Factors Affecting Arsenic Methylation in Contaminated Italian Areas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Areas
2.2. Study Sample
2.3. Urine Sample Collection and Arsenic Analysis
2.4. Genetic Susceptibility
2.5. Urine Arsenic Indicators
2.6. Data Collection and Questionnaire Variables Selection
2.7. Statistical Methods
3. Results
3.1. Effects of Gender on Arsenic Methylation
3.2. Effects of Age on Arsenic Methylation
3.3. Effects of Aqueduct Water Consumption for Drinking Purpose on Arsenic Methylation
3.4. Effects of Occupational Exposure to Chemicals on Arsenic Methylation
3.5. Effects of Smoking 0n Arsenic Methylation
3.6. Effects of Wine Consumption on Arsenic Methylation
3.7. Effects of Whole Milk Consumption on Arsenic Methylation
3.8. Effects of Meat Consumption on Arsenic Methylation
3.9. Effects of Fish Consumption on Arsenic Methylation
3.10. Effects of the Polymorphism of the As3mt Gene on Arsenic Methylation
3.11. Effects of The Polymorphism of The Gstt Gene on Arsenic Methylation
3.12. Summary of The Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO Arsenic. Available online: http://www.who.int/ipcs/assessment/public_health/arsenic/en/ (accessed on 7 June 2020).
- IARC. Arsenic, Metals, Fibres, and Dusts. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 100 C; International Agency for Research on Cancer, Weltgesundheitsorganisation, Eds.; IARC: Lyon, France, 2012; ISBN 978–92–832–1320–8. [Google Scholar]
- Naujokas, M.F.; Anderson, B.; Ahsan, H.; Aposhian, H.V.; Graziano, J.H.; Thompson, C.; Suk, W.A. The Broad Scope of Health Effects from Chronic Arsenic Exposure: Update on a Worldwide Public Health Problem. Environ. Health Perspect. 2013, 121, 295–302. [Google Scholar] [CrossRef]
- Moon, K.A.; Oberoi, S.; Barchowsky, A.; Chen, Y.; Guallar, E.; Nachman, K.E.; Rahman, M.; Sohel, N.; D’Ippoliti, D.; Wade, T.J.; et al. A dose–response meta–analysis of chronic arsenic exposure and incident cardiovascular disease. Int. J. Epidemiol. 2017, 46, 1924–1939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Exposure to arsenic: A major public health concern. In Preventing Disease through Healthy Environments; Department of Public Health, Environmental and Social Determinants of Health, World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on Arsenic in Food. EFSA J. 2009, 7, 1351. [Google Scholar] [CrossRef]
- Cubadda, F.; D’Amato, M.; Mancini, F.R.; Aureli, F.; Raggi, A.; Busani, L.; Mantovani, A. Assessing human exposure to inorganic arsenic in high–arsenic areas of Latium: A biomonitoring study integrated with indicators of dietary intake. Ann. Ig. 2015, 27, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.G.M.; Allinson, G.; Stagnitti, F.; Tanaka, A.; Westbrooke, M. Arsenic contamination in Bangladesh groundwater: A major environmental and social disaster. Int. J. Environ. Health Res. 2002, 12, 235–253. [Google Scholar] [CrossRef]
- Centeno, J.A.; Tseng, C.-H.; Van der Voet, G.B.; Finkelman, R.B. Global impacts of geogenic arsenic: A medical geology research case. Ambio 2007, 36, 78–81. [Google Scholar] [CrossRef]
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Tseng, C.-H.; Huang, Y.-K.; Huang, Y.-L.; Chung, C.-J.; Yang, M.-H.; Chen, C.-J.; Hsueh, Y.-M. Arsenic exposure, urinary arsenic speciation, and peripheral vascular disease in blackfoot disease–hyperendemic villages in Taiwan. Toxicol. Appl. Pharmacol. 2005, 206, 299–308. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Directorate General for Health & Consumers. In Derogation on the Drinking Water Directive 98/83/EC; European Commission: Brussels, Belgium, 2010; ISBN 978-92-79-12752-6. [Google Scholar]
- Scientific Committee on Health and Environmental Risks (SCHER) Derogation on the Drinking Water Directive 98/83/EC. 2010. Available online: https://ec.europa.eu/health/scientific_committees/environmental_risks/docs/scher_o_120.pdf (accessed on 7 June 2020).
- Cubadda, F.; Ciardullo, S.; D’Amato, M.; Raggi, A.; Aureli, F.; Carcea, M. Arsenic contamination of the environment–food chain: A survey on wheat as a test plant to investigate phytoavailable arsenic in Italian agricultural soils and as a source of inorganic arsenic in the diet. J. Agric. Food Chem. 2010, 58, 10176–10183. [Google Scholar] [CrossRef] [PubMed]
- Bustaffa, E.; Minichilli, F.; Andreassi, M.G.; Carone, S.; Coi, A.; Cori, L.; Faita, F.; Faita, F.; Grecchi, S.; Minoia, C.; et al. Sorveglianza epidemiologica in aree interessate da inquinamento ambientale da arsenico di origine naturale o antropica (SEpiAs CCM 2010). Epidemiol. Prev. 2014, 38, 68. [Google Scholar]
- Vahter, M. Methylation of Inorganic Arsenic in Different Mammalian Species and Population Groups. Sci. Prog. 1999, 82, 69–88. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, C.A.; Aposhian, H.V.; Cebrian, M.E.; Yamauchi, H.; Silbergeld, E.K. Variability in human metabolism of arsenic. Environ. Res. 2003, 92, 85–91. [Google Scholar] [CrossRef]
- Tseng, C.-H. Metabolism of inorganic arsenic and non–cancerous health hazards associated with chronic exposure in humans. J. Environ. Biol. 2007, 28, 349–357. [Google Scholar] [PubMed]
- Vahter, M. Genetic polymorphism in the biotransformation of inorganic arsenic and its role in toxicity. Toxicol. Lett. 2000, 112–113, 209–217. [Google Scholar] [CrossRef]
- Cohen, S.M.; Arnold, L.L.; Eldan, M.; Lewis, A.S.; Beck, B.D. Methylated arsenicals: The implications of metabolism and carcinogenicity studies in rodents to human risk assessment. Crit. Rev. Toxicol. 2006, 36, 99–133. [Google Scholar] [CrossRef] [PubMed]
- Zakharyan, R.A.; Sampayo–Reyes, A.; Healy, S.M.; Tsaprailis, G.; Board, P.G.; Liebler, D.C.; Aposhian, H.V. Human monomethylarsonic acid (MMA(V)) reductase is a member of the glutathione–S–transferase superfamily. Chem. Res. Toxicol. 2001, 14, 1051–1057. [Google Scholar] [CrossRef]
- Tseng, C.-H. A review on environmental factors regulating arsenic methylation in humans. Toxicol. Appl. Pharmacol. 2009, 235, 338–350. [Google Scholar] [CrossRef]
