Effect of Supplements on Endurance Exercise in the Older Population: Systematic Review
Abstract
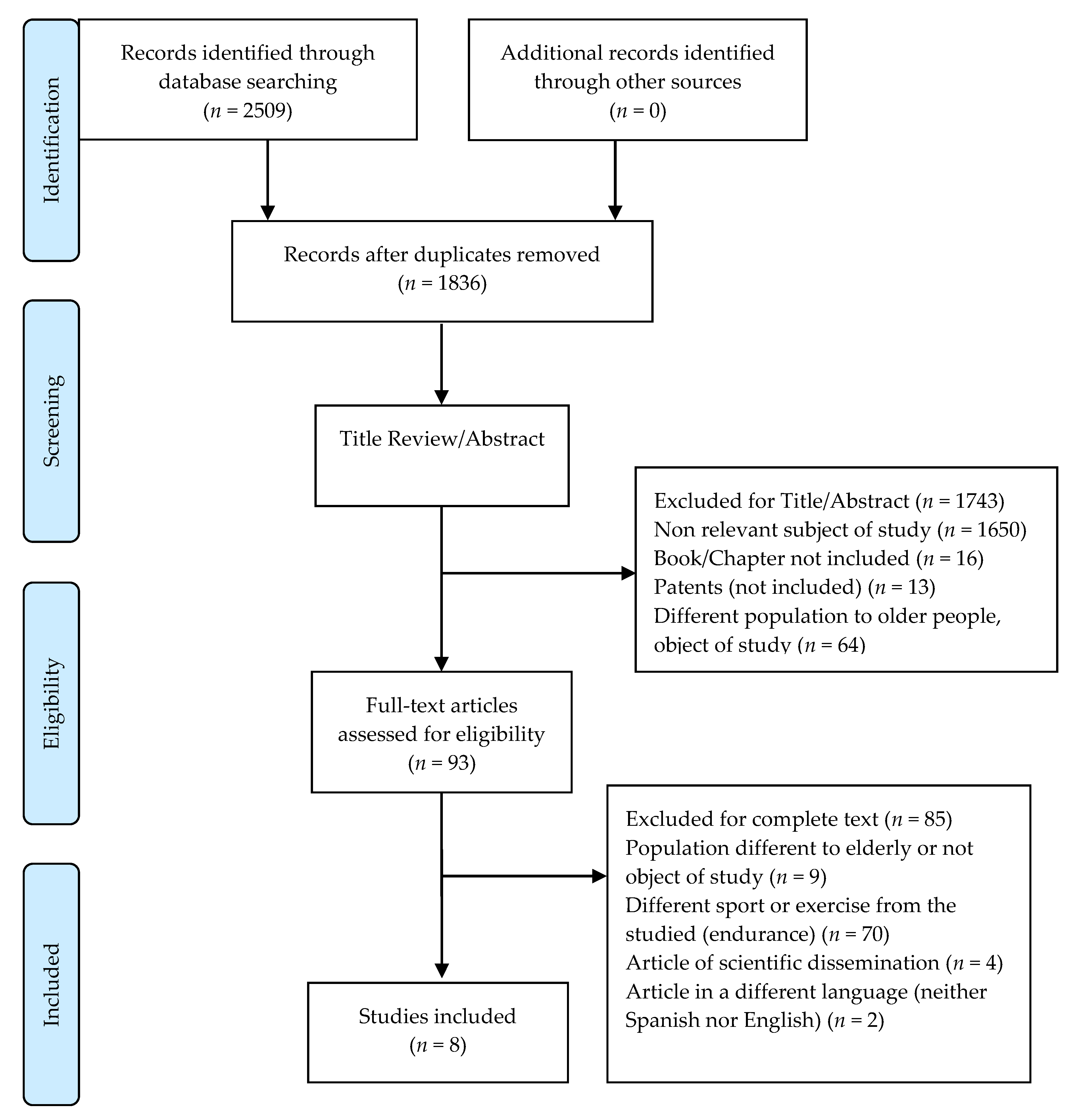
:1. Introduction
2. Materials and Methods.
2.1. Data Sources and Searches
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection and Data Extraction
3. Results
3.1. General Characteristics of Studies
3.2. Risk-of-Bias Assessment
3.3. Intervention
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Christensen, K.; Doblhammer, G.; Rau, R.; Vaupel, J.W. Ageing populations: The challenges ahead. Lancet 2009, 74, 1196–1208. [Google Scholar] [CrossRef] [Green Version]
- WHO. Ageing and Life Course. WHO, 2019. Available online: https://www.who.int/ageing/en/ (accessed on 16 July 2020).
