390/ijerph17145220