- Shen, H.; Niu, Q.; Xu, M.; Rui, D.; Xu, S.; Feng, G.; Ding, Y.; Li, S.; Jing, M. Factors Affecting Arsenic Methylation in Arsenic–Exposed Humans: A Systematic Review and Meta–Analysis. IJERPH 2016, 13, 205. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, T.; Kobayashi, Y.; Cui, X.; Hirano, S. A new metabolic pathway of arsenite: Arsenic glutathione complexes are substrates for human arsenic methyltransferase Cyt19. Arch. Toxicol. 2005, 79, 183–191. [Google Scholar] [CrossRef]
- Aposhian, H.V.; Zakharyan, R.A.; Avram, M.D.; Kopplin, M.J.; Wollenberg, M.L. Oxidation and detoxification of trivalent arsenic species. Toxicol. Appl. Pharmacol. 2003, 193, 1–8. [Google Scholar] [CrossRef]
- Petrick, J.S.; Ayala–Fierro, F.; Cullen, W.R.; Carter, D.E.; Vasken Aposhian, H. Monomethylarsonous acid (MMA(III)) is more toxic than arsenite in Chang human hepatocytes. Toxicol. Appl. Pharmacol. 2000, 163, 203–207. [Google Scholar] [CrossRef]
- Kitchin, K.T. Recent advances in arsenic carcinogenesis: Modes of action, animal model systems, and methylated arsenic metabolites. Toxicol. Appl. Pharmacol. 2001, 172, 249–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stýblo, M.; Drobná, Z.; Jaspers, I.; Lin, S.; Thomas, D.J. The Role of Biomethylation in Toxicity and Carcinogenicity of Arsenic: A Research Update. Environ. Health Perspect. 2002, 110, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bredfeldt, T.; Jagadish, B.; Eblin, K.; Mash, E.; Gandolfi, A. Monomethylarsonous acid induces transformation of human bladder cells. Toxicol. Appl. Pharmacol. 2006, 216, 69–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vahter, M.; Concha, G. Role of Metabolism in Arsenic Toxicity. Pharmacol. Toxicol. 2001, 89, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Concha, G.; Nermell, B.; Vahter, M. Spatial and Temporal Variations in Arsenic Exposure via Drinking–water in Northern Argentina. J. Health Popul. Nutr. 2006, 24, 317–326. [Google Scholar]
- Tseng, C.-H. Arsenic methylation, urinary arsenic metabolites and human diseases: Current perspective. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2007, 25, 1–22. [Google Scholar] [CrossRef]
- Ahsan, H.; Chen, Y.; Kibriya, M.G.; Slavkovich, V.; Parvez, F.; Jasmine, F.; Gamble, M.V.; Graziano, J.H. Arsenic Metabolism, Genetic Susceptibility, and Risk of Premalignant Skin Lesions in Bangladesh. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1270–1278. [Google Scholar] [CrossRef] [Green Version]
- Lindberg, A.-L.; Rahman, M.; Persson, L.-Å.; Vahter, M. The risk of arsenic induced skin lesions in Bangladeshi men and women is affected by arsenic metabolism and the age at first exposure. Toxicol. Appl. Pharmacol. 2008, 230, 9–16. [Google Scholar] [CrossRef]
- Wen, J.; Wen, W.; Li, L.; Liu, H. Methylation capacity of arsenic and skin lesions in smelter plant workers. Environ. Toxicol. Pharmacol. 2012, 34, 624–630. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Liu, J.; Wang, D.; Zheng, Q.; Sun, G. Differences of Urinary Arsenic Metabolites and Methylation Capacity between Individuals with and without Skin Lesions in Inner Mongolia, Northern China. IJERPH 2014, 11, 7319–7332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, R.C.; Hsu, K.-H.; Chen, C.-J.; Froines, J.R. Arsenic Methylation Capacity and Skin Cancer. Cancer Epidemiol. Biomark. Prev. 2000, 9, 1259–1262. [Google Scholar]
- Chen, Y.-C.; Guo, Y.-L.L.; Su, H.-J.J.; Hsueh, Y.-M.; Smith, T.J.; Ryan, L.M.; Lee, M.-S.; Chao, S.-C.; Lee, J.Y.-Y.; Christiani, D.C. Arsenic methylation and skin cancer risk in southwestern Taiwan. J. Occup. Environ. Med. 2003, 45, 241–248. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Su, H.-J.J.; Guo, Y.-L.L.; Hsueh, Y.-M.; Smith, T.J.; Ryan, L.M.; Lee, M.-S.; Christiani, D.C. Arsenic methylation and bladder cancer risk in Taiwan. Cancer Causes Control. 2003, 14, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Su, H.-J.J.; Guo, Y.-L.L.; Houseman, E.A.; Christiani, D.C. Interaction between environmental tobacco smoke and arsenic methylation ability on the risk of bladder cancer. Cancer Causes Control. 2005, 16, 75–81. [Google Scholar] [CrossRef]
- Steinmaus, C.; Bates, M.N.; Yuan, Y.; Kalman, D.; Atallah, R.; Rey, O.A.; Biggs, M.L.; Hopenhayn, C.; Moore, L.E.; Hoang, B.K.; et al. Arsenic Methylation and Bladder Cancer Risk in Case–Control Studies in Argentina and the United States. J. Occup. Environ. Med. 2006, 48, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Steinmaus, C.; Moore, L.E.; Shipp, M.; Kalman, D.; Rey, O.A.; Biggs, M.L.; Hopenhayn, C.; Bates, M.N.; Zheng, S.; Wiencke, J.K.; et al. Genetic polymorphisms in MTHFR 677 and 1298, GSTM1 and T1, and metabolism of arsenic. J. Toxicol. Environ. Health Part A 2007, 70, 159–170. [Google Scholar] [CrossRef]
- Steinmaus, C.; Yuan, Y.; Kalman, D.; Rey, O.A.; Skibola, C.F.; Dauphine, D.; Basu, A.; Porter, K.E.; Hubbard, A.; Bates, M.N. Individual differences in arsenic metabolism and lung cancer in a case–control study in Cordoba, Argentina. Toxicol. Appl. Pharmacol. 2010, 247, 138–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melak, D.; Ferreccio, C.; Kalman, D.; Parra, R.; Acevedo, J.; Pérez, L.; Cortés, S.; Smith, A.H.; Yuan, Y.; Liaw, J.; et al. Arsenic methylation and lung and bladder cancer in a case–control study in northern Chile. Toxicol. Appl. Pharmacol. 2014, 274, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-K.; Tseng, C.-H.; Huang, Y.-L.; Yang, M.-H.; Chen, C.-J.; Hsueh, Y.-M. Arsenic methylation capability and hypertension risk in subjects living in arseniasis–hyperendemic areas in southwestern Taiwan. Toxicol. Appl. Pharmacol. 2007, 218, 135–142. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Hsueh, Y.-M.; Huang, Y.-K.; Yip, P.-K.; Yang, M.-H.; Chen, C.-J. Urinary arsenic methylation capability and carotid atherosclerosis risk in subjects living in arsenicosis–hyperendemic areas in southwestern Taiwan. Sci. Total Environ. 2009, 407, 2608–2614. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, F.; Liu, M.; Parvez, F.; Slavkovich, V.; Eunus, M.; Ahmed, A.; Argos, M.; Islam, T.; Rakibuz–Zaman, M.; et al. A Prospective Study of Arsenic Exposure, Arsenic Methylation Capacity, and Risk of Cardiovascular Disease in Bangladesh. Environ. Health Perspect. 2013, 121, 832–838. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Wang, Y.; Zheng, Q.; Li, X.; Li, B.; Jin, Y.; Sun, X.; Sun, G. Association of oxidative stress with arsenic methylation in chronic arsenic–exposed children and adults. Toxicol. Appl. Pharmacol. 2008. [Google Scholar] [CrossRef] [PubMed]