- Tarnopolsky, M.A. Nutritional Consideration in the Aging Athlete. Clin. J. Sport Med. 2008, 18, 531–538. [Google Scholar] [CrossRef]
- Walrand, S.; Guillet, C.; Salles, J.; Cano, N.; Boirie, Y. Physiopathological mechanism of sarcopenia. Clin. Geriatr. Med. 2011, 27, 365–385. [Google Scholar] [CrossRef]
- Narici, M.V.; Maffulli, N. Sarcopenia: Characteristics, mechanisms and functional significance. Br. Med. Bull. 2010, 95, 139–159. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Vetta, F.; Ronzoni, S.; Taglieri, G.; Bollea, M.R. The impact of malnutrition on the quality of life in the elderly. Clin. Nutr. 1999, 18, 259–267. [Google Scholar] [CrossRef]
- Rolland, Y.; Czerwinski, S.; Van Kan, G.A.; Morley, J.E.; Cesari, M.; Onder, G.; Woo, J.; Baumgartner, R.; Pillard, F.; Boirie, Y.; et al. Sarcopenia: Its assessment, etiology, pathogenesis, consequences and future perspectives. J. Nutr. Health Aging 2008, 12, 433–450. [Google Scholar] [CrossRef] [Green Version]
- Wilmore, J.H.; Costill, D.L. Fisiología del esfuerzo y del deporte; Paidotribo: Barcelona, Spain, 2004. [Google Scholar]
- Chodzko-Zajko, W.J.; Proctor, D.N.; Fiatarone Singh, M.A.; Minson, C.T.; Nigg, C.R.; Salem, G.J.; Skinner, J.S. Exercise and physical activity for older adults. Med. Sci. Sport. Exer. 2009, 41, 1510–1530. [Google Scholar] [CrossRef]
- Chou, C.-H.; Hwang, C.-L.; Wu, Y.-T. Effect of exercise on physical function, activities of daily living, and quality of life in the frail elderly: A meta-analysis. Arch. Phys. Med. Rehabil. 2012, 93, 237–244. [Google Scholar] [CrossRef]
- Dallosso, H.M.; McGrother, C.W.; Matthews, R.J.; Donaldson, M.M.K.; Leicestershire MRC Incontinence Study Group. The association of diet and other lifestyle factors with overactive bladder and stress incontinence: A longitudinal study in women. BJU Int. 2003, 92, 69–77. [Google Scholar] [CrossRef]
- CDC. Data and Statistics. Available online: https://www.cdc.gov/physicalactivity/data/ (accessed on 16 July 2020).
- Oman, D.; Reed, D.; Ferrara, A. Do elderly women have more physical disability than men do? Am. J. Epidemiol. 1999, 150, 834–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drewnowski, A.; Warren-Mears, V.A. Does aging change nutrition requirements? J. Nutr. Health Aging. 2001, 5, 70–74. [Google Scholar] [PubMed]
- Baumgartner, R.N.; Waters, D.L.; Gallagher, D.; Morley, J.E.; Garry, P.J. Predictors of skeletal muscle mass in elderly men and women. Mech. Ageing Dev. 1999, 107, 123–136. [Google Scholar] [CrossRef]
- Houston, D.K.; Nicklas, B.J.; Ding, J.; Harris, T.B.; Tylavsky, F.A.; Newman, A.B.; Lee, J.S.; Sahyoun, N.R.; Visser, M.; Kritchevsky, S.B.; et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: The Health, Aging, and Body Composition (Health ABC) study. Am. J. Clin. Nutr. 2008, 87, 150–155. [Google Scholar] [CrossRef] [Green Version]
- Geirsdottir, O.G.; Arnarson, A.; Ramel, A.; Jonsson, P.V.; Thorsdottir, I. Dietary protein intake is associated with lean body mass in community-dwelling older adults. Nutr. Res. 2013, 33, 608–612. [Google Scholar] [CrossRef]
- Sociedad Española de Nutrición Parenteral y Enteral (SENPE); Sociedad Española de Geriatría y Gerontología(SEGG). Valoración Nutricional en el Anciano. Available online: https://www.segg.es/media/descargas/Acreditacion%20de%20Calidad%20SEGG/Residencias/valoracion_nutricional_anciano.pdf (accessed on 16 July 2020).
- Casimiro, C.; García De Lorenzo, A.; Usán, L.; El Grupo De Estudio, Y.; Geriátrico, C. Evaluación del riesgo nutricional en pacientes ancianos ambulatorios. Nutr. Hosp. 2001, 3, 97–103. (In Spanish) [Google Scholar]
- Wolfe, R.R. The underappreciated role of muscle in health and disease. Am. J. Clin. Nutr. 2006, 84, 475–482. [Google Scholar] [CrossRef]
- Demling, R.H. Nutrition, anabolism, and the wound healing process: An overview. Eplasty 2009, 9, 9. [Google Scholar]
- Rondanelli, M.; Opizzi, A.; Antoniello, N.; Boschi, F.; Iadarola, P.; Pasini, E.; Aquilani, R.; Dioguardi, F.S. Effect of essential amino acid supplementation on quality of life, Amino acid profile and strength in institutionalized elderly patients. Clin. Nutr. 2011, 30, 571–577. [Google Scholar] [CrossRef]
- Rolland, Y.; Onder, G.; Morley, J.E.; Gillette-Guyonet, S.; Abellan van Kan, G.; Vellas, B. Current and future pharmacologic treatment of sarcopenia. Clin. Geriat. Med. 2011, 27, 423–447. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. BMC 2016, 20, 148–160. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, M.J.; Clarke, N.D.; Tallis, J.; Guimarães-Ferreira, L.; Leddington Wright, S. The effect of caffeine ingestion on functional performance in older adults. J. Nutr. Heal. Aging 2014, 18, 883–887. [Google Scholar] [CrossRef] [PubMed]
- McCormack, W.P.; Stout, J.R.; Emerson, N.S.; Scanlon, T.C.; Warren, A.M.; Wells, A.J.; Gonzalez, A.M.; Mangine, G.T.; Robinson, E.H.; Fragala, M.S.; et al. Oral nutritional supplement fortified with beta-alanine improves physical working capacity in older adults: A randomized, placebo-controlled study. Exp. Gerontol. 2013, 48, 933–939. [Google Scholar] [CrossRef] [Green Version]
- Verschueren, S.M.; Bogaerts, A.; Delecluse, C.; Claessens, A.L.; Haentjens, P.; Vanderschueren, D.; Boonen, S. The effects of whole-body vibration training and vitamin D supplementation on muscle strength, muscle mass, and bone density in institutionalized elderly women: A 6-month randomized, controlled trial. J. Bone. Miner. Res. 2011, 26, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Sillanpää, E.; Häkkinen, A.; Laaksonen, D.E.; Karavirta, L.; Kraemer, W.J.; Häkkinen, K. Serum basal hormone concentrations, nutrition and physical fitness during strength and/or endurance training in 39-64-year-old women. Int. J. Sports Med. 2010, 31, 110–117. [Google Scholar] [CrossRef]
- Dawson-Hughes, B.; Castaneda-Sceppa, C.; Harris, S.S.; Palermo, N.J.; Ceglia, L.; Cloutier, G.; Dallal, G.E. Impact of supplementation with bicarbonate on lower-extremity muscle performance in older men and women. Osteoporos. Int. 2010, 21, 1171–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norager, C.B.; Jensen, M.B.; Madsen, M.R.; Laurberg, S. Caffeine improves endurance in 75-yr-old citizens: A randomized, double-blind, placebo-controlled, crossover study. J. Appl. Physiol. 2005, 99, 2302–2306. [Google Scholar] [CrossRef]
- Flakoll, P.; Sharp, R.; Baier, S.; Levenhagen, D.; Carr, C.; Nissen, S. Effect of β-hydroxy-β-methylbutyrate, arginine, and lysine supplementation on strength, functionality, body composition, and protein metabolism in elderly women. Nutrition 2004, 20, 445–451. [Google Scholar] [CrossRef]
- Laaksonen, R.; Fogelholm, M.; Himberg, J.J.; Laakso, J.; Salorinne, Y. Ubiquinone supplementation and exercise capacity in trained young and older men. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 72, 95–100. [Google Scholar] [CrossRef]
- PEDro scale (English). Available online: https://www.pedro.org.au/english/downloads/pedro-scale/ (accessed on 16 July 2020).