- Nizam, S.; Kato, M.; Yatsuya, H.; Khalequzzaman, M.; Ohnuma, S.; Naito, H.; Nakajima, T. Differences in Urinary Arsenic Metabolites between Diabetic and Non–Diabetic Subjects in Bangladesh. IJERPH 2013, 10, 1006–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendez, M.A.; González–Horta, C.; Sánchez–Ramírez, B.; Ballinas–Casarrubias, L.; Cerón, R.H.; Morales, D.V.; Terrazas, F.A.B.; Ishida, M.C.; Gutiérrez–Torres, D.S.; Saunders, R.J.; et al. Chronic Exposure to Arsenic and Markers of Cardiometabolic Risk: A Cross–Sectional Study in Chihuahua, Mexico. Environ. Health Perspect. 2016, 124, 104–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grau-Perez, M.; Kuo, C.-C.; Gribble, M.O.; Balakrishnan, P.; Jones Spratlen, M.; Vaidya, D.; Francesconi, K.A.; Goessler, W.; Guallar, E.; Silbergeld, E.K.; et al. Association of Low–Moderate Arsenic Exposure and Arsenic Metabolism with Incident Diabetes and Insulin Resistance in the Strong Heart Family Study. Environ. Health Perspect. 2017, 125, 127004. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-C.; Moon, K.A.; Wang, S.-L.; Silbergeld, E.; Navas-Acien, A. The Association of Arsenic Metabolism with Cancer, Cardiovascular Disease, and Diabetes: A Systematic Review of the Epidemiological Evidence. Environ. Health Perspect. 2017, 125, 087001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pace, C.; Smith–Gagen, J.; Angermann, J. Arsenic Methylation Capacity and Metabolic Syndrome in the 2013–2014 U.S. National Health and Nutrition Examination Survey (NHANES). IJERPH 2018, 15, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, B.; Yu, J.; Li, H.; Yang, L.; Xia, Y.; Wu, K.; Gao, J.; Guo, Z.; Cui, N. Arsenic Metabolites and Methylation Capacity Among Individuals Living in a Rural Area with Endemic Arseniasis in Inner Mongolia, China. Biol. Trace Elem. Res. 2016, 170, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Gamble, M.V.; Liu, X.; Ahsan, H.; Pilsner, J.R.; Ilievski, V.; Slavkovich, V.; Parvez, F.; Levy, D.; Factor–Litvak, P.; Graziano, J.H. Folate, Homocysteine, and Arsenic Metabolism in Arsenic–Exposed Individuals in Bangladesh. Environ. Health Perspect. 2005, 113, 1683–1688. [Google Scholar] [CrossRef] [Green Version]
- Kurzius-Spencer, M.; da Silva, V.; Thomson, C.A.; Hartz, V.; Hsu, C.-H.; Burgess, J.L.; O’Rourke, M.K.; Harris, R.B. Nutrients in one–carbon metabolism and urinary arsenic methylation in the National Health and Nutrition Examination Survey (NHANES) 2003–2004. Sci. Total Environ. 2017, 607–608, 381–390. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-W.; Wang, S.-L.; Wang, Y.-H.; Sun, C.-W.; Huang, Y.-L.; Chen, C.-J.; Li, W.-F. Arsenic methylation, GSTO1 polymorphisms, and metabolic syndrome in an arseniasis endemic area of southwestern Taiwan. Chemosphere 2012, 88, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, R.; Shao, K.; Thomas, D.J.; Sams, R.; Cowden, J. AS3MT, GSTO, and PNP polymorphisms: Impact on arsenic methylation and implications for disease susceptibility. Environ. Res. 2014, 132, 156–167. [Google Scholar] [CrossRef]
- Engström, K.S.; Vahter, M.; Fletcher, T.; Leonardi, G.; Goessler, W.; Gurzau, E.; Koppova, K.; Rudnai, P.; Kumar, R.; Broberg, K. Genetic variation in arsenic (+3 oxidation state) methyltransferase (AS3MT ), arsenic metabolism and risk of basal cell carcinoma in a European population: AS3MT Polymorphisms, Arsenic Metabolism, BCC Risk. Environ. Mol. Mutagen. 2015, 56, 60–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Yan, L.; Zhang, M.; Wang, Y.; Wang, C.; Xiang, Q. Associations between the polymorphisms of GSTT1, GSTM1 and methylation of arsenic in the residents exposed to low–level arsenic in drinking water in China. J. Hum. Genet. 2015, 60, 387–394. [Google Scholar] [CrossRef] [PubMed]
- de la Rosa, R.; Steinmaus, C.; Akers, N.K.; Conde, L.; Ferreccio, C.; Kalman, D.; Zhang, K.R.; Skibola, C.F.; Smith, A.H.; Zhang, L.; et al. Associations between arsenic (+3 oxidation state) methyltransferase (AS3MT) and N–6 adenine–specific DNA methyltransferase 1 ( N6AMT1 ) polymorphisms, arsenic metabolism, and cancer risk in a chilean population: Genetic Polymorphisms Associated with Arsenic Metabolism and Cancer Risk. Environ. Mol. Mutagen. 2017, 58, 411–422. [Google Scholar] [CrossRef]
- Musmeci, L.; Bianchi, F.; Carere, M.; Cori, L. Environment and health in Gela (Sicily): Present knowledge and prospects for future studies. Epidemiol. Prev. 2009, 33, 7–12. [Google Scholar]
- Musmeci, L.; Carere, M.; Fallenti, F. Environmental pollution in the area of Gela. Epidemiol. Prev. 2009, 33, 18–23. [Google Scholar]
- Comba, P.; Pirastu, R.; Conti, S.; De Santis, M.; Iavarone, I.; Marsili, G.; Mincuzzi, A.; Minelli, G.; Manno, V.; Minerba, S.; et al. Environment and health in Taranto, southern Italy: Epidemiological studies and public health recommendations. Epidemiol. Prev. 2012, 36, 305–320. [Google Scholar]
- Method Validation. Available online: https://www.eurachem.org/index.php/publications/guides/mv (accessed on 7 June 2020).
- Centers for Disease Control and Prevention (CDC)—Fourth National Report on Human Exposure to Environmental Chemicals. Available online: https://www.cdc.gov/exposurereport/pdf/fourthreport.pdf (accessed on 7 June 2020).
- Chung, C.-J.; Hsueh, Y.-M.; Bai, C.-H.; Huang, Y.-K.; Huang, Y.-L.; Yang, M.-H.; Chen, C.-J. Polymorphisms in arsenic metabolism genes, urinary arsenic methylation profile and cancer. Cancer Causes Control. 2009, 20, 1653–1661. [Google Scholar] [CrossRef]
- Schläwicke Engström, K.; Nermell, B.; Concha, G.; Strömberg, U.; Vahter, M.; Broberg, K. Arsenic metabolism is influenced by polymorphisms in genes involved in one–carbon metabolism and reduction reactions. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2009, 667, 4–14. [Google Scholar] [CrossRef] [Green Version]
- Valenzuela, O.L.; Drobná, Z.; Hernández–Castellanos, E.; Sánchez–Peña, L.C.; García–Vargas, G.G.; Borja–Aburto, V.H.; Stýblo, M.; Del Razo, L.M. Association of AS3MT polymorphisms and the risk of premalignant arsenic skin lesions. Toxicol. Appl. Pharmacol. 2009, 239, 200–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minichilli, F.; Bianchi, F.; Ronchi, A.; Gorini, F.; Bustaffa, E. Urinary Arsenic in Human Samples from Areas Characterized by Natural or Anthropogenic Pollution in Italy. IJERPH 2018, 15, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasserstein, R.L.; Schirm, A.L.; Lazar, N.A. Moving to a World Beyond “p <0.05”. Am. Stat. 2019, 73, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Tondel, M.; Rahman, M.; Magnuson, A.; Ahmad, S.A. The Relationship of Arsenic Levels in Drinking Water and the Prevalence Rate of Skin Lesions in Bangladesh. Environ. Health Perspect. 1999, 107, 3. [Google Scholar] [CrossRef]
- Fatmi, Z.; Abbasi, I.N.; Ahmed, M.; Kazi, A.; Kayama, F. Burden of skin lesions of arsenicosis at higher exposure through groundwater of taluka Gambat district Khairpur, Pakistan: A cross–sectional survey. Environ. Geochem. Health 2013, 35, 341–346. [Google Scholar] [CrossRef]
- Balakumar, P.; Kaur, J. Arsenic Exposure and Cardiovascular Disorders: An Overview. Cardiovasc. Toxicol. 2009, 9, 169–176. [Google Scholar] [CrossRef]
- Agusa, T.; Kunito, T.; Kubota, R.; Inoue, S.; Fujihara, J.; Minh, T.B.; Ha, N.N.; Tu, N.P.C.; Trang, P.T.K.; Chamnan, C.; et al. Exposure, metabolism, and health effects of arsenic in residents from arsenic–contaminated groundwater areas of Vietnam and Cambodia: A review. Rev. Environ. Health 2010, 25, 193–220. [Google Scholar] [CrossRef]
- Abhyankar, L.N.; Jones, M.R.; Guallar, E.; Navas–Acien, A. Arsenic Exposure and Hypertension: A Systematic Review. Environ. Health Perspect. 2012, 120, 494–500. [Google Scholar] [CrossRef] [Green Version]
- Ameer, S.S.; Engström, K.; Harari, F.; Concha, G.; Vahter, M.; Broberg, K. The effects of arsenic exposure on blood pressure and early risk markers of cardiovascular disease: Evidence for population differences. Environ. Res. 2015, 140, 32–36. [Google Scholar] [CrossRef]
- Pan, W.-C.; Seow, W.J.; Kile, M.L.; Hoffman, E.B.; Quamruzzaman, Q.; Rahman, M.; Mahiuddin, G.; Mostofa, G.; Lu, Q.; Christiani, D.C. Association of Low to Moderate Levels of Arsenic Exposure With Risk of Type 2 Diabetes in Bangladesh. Am. J. Epidemiol. 2013, 178, 1563–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Giovanni, P.; Di Martino, G.; Scampoli, P.; Cedrone, F.; Meo, F.; Lucisano, G.; Romano, F.; Staniscia, T. Arsenic Exposure and Risk of Urothelial Cancer: Systematic Review and Meta–Analysis. Int. J. Environ. Res. Public Health 2020, 17, 3105. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.-J.; Huang, C.-J.; Pu, Y.-S.; Su, C.-T.; Huang, Y.-K.; Chen, Y.-T.; Hsueh, Y.-M. Urinary 8–hydroxydeoxyguanosine and urothelial carcinoma risk in low arsenic exposure area. Toxicol. Appl. Pharmacol. 2008, 226, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-K.; Huang, Y.-L.; Hsueh, Y.-M.; Yang, M.-H.; Wu, M.-M.; Chen, S.-Y.; Hsu, L.-I.; Chen, C.-J. Arsenic exposure, urinary arsenic speciation, and the incidence of urothelial carcinoma: A twelve–year follow–up study. Cancer Causes Control. 2008, 19, 829–839. [Google Scholar] [CrossRef]
- Huang, Y.-K.; Pu, Y.-S.; Chung, C.-J.; Shiue, H.-S.; Yang, M.-H.; Chen, C.-J.; Hsueh, Y.-M. Plasma folate level, urinary arsenic methylation profiles, and urothelial carcinoma susceptibility. Food Chem. Toxicol. 2008, 46, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, A.-L.; Ekström, E.-C.; Nermell, B.; Rahman, M.; Lönnerdal, B.; Persson, L.-A.; Vahter, M. Gender and age differences in the metabolism of inorganic arsenic in a highly exposed population in Bangladesh. Environ. Res. 2008, 106, 110–120. [Google Scholar] [CrossRef]