- Burke, L. Nutricion En El Deporte/Nutrition in Sport: Un Enfoque Practico/a Practical Approach; Editorial Medica Panamericana: Ciudad, Mexico, 2009. [Google Scholar]
- Wilborn, C.; Beckham, J.; Campbell, B.; Harvey, T.; Galbreath, M.; La Bounty, P.; Nassar, E.; Wismann, J.; Kreider, R. Obesity: Prevalence, Theories, Medical Consequences, Management, and Research Directions. J. Int. Soc. Sports Nutr. 2005, 2, 4–31. [Google Scholar] [CrossRef] [Green Version]
- Perna, S.; Peroni, G.; Anna, F.M.; Bartolo, A.; Naso, M.; Miccono, A.; Rondanelli, M. Sarcopenia and sarcopenic obesity in comparison: Prevalence, metabolic profile, and key differences. A cross-sectional study in Italian hospitalized elderly. Aging Clin. Exp. Res. 2017, 29, 1–10. [Google Scholar]
- Baccaro, L.F.; Conde, D.M.; Costa-Paiva, L.; Pinto-Neto, A.M. The epidemiology and management of postmenopausal osteoporosis: A viewpoint from Brazil. Clin. Interv. Aging 2015, 10, 583–591. [Google Scholar] [CrossRef] [Green Version]
- Marzetti, E.; Calvani, R.; Tosato, M.; Cesari, M.; Di Bari, M.; Cherubini, A.; Broccatelli, M.; Savera, G.; D’Elia, M.; Pahor, M.; et al. Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging Clin. Exp. Res. 2017, 29, 35–42. [Google Scholar] [CrossRef]
- Deutz, N.E.P.; Bauer, J.M.; Barazzoni, R.; Biolo, G.; Boirie, Y.; Bosy-Westphal, A.; Cederholm, T.; Cruz-Jentoft, A.; Krznariç, Z.; Nair, K.S.; et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group. Clin. Nutr. 2014, 33, 929–936. [Google Scholar] [CrossRef] [Green Version]
- Volpi, E.; Campbell, W.W.; Dwyer, J.T.; Johnson, M.A.; Jensen, G.L.; Morley, J.E.; Wolfe, R.R. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 677–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the prot-age study group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R. Dietary protein and exercise training in ageing. Proc. Nutr. Soc. 2011, 70, 104–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaffney-Stomberg, E.; Insogna, K.L.; Rodriguez, N.R.; Kerstetter, J.E. Increasing dietary protein requirements in elderly people for optimal muscle and bone health. J. Am. Geri. Soc. 2009, 57, 1073–1079. [Google Scholar] [CrossRef]
- Morley, J.E.; Argiles, J.M.; Evans, W.J.; Bhasin, S.; Cella, D.; Deutz, N.E.P.; Doehner, W.; Fearon, F.C.; Ferrucci, L.; Hellerstein, M.K.; et al. Nutritional recommendations for the management of sarcopenia. J. Am. Med. Dir. Assoc. 2010, 11, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Durham, W.J.; Casperson, S.L.; Dillon, E.L.; Keske, M.A.; Paddon-Jones, D.; Sanford, A.P.; Hickner, R.C.; Grady, J.J.; Sheffield-Moore, M. Age-related anabolic resistance after endurance-type exercise in healthy humans. FASEB J. 2010, 24, 4117–4127. [Google Scholar] [CrossRef] [PubMed]
- Churchward-Venne, T.A.; Holwerda, A.M.; Phillips, S.M.; Van Loon, L.J.C. What is the Optimal Amount of Protein to Support Post-Exercise Skeletal Muscle Reconditioning in the Older Adult? Sports Med. 2016, 46, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Cherniack, E.P. Ergogenic dietary aids for the elderly. Nutrition 2012, 28, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, Z.V.; Nelsonsteen, S.; Scafidi, K. Exercise, Aging, and Nutrition. South Med. J. 1994, 87, 50–60. [Google Scholar] [CrossRef]
- Palacios, N.; Manonelles, P.; Blasco, R.; Franco, L.; Teresa Gaztañaga, B.M.; García, J.A.V. Documento de consenso de la Federación Española de Medicina del Deporte, 2019. Available online: http://archivosdemedicinadeldeporte.com/articulos/upload/Doc-consenso-ayudas-2019.pdf (accessed on 15 July 2020).
- Davis, J.K.; Green, J.M. Caffeine and anaerobic performance: Ergogenic value and mechanisms of action. Sports Med. 2009, 39, 813–832. [Google Scholar] [CrossRef]
- Astorino, T.A.; Roberson, D.W. Efficacy of acute caffeine ingestion for short-term high-intensity exercise performance: A systematic review. J. Strength. Cond. Res. 2010, 24, 257–265. [Google Scholar] [CrossRef]
- Astorino, T.A.; Terzi, M.N.; Roberson, D.W.; Burnett, T.R. Effect of two doses of caffeine on muscular function during isokinetic exercise. Med. Sci. Sports Exerc. 2010, 42, 2205–2210. [Google Scholar] [CrossRef]
- Campbell, W.W.; Geik, R.A. Nutritional considerations for the older athlete. Nutrition 2004, 20, 603–608. [Google Scholar] [CrossRef]
| Lead Author, Year | 1. Selection Criteria | 2. Random Assignment | 3. Hidden Assignment | 4. Similar Groups | 5. Blinded Subjects | 6. Blinded Therapists | 7. Blinded Evaluators | 8. Adequate Follow-up | 9. Intention to Treat | 10. Comparison between Groups | 11. Punctual Measures of Variability | Total Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ducan et al., 2014 [27] | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | 8 |
| McCormark et al., 2013 [28] | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | 7 |
| Verschueren et al., 2010 [29] | Yes | Yes | No | Yes | No | No | Yes | Yes | Yes | Yes | Yes | 7 |
| Sillanpää et al., 2010 [30] | Yes | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | 6 |
| Dawson-Hughes et al., 2010 [31] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 9 |
| Norager et al. 2005 [32] | Yes | Yes | Yes | Yes | Yes | Yes | no | No | Yes | Yes | Yes | 8 |
| Flakoll et al., 2004 [33] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | 9 |
| Laaksonen et al., 1995 [34] | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | 9 |
| Author and Year | Characteristics | Period | Intervention | Measures | Results | Conclusion | Type of Exercise | Corporal Composition |
|---|---|---|---|---|---|---|---|---|
| Duncan et al., 2014 [27] | N = 19 Sex: M = 9; F = 10 Age: 66 ± 2 years Non usual caffeine consumers | The sup. is given 60 min before the measurements | G 1: Caffeine (3 mg of caffeine x kg−1 body mass) G 2: Placebo (3 mg dextrose x kg−1 body mass) | Test fitness The Rikli and Jones Senior fitnessManual Dexterity Turning Test | G1 significantly improved performance compared to G2. In all analyses, gender was not significant | Acute caffeine intake improves functional performance and manual dexterity in older people. | Non training. The participants were physically active. | There were no pre- or post-intervention data regarding BC. |
| McCormack et al., 2013 [28] | N = 44 Age: 70.7 ± 6.2 years G1: N = 16 / Sex: M = 11; F = 5 G2: N = 15 / Sex: M = 5; F = 10 G3: N = 13 / Sex: M = 6; F = 7 | 12 weeks | G1: twice a day, Ensure high protein (ONS) G2: twice a day, ONS plus 800 mg beta-alanine G3: twice a day, ONS plus 1200 mg beta-alanine | Submaximal discontinuous test in cyclo-ergometer Manual grip dynamometer 30-sec sit-to stand (STS)DEXA | G2 and G3 show significant improvements in their PWCFT and were not significantly different. No improvements in GRIP G3: improvement in 30s STS test There were no significant changes in BC for any G. | ONS strengthened with beta-alanine can improve physical work capacity, muscle quality, and its function in older men and women. | No training | There were no differences in body mass, fat free mass, or fat mass. |