- Pu, Y.-S.; Yang, S.-M.; Huang, Y.-K.; Chung, C.-J.; Huang, S.K.; Chiu, A.W.-H.; Yang, M.-H.; Chen, C.-J.; Hsueh, Y.-M. Urinary arsenic profile affects the risk of urothelial carcinoma even at low arsenic exposure. Toxicol. Appl. Pharmacol. 2007, 218, 99–106. [Google Scholar] [CrossRef]
- Fischer, L.M.; da Costa, K.A.; Kwock, L.; Stewart, P.W.; Lu, T.-S.; Stabler, S.P.; Allen, R.H.; Zeisel, S.H. Sex and menopausal status influence human dietary requirements for the nutrient choline. Am. J. Clin. Nutr. 2007, 85, 1275–1285. [Google Scholar] [CrossRef] [Green Version]
- Vahter, M.E. Interactions between Arsenic–Induced Toxicity and Nutrition in Early Life. J. Nutr. 2007, 137, 2798–2804. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Wang, D.; Zheng, Q.; Zheng, Y.; Wang, H.; Xu, Y.; Li, X.; Sun, G. Joint effects of urinary arsenic methylation capacity with potential modifiers on arsenicosis: A cross–sectional study from an endemic arsenism area in Huhhot Basin, northern China. Environ. Res. 2014, 132, 281–289. [Google Scholar] [CrossRef]
- Xi, S.; Zheng, Q.; Zhang, Q.; Sun, G. Metabolic profile and assessment of occupational arsenic exposure in copper– and steel–smelting workers in China. Int. Arch. Occup. Environ. Health 2011, 84, 347–353. [Google Scholar] [CrossRef]
- Wang, X.; Jin, P.; Zhou, Q.; Liu, S.; Wang, F.; Xi, S. Metal Biomonitoring and Comparative Assessment in Urine of Workers in Lead–Zinc and Steel–Iron Mining and Smelting. Biol. Trace Elem. Res. 2019, 189, 1–9. [Google Scholar] [CrossRef]
- Shuhua, X.; Qingshan, S.; Fei, W.; Shengnan, L.; Ling, Y.; Lin, Z.; Yingli, S.; Nan, Y.; Guifan, S. The factors influencing urinary arsenic excretion and metabolism of workers in steel and iron smelting foundry. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 36–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hata, A.; Endo, Y.; Nakajima, Y.; Ikebe, M.; Ogawa, M.; Fujitani, N.; Endo, G. HPLC-ICP-MS Speciation Analysis of Arsenic in Urine of Japanese Subjects without Occupational Exposure. J. Occup. Health 2007, 49, 217–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soleo, L.; Lovreglio, P.; Iavicoli, S.; Antelmi, A.; Drago, I.; Basso, A.; Di Lorenzo, L.; Gilberti, M.E.; De Palma, G.; Apostoli, P. Significance of urinary arsenic speciation in assessment of seafood ingestion as the main source of organic and inorganic arsenic in a population resident near a coastal area. Chemosphere 2008, 73, 291–299. [Google Scholar] [CrossRef]
- Vimercati, L.; Carrus, A.; Sciannamblo, G.; Caputo, F.; Minunni, V.; de Nichilo, G.; Bellotta, M.R.; Gagliardi, T.; Bisceglia, L.; Assennato, G. A study of factors influencing urinary arsenic excretion in exposed workers. Int. J. Environ. Health Res. 2009, 19, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Graziano, J.H.; Parvez, F.; Liu, M.; Slavkovich, V.; Kalra, T.; Argos, M.; Islam, T.; Ahmed, A.; Rakibuz–Zaman, M.; et al. Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: Prospective cohort study. BMJ 2011, 342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopenhayn–Rich, C.; Biggs, M.L.; Smith, A.H.; Kalman, D.A.; Moore, L.E. Methylation Study of a Population Environmentally Exposed to Arsenic in Drinking Water. Environ. Health Perspect. 1996, 104, 9. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.-S.; Ryu, D.-Y.; Choi, B.-S.; Park, J.-D. Urinary Arsenic Concentrations and their Associated Factors in Korean Adults. Toxicol. Res. 2013, 29, 137–142. [Google Scholar] [CrossRef]
- Jain, R.B. Association of arsenic exposure with smoking, alcohol, and caffeine consumption: Data from NHANES 2005–2010. Environ. Toxicol. Pharmacol. 2015, 39, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Bozack, A.K.; Saxena, R.; Gamble, M.V. Nutritional Influences on One–Carbon Metabolism: Effects on Arsenic Methylation and Toxicity. Annu. Rev. Nutr. 2018, 38, 401–429. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.J.; Li, J.; Waters, S.B.; Xing, W.; Adair, B.M.; Devesa, V.; Styblo, M. Arsenic (+3 Oxidation State) Methyltransferase and the Methylation of Arsenicals. Exp. Biol. Med. 2008, 232, 3–13. [Google Scholar]
- Hall, M.N.; Gamble, M.V. Nutritional manipulation of one–carbon metabolism: Effects on arsenic methylation and toxicity. J. Toxicol. 2012, 2012, 595307. [Google Scholar] [CrossRef] [Green Version]
- Spratlen, M.J.; Gamble, M.V.; Grau-Perez, M.; Kuo, C.-C.; Best, L.G.; Yracheta, J.; Francesconi, K.; Goessler, W.; Mossavar-Rahmani, Y.; Hall, M.; et al. Arsenic metabolism and one–carbon metabolism at low–moderate arsenic exposure: Evidence from the Strong Heart Study. Food Chem. Toxicol. 2017, 105, 387–397. [Google Scholar] [CrossRef]
- López-Carrillo, L.; Gamboa-Loira, B.; Becerra, W.; Hernández-Alcaraz, C.; Hernández-Ramírez, R.U.; Gandolfi, A.J.; Franco-Marina, F.; Cebrián, M.E. Dietary micronutrient intake and its relationship with arsenic metabolism in Mexican women. Environ. Res. 2016, 151, 445–450. [Google Scholar] [CrossRef]
- Heck, J.E.; Gamble, M.V.; Chen, Y.; Graziano, J.H.; Slavkovich, V.; Parvez, F.; Baron, J.A.; Howe, G.R.; Ahsan, H. Consumption of folate–related nutrients and metabolism of arsenic in Bangladesh. Am. J. Clin. Nutr. 2007, 85, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Aposhian, H.V.; Aposhian, M.M. Arsenic toxicology: Five questions. Chem. Res. Toxicol. 2006, 19, 1–15. [Google Scholar] [CrossRef]
- Strange, R.C.; Jones, P.W.; Fryer, A.A. Glutathione S–transferase: Genetics and role in toxicology. Toxicol. Lett. 2000, 112–113, 357–363. [Google Scholar] [CrossRef]
- Hayes, J.D.; Pulford, D.J. The glutathione S–transferase supergene family: Regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 445–600. [Google Scholar] [CrossRef]
- Del Razo, L.M.; Styblo, M.; Cullen, W.R.; Thomas, D.J. Determination of trivalent methylated arsenicals in biological matrices. Toxicol. Appl. Pharmacol. 2001, 174, 282–293. [Google Scholar] [CrossRef]
- Su, L.; Jin, Y.; Cheng, Y.; Lin, S. Study on the relationship between GSTM1, GSTT1 gene polymorphisms and arsenic methylation level. Wei Sheng Yan Jiu 2008, 37, 432–434. [Google Scholar] [PubMed]
- Agusa, T.; Iwata, H.; Fujihara, J.; Kunito, T.; Takeshita, H.; Minh, T.B.; Trang, P.T.K.; Viet, P.H.; Tanabe, S. Genetic polymorphisms in glutathione S–transferase (GST) superfamily and arsenic metabolism in residents of the Red River Delta, Vietnam. Toxicol. Appl. Pharmacol. 2010, 242, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Chiou, H.Y.; Hsueh, Y.M.; Hsieh, L.L.; Hsu, L.I.; Hsu, Y.H.; Hsieh, F.I.; Wei, M.L.; Chen, H.C.; Yang, H.T.; Leu, L.C.; et al. Arsenic methylation capacity, body retention, and null genotypes of glutathione S–transferase M1 and T1 among current arsenic–exposed residents in Taiwan. Mutat. Res. 1997, 386, 197–207. [Google Scholar] [CrossRef]
- Kile, M.L.; Houseman, E.A.; Quamruzzaman, Q.; Rahman, M.; Mahiuddin, G.; Mostofa, G.; Hsueh, Y.-M.; Christiani, D.C. Influence of GSTT1 Genetic Polymorphisms on Arsenic Metabolism. J. Indian Soc. Agric. Stat. 2013, 67, 197–207. [Google Scholar]
- Gomez–Rubio, P.; Roberge, J.; Arendell, L.; Harris, R.B.; O’Rourke, M.K.; Chen, Z.; Cantu–Soto, E.; Meza–Montenegro, M.M.; Billheimer, D.; Lu, Z.; et al. Association between body mass index and arsenic methylation efficiency in adult women from southwest U.S. and northwest Mexico. Toxicol. Appl. Pharmacol. 2011, 252, 176–182. [Google Scholar] [CrossRef] [Green Version]
- Gribble, M.O.; Crainiceanu, C.M.; Howard, B.V.; Umans, J.G.; Francesconi, K.A.; Goessler, W.; Zhang, Y.; Silbergeld, E.K.; Guallar, E.; Navas–Acien, A. Body Composition and Arsenic Metabolism: A Cross–Sectional Analysis in the Strong Heart Study. Environ. Health. 2013, 12, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudgens, E.E.; Drobna, Z.; He, B.; Le, X.C.; Styblo, M.; Rogers, J.; Thomas, D.J. Biological and behavioral factors modify urinary arsenic metabolic profiles in a U.S. population. Environ. Health 2016, 15, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, R.J.; Argos, M.; Tong, L.; Li, J.; Rakibuz–Zaman, M.; Islam, M.T.; Slavkovich, V.; Ahmed, A.; Navas–Acien, A.; Parvez, F.; et al. Determinants and Consequences of Arsenic Metabolism Efficiency among 4,794 Individuals: Demographics, Lifestyle, Genetics, and Toxicity. Cancer Epidemiol. Biomark. Prev. 2016, 25, 381–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bommarito, P.A.; Xu, X.; González-Horta, C.; Sánchez-Ramirez, B.; Ballinas-Casarrubias, L.; Luna, R.S.; Pérez, S.R.; Ávila, J.E.H.; García-Vargas, G.G.; Del Razo, L.M.; et al. One–carbon metabolism nutrient intake and the association between body mass index and urinary arsenic metaboliyes in adults in the Chihuahua cohort. Environ. Int. 2019, 123, 292–300. [Google Scholar] [CrossRef]
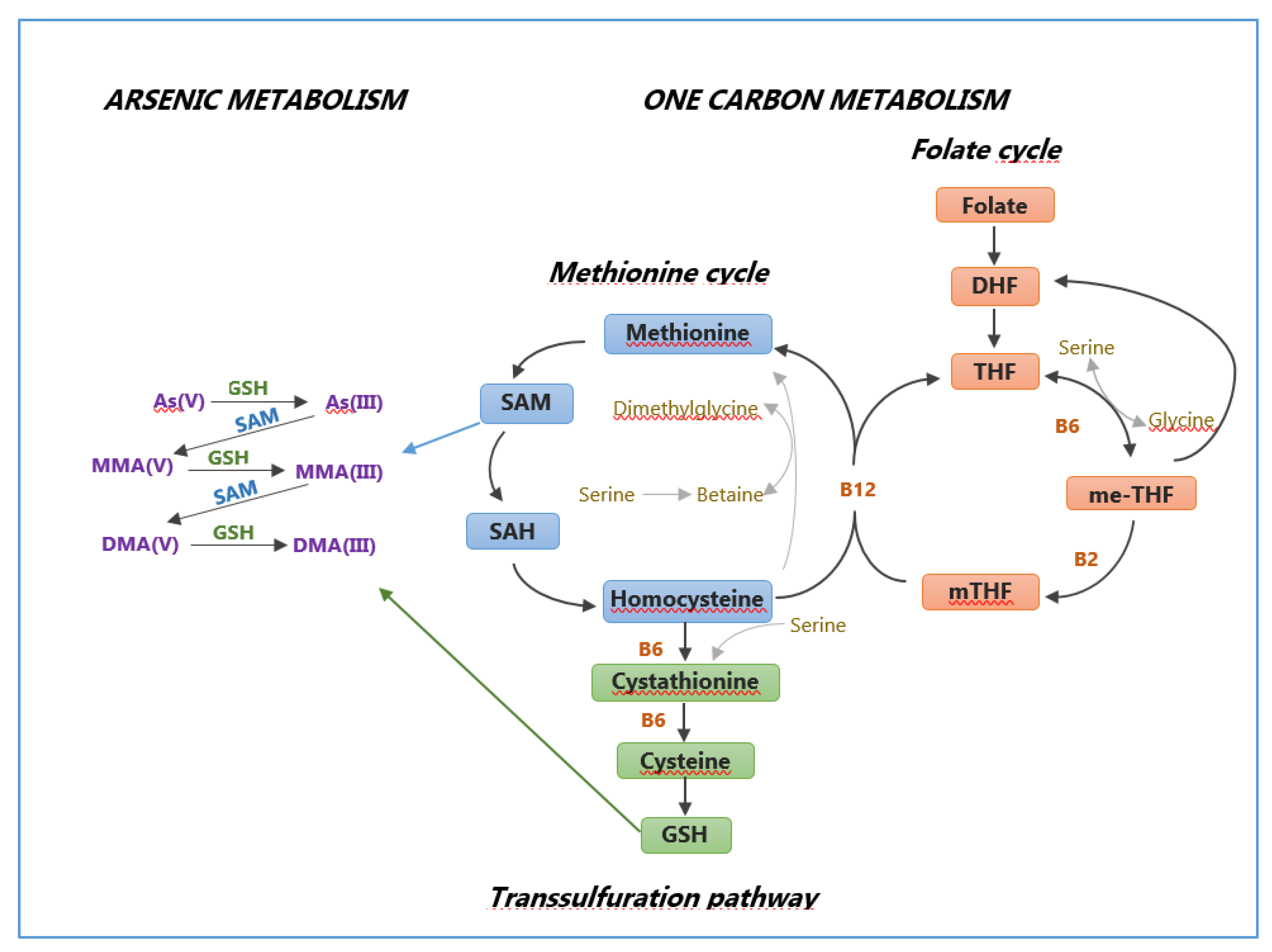