| Verschuerenet al., 2011 [29] | N: 113 Sex: F Age range: 70–80 years G1: N = 28/Age: 79.8 ± 5.3 G2: N = 26/Age: 80.3 ± 5.3 G3: N = 28/Age: 79.6 ± 5.2 G4: N = 29/Age: 78.7 ± 5.6 | 6 months | G1: VPT 3 times / week + 880 IU Vit D G2: VPT 3 times / week + 1600 IU Vit D G3 (control): 880 IU Vit D G4 (control): 1600 IU vitamin D | Muscle strength: knee extension with dynamometer CT scan of the thigh | The VPT program did not increase muscle strength, the FM, hip BMD, or serum Vit. D levels compared to a program without exercises. Ingestion of 1600 IU of Vit. D produced a greater increase in serum Vit D levels compared to a dose of 880 IU, but there were no differences in muscle strength between both G. | The strength and MM. do not change significantly The VPT does not offer additional improvements than that provided by vitamin D. A higher dose of vitamin D does not demonstrate muscle benefits in dynamic muscle strength, hip BMD, or serum vitamin D levels compared to conventional doses. | Static and dynamic exercises on a vibrating platform 3 times per week. | No significant differences in FM. G1: BMI: 27.5 (SD 2.7), FM, (cm3): 69.6 (SD 11.3) G2: BMI: 26.4 (SD 4.4), FM. (cm3): 67.3 (SD 8.0) G3: BMI: 27.4 (SD 3.7), FM. (Cm3): 72.1 ± 10.2 |
| Sillanpää et al., 2010 [30] | G1: N = 21/Age: 53 ± 8/Sex: F G2: (control) N = 9/Age: 53 ± 8/Sex: F | 21 weeks | G1: endurance training (cycle ergometer) 2x week + FNR: 47 ± 6% CH, 19 ± 3% protein, 32 ± 4% fat. G2 (control): No training + FNR | Maximum pedaling force Knee Extender Force DEXA | No improvements in knee extender strength. There is an increase in blood cortisol (32.7 ± 51.3%) | Endurance training with a bicycle twice a week increases the maximum pedaling power, but not muscle strength. | Cycle ergometer: Periodic training in two cycles: Increasing intensity and volume (from 30 min aerobic to 90 min) | G1: significant decrease in BMI and increase in MM in legs. Initial data: G1: Body Mass: 66 ± 9 kg/BMI: 25.1 ± 2.6/% fat 37.4 ± 5.1 G2: Body Mass: 66 ± 8 kg/BMI: 23.4 ± 2.0/% fat: 32.1 ± 6.1 |
| Dawson-Hughes et al., 2010 [31] | Sex: F G3 (control): N = 49/ Age: 62.7 ± 7.4 G4: N = 42/Age: 61.7 ± 7.8 | 3 months | G1: (control) microcrystalline cellulose capsules G 2: (treatment) 67.5 mmol/day of sodium bicarbonate (sodium, potassium or sodium chloride) in gelatin capsules. Everyone took a calcium triphosphate sup. and a multivitamin with Vit. D3 daily with breakfast | 1-RM/Leg press and knee extension Manual dynamometry Cybex II isokinetic dynamometer Blood and urine tests | Sodium bicarbonate sup. was well tolerated and the NAE decreased. NAE was inversely correlated with the change in performance measures. Sodium bicarbonate increased leg press power to 70% of an 1-RM and improved other performance measures. | Sodium bicarbonate ingestion decreased nitrogen excretion and sodium bicarbonate-induced decrease in NAE was associated with a reduction in nitrogen excretion. Sodium bicarbonate can reduce age-related loss of muscle performance and mass in older women. | No Training | There were no data regarding BC |
| Sex: M G1 (control): N = 35/Age: 64.2 ± 8.2 G2: N = 36/Age: 63.8 ± 8.3 | Sodium bicarbonate sup. was well tolerated and decreased excretion of NAE. The NAE was not correlated with any of the performance measures. The sodium bicarbonate treatment did not have a significant effect on muscle performance in men. | Sodium bicarbonate sup. was well tolerated but had no favorable effects on selected measures of muscle performance in men. The reason may be due to the dose in relation to body size. | ||||||