| AREA | MALE | FEMALE | TOTAL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20–29 (%) | 30–39 (%) | 40–44 (%) | TOTAL (%) | 20–29 (%) | 30–39 (%) | 40–44 (%) | Total (%) | 20–29 (%) | 30–39 (%) | 40–44 (%) | Total (%) | |
| Amiata | 10 (62.5) | 12 (75.0) | 6 (75.0) | 28 (70.0) | 11 (68.7) | 11 (68.7) | 8 (100.0) | 30 (75.0) | 21 (65.6) | 23 (71.9) | 14 (87.5) | 58 (72.5) |
| Viterbese | 15 (88.2) | 11 (68.8) | 6 (66.7) | 32 (76.2) | 16 (94.1) | 15 (93.8) | 9 (100.0) | 40 (95.2) | 31 (91.2) | 26 (81.3) | 15 (83.3) | 72 (85.7) |
| Taranto | 11 (84.6) | 9 (69.2) | 4 (66.7) | 24 (75.0) | 11 (84.6) | 10 (76.9) | 5 (83.3) | 26 (81.3) | 22 (84.6) | 19 (73.1) | 9 (75.0) | 50 (78.1) |
| Gela | 16 (69.6) | 20 (86.9) | 12 (100.0) | 48 (82.8) | 23 (100.0) | 12 (52.2) | 8 (66.7) | 43 (74.1) | 39 (84.8) | 32 (69.6) | 20 (83.3) | 91 (78.4) |
| TOTAL | 52 (75.4) | 52 (76.5) | 28 (80.0) | 132 (75.9) | 61 (88.4) | 48 (70.6) | 30 (85.7) | 139 (80.8) | 113 (81.9) | 100 (73.5) | 58 (82.6) | 271 (78.8) |
| Description | Value |
|---|---|
| Personal data | |
| “Is the subject female?” | Dichotomous |
| Age class | 20–29, 30–39, 40–44 (years) |
| Residence area | Amiata, Viterbo, Taranto, Gela |
| “Has the subject a body mass index>25?” | Dichotomous |
| Water | |
| “The subjects habitually drinks water from public aqueduct” | Dichotomous |
| Exposure to chemical and physical agents | |
| “Has the subject ever worked in a chemical industry?” | Dichotomous |
| Smoking | |
| Lifetime tobacco exposure calculated as pack/year=(number of cigarettes smoked per day*number of years the person has smoked)/20 | Continuous |
| Alcohol consumption | |
| “Does the subject consume at least 1–2 glasses of wine a day?” | Dichotomous |
| “Does the subject consume at least 1–2 glasses of any kind of alcoholic beverages * a day?” *(beer/super alcoholic beverages/aperitifs or mixture of non-alcoholic and super alcoholic beverages/grappa/whiskey/cognac and similar) | Dichotomous |
| Dietary habits | |
| “Does the subject consume at least 1–2 times a week whole milk?” | Dichotomous |
| “Does the subject consume at least once or twice a week these types of meat: poultry, beef, pork, lamb, horse, pork products, liver ?” | Dichotomous |
| “Does the subject consume at least 1–2 times a week fish, mollusks or crustaceans?” | Dichotomous |
| ”Does the subject consume at least 1–2 times a week cereals, pasta and bread?” | Dichotomous |
| “Does the subject consume wine at least 1–2 times a week?” | Dichotomous |
| Genetic polymorphisms | |
| “The subject has at least one mutated allele of the As (+3 oxidation state) methyltransferase gene” | Dichotomous |
| “The subject carries the wild type genotype of the glutathione S-transferase gene” | Dichotomous |
| Gender–Men vs. Women | |||||||||||
| iAs% | p | MMA% | p | DMA% | p | PMI | p | SMI | p | ||
| AV area | SMD [95%CI] | −0.64 [−4.90–3.63] | 0.767 | 0.47 [−3.14–4.09] | 0.434 | 1.72 [−2.31–5.74] | 0.400 | 0.02 [−0.28–0.32] | 0.904 | 0.14 [−0.25–0.53] | 0.482 |
| TG area | −2.25 [−7.03–2.53] | 0.353 | 5.64 [2.54–8.74] | <0.001 | −3.39 [−7.84–1.06] | 0.134 | 0.39 [0.16–0.67] | 0.001 | −1.39 [−2.62–(–0.17)] | 0.026 | |
| Total of 4 areas | −1.62 [−4.82–1.59] | 0.321 | 2.48 [0.38–4.59] | 0.021 | −0.87 [−3.87–2.13] | 0.570 | 0.22 [0.03–0.41] | 0.026 | −0.70 [−1.38–(−0.03)] | 0.040 | |
| Age class—class 2 (30–39) vs. class 1 (20–29) (1st row) and class 3(40–49) vs. class 1 (20–29) (2nd row) | |||||||||||
| iAs% | p | MMA% | p | DMA% | p | PMI | p | SMI | p | ||
| AV area | SMD [95%CI] | 4.44 [−0.28–9.16] −2.61 [−8.11–2.88] | 0.065 0.349 | 1.10 [−1.98–4.17] 1.90 [−1.68–5.48] | 0.481 0.297 | −5.54 [−9.98–(–1.09)] 0.72 [−4.46–5.89] | 0.015 0.785 | −0.20 [−0.54–0.15] 0.14 [−0.26–0.54] | 0.258 0.483 | −0.54 [0.98–(−0.11)] −0.14 [−0.97–0.36] | 0.014 0.570 |
| TG area | 0.92 [−4.55–6.40] −0.19 [−6.49–6.11] | 0.739 0.952 | −2.62 [−6.31–1.06] −0.83 [−5.07–3.41] | 0.161 0.699 | 1.70 [−3.42–6.82] 1.02 [−4.87–6.92] | 0.513 0.732 | −0.10 [−0.37–0.17] −0.14 [−0.45–0.18] | 0.466 0.390 | 1.45 [0.041–2.86] 0.77 [−0.85–2.39] | 0.044 0.346 | |
| Total of 4 areas | 2.61 [−1.02–6.24] −1.37 [−5.57–2.83] | 0.158 0.522 | −0.78 [−3.19–1.64] 0.49 [−2.31–3.28] | 0.527 0.731 | −1.83 [−5.24–1.57] 0.88 [−3.06–4.82] | 0.290 0.660 | −0.15 [−0.36–0.07] −0.00 [−0.25–0.25] | 0.187 0.985 | 0.46 [−0.31–1.23] 0.33 [−0.56–1.22] | 0.240 0.466 | |
| Body Mass Index—Overweight (≥25) vs. Normal Weight (<25) | |||||||||||
| iAs% | p | MMA% | p | DMA% | p | PMI | p | SMI | p | ||