| Norager et al., 2005 [32] | N = 30 Age: 74.7 ± 5.5 N = 15/Sex: F N = 15/Sex: M | G1: caffeine 1h before exercise (6 mg/kg) G2 (control): placebo G1 and G2: No caffeine 48 h before. High carb diet 1 day before. | Maximum voluntary isometric force of arm flexion Cycloergometer test Walking speed: 15 m. Perceived effort | G1: improves submaximal isometric strength by 54% and reduces the perceived effort in 5 min by pedaling by 11%. G1 had no significant effect on reaction times or movements | Caffeine sup. increases cycling endurance, isometric flexural strength of the arm, and perceived exertion during cycling in older people. | There was no training | There were no differences. Initial data: Height: 164.3 ± 9.2 m Body Mass: 72.1 ± 13.4 kg | |
| Flakoll et al., 2004 [33] | Study 1: G1: N = 13/Age: 84.2 ± 1.6/Sex: F G2: (control) N = 10/Age: 81.1 ± 1.8/Sex: F Study 2: G1: N = 14/Age: 71.5 ± 1/Sex: F G2: (control) N = 13/Age: 71.5 ± 1.5/Sex: F | 12 weeks | Study 1 and 2: G1: Orange flavor drink sup. (calcium HMB; arginine; lysine hydrochloride, and ascorbic Ac) Study 1: G2 (control): Drink (maltodextrin and ascorbic acid) Study 2: G2 (control): Drink (nitrogen + non-essential amino acids and ascorbic Ac) | “Get-up-and-go” functionality test Knee extender and knee flexor force Grip Force: Handgrip | G1 obtained a 17% improvement in the “get-up-and-go” test. G1 increased the circumference, leg strength, and grip strength | Sup.with HMB, arginine, and lysine improve functionality, strength, FFM, and protein synthesis, suggesting that this nutritional strategy affects muscle health in older women. | No training | G1: increases FFM (0.7 ± 0.3 kg) compared to subjects with a placebo sup. (0.0–0.3 kg) |
| Laaksonen et al., 1995 [34] | N = 19 Elderly men: N = 8/Age: 60–74 y/Sex: M Young men: N = 11/Age: 22–38/Sex: M | 6 weeks separated by a 4 week rest period | G1: Ubiquinone + Placebo G2: Placebo + Ubiquinone Treatment phase: 120 mg ubiquinone + fish oil. Placebo phase: 120 mg of placebo + fish oil. | Ergometer endurance exercise tests Muscle biopsy to determine ubiquinone. | The concentration of ubiquinone in blood increased after sup. in older and younger people. The time to fatigue was longer after placebo intake than after the treatment phase. | Ubiquinone was ineffective in reducing fatigue and improving aerobic and anaerobic function and was also ineffective as an ergogenic aid in trained young and older men. | There was no training of the study, but they were trained subjects. | There were no data regarding BC. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Rodríguez, A.; Cuestas-Calero, B.J.; Hernández-García, M.; Martíez-Olcina, M.; Vicente-Martínez, M.; Rubio-Arias, J.Á. Effect of Supplements on Endurance Exercise in the Older Population: Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 5224. https://doi.org/10.3390/ijerph17145224
Martínez-Rodríguez A, Cuestas-Calero BJ, Hernández-García M, Martíez-Olcina M, Vicente-Martínez M, Rubio-Arias JÁ. Effect of Supplements on Endurance Exercise in the Older Population: Systematic Review. International Journal of Environmental Research and Public Health. 2020; 17(14):5224. https://doi.org/10.3390/ijerph17145224
Chicago/Turabian StyleMartínez-Rodríguez, Alejandro, Bernardo J. Cuestas-Calero, María Hernández-García, María Martíez-Olcina, Manuel Vicente-Martínez, and Jacobo Á. Rubio-Arias. 2020. "Effect of Supplements on Endurance Exercise in the Older Population: Systematic Review" International Journal of Environmental Research and Public Health 17, no. 14: 5224. https://doi.org/10.3390/ijerph17145224
APA StyleMartínez-Rodríguez, A., Cuestas-Calero, B. J., Hernández-García, M., Martíez-Olcina, M., Vicente-Martínez, M., & Rubio-Arias, J. Á. (2020). Effect of Supplements on Endurance Exercise in the Older Population: Systematic Review. International Journal of Environmental Research and Public Health, 17(14), 5224. https://doi.org/10.3390/ijerph17145224