| AV area | SMD [95%CI] | −0.62 [−1.77–0.52] | 0.279 | 0.60 [−0.36–1.56] | 0.213 | 0.02 [−1.19–1.24] | 0.970 | −0.041 [−0.17–0.08] | 0.510 | −0.33 [−0.14–0.07] | 0.540 |
| TG area | −0.07 [−0.50–0.36] | 0.739 | 0.068 [−0.22–0.36] | 0.645 | 0.00 [−0.40–0.40] | 0.982 | 0.00 [−0.02–0.021] | 0.982 | 0.00 [−0.12–0.11] | 0.949 | |
| Total of 4 areas | −0.05 [−0.43–0.34] | 0.814 | 0.00 [−0.27–0.29] | 0.959 | 0.04 [−0.33–0.40] | 0.835 | −0.01 [−0.03–0.02] | 0.443 | 0.02 [−0.08–0.11] | 0.714 | |
| Water Consumption—Yes vs. No | |||||||||||
| iAs% | p | MMA% | p | DMA% | p | PMI | p | SMI | p | ||
| AV area | SMD [95%CI] | −0.72 [−5.30–3.86] | 0.755 | 6.40 [3.71–9.10] | <0.001 | −5.68 [−9.89–(–1.46)] | 0.009 | 0.40 [0.08–0.71] | 0.015 | −0.77 [−1.17–(−0.37)] | <0.001 |
| TG area | −2.65 [−8.94–3.64] | 0.405 | 2.81 [−1.43–7.10] | 0.192 | −0.16 [−6.06–5.74] | 0.957 | 0.18 [−0.13–0.50]SM | 0.255 | −1.06 [−2.69–0.58] | 0.203 | |
| Total of 4 areas | −2.06 [−5.80–1.67] | 0.277 | 5.21 [2.81–7.60] | <0.001 | −3.15 [−6.62–0.33] | 0.076 | 0.33 [0.11–0.55] | 0.003 | −1.07 [−1.85−(–0.30)] | 0.007 | |
| Occupational Exposure to Chemicals—Yes vs. No | |||||||||||
| iAs% | p | MMA% | p | DMA% | PMI | SMI | |||||
| AV area | SMD [95%CI] | 0.46 [−13.71–14.64] | 0.949 | −0.32 [−9.35–8.72] | 0.945 | −0.14 [−13.54–13.26] | 0.983 | −0.23 [−1.24–0.77] | 0.645 | 0.05 [−1.24–1.35] | 0.936 |
| TG area | −3.99 [−10.89–2.91] | 0.255 | −8.34 [−12.81–(−3.86)] | <0.001 | 12.32 [6.17–18.47] | <0.001 | −0.32 [−0.66–0.02] | 0.068 | 3.64 [1.94–5.34] | <0.001 | |
| Total of 4 areas | −2.00 [−7.83–3.84] | 0.501 | −7.65 [−11.40–(−3.90)] | <0.001 | 9.65 [4.32–14.98] | <0.001 | −0.36 [−0.71–(−0.02)] | 0.041 | 3.38 [2.22–4.54] | <0.001 | |
| Smoking—Increment of 1 Pack-Year of Cigarettes | |||||||||||
| iAs% | p | MMA% | p | DMA% | p | PMI | p | SMI | p | ||
| AV area | SMD [95%CI] | 0.14 [−0.22–0.50] | 0.438 | 0.03 [−0.20–0.26] | 0.808 | −0.17 [−0.51–0.17] | 0.324 | −0.00 [−0.03–0.02] | 0.800 | −0.02 [−0.85–0.02] | 0.339 |
| TG area | 0.16 [0.16–0.49] | 0.323 | 0.14 [−0.08–0.36] | 0.219 | −0.30 [−0.60–0.00] | 0.050 | 0.00 [−0.01–0.02] | 0.803 | −0.07 [−0.15–0.01)] | 0.099 | |
| Total of 4 areas | 0.16 [−0.77–0.40] | 0.183 | 0.09 [−0.69–0.25] | 0.263 | −0.25 [−0.47–(−0.03)] | 0.026 | 0.00 [−0.01–0.01] | 0.954 | −0.05 [−0.98–0.00] | 0.069 | |
| Wine consumption—Yes (at least 1–2 Glasses Per Day) vs. No | |||||||||||
| iAs% | p | MMA% | p | DMA% | p | PMI | p | SMI | p | ||
| AV area | SMD [95%CI] | 6.27 [−1.00–13.54] | 0.090 | 1.45 [−3.23–6.13] | 0.541 | −7.72 [−14.54–(−0.90)] | 0.027 | −2.23 [−0.75–0.29] | 0.384 | −0.70 [−1.36–(−0.41)] | 0.037 |
| TG area | 3.79 [−5.40–12.98] | 0.416 | 0.42 [−5.82–6.66] | 0.895 | −4.21 [−12.81–4.39] | 0.335 | −0.22 [−0.68–0.24] | 0.344 | −0.49 [−2.89–1.92] | 0.690 | |
| Total of 4 areas | 4.93 [−0.88-10.74] | 0.096 | 1.11 [−2.75–4.98] | 0.570 | −6.04 [−11.45–(−0.64)] | 0.029 | −0.21 [−0.56–0.13] | 0.226 | −0.67 [−1.90–0.57] | 0.282 | |
| Alcohol Consumption—Yes (at least 1–2 Glasses Per Day) vs. No | |||||||||||
| iAs% | p | MMA% | p | DMA% | p | PMI | p | SMI | p | ||
| AV area | SMD [95%CI] | −2.64 [−16.81–11.53] | 0.713 | 3.75 [−5.25–12.76] | 0.411 | −1.11 [−14.51–12.28] | 0.870 | 0.16 [−0.85–1.16] | 0.757 | −0.64 [−1.93–0.65] | 0.329 |
| TG area | 3.80 [−6.40–14.00] | 0.463 | 2.22 [−4.69–9.13] | 0.526 | −6.02 [−15.54–3.49] | 0.213 | −0.03 [−0.55–0.47] | 0.893 | −0.95 [−3.61–1.72] | 0.483 | |
| Total of 4 areas | 2.66 [−5.41–10.74] | 0.516 | 2.14 [−3.20–7.48] | 0.432 | −4.80 [−12.33–2.73] | 0.210 | −0.02 [−0.50–0.46] | 0.932 | −0.61 [−2.31–1.09] | 0.483 | |
| Whole Milk Consumption—Yes (at Least 1–2 Glasses Per Week) vs. No | |||||||||||
| iAs% | p | MMA% | p | DMA% | p | PMI | p | SMI | p | ||
| AV area | SMD [95%CI] | 2.10 [−2.41–6.61] | 0.359 | −2.48 [−5.33–0.37] | 0.087 | 0.38 [−3.89–4.66] | 0.859 | −0.32 [−0.64–(−0.01)] | 0.045 | 0.24 [−0.17–0.65] | 0.252 |
| TG area | 6.14 [−0.30–12.58] | 0.062 | −3.39 [−7.77–1.00] | 0.129 | −2.75 [−8.85–3.34] | 0.373 | −0.26 [−0.58–0.06] | 0.114 | 0.48 [−1.22–2.18] | 0.575 | |
| Total of 4 areas | 2.83 [−0.90–6.55] | 0.136 | −2.21 [−4.67–0.25] | 0.078 | −0.61 [−4.11–2.88] | 0.730 | −0.25 [−0.47–(−0.03)] | 0.029 | 0.07 [−0.72–0.86] | 0.868 | |
| Meat Consumption—Yes (at Least 1–2 Times a Week) vs. No | |||||||||||
| iAs% | p | MMA% | p | DMA% | p | PMI | p | SMI | p | ||
| AV area | SMD [95%CI] | −1.18 [−5.55–3.19] | 0.595 | −1.47 [−4.24–1.31] | 0.298 | 2.64 [−1.47–6.75] | 0.206 | −0.05 [−0.36–0.26] | 0.766 | 0.13 [−0.27–0.53] | 0.516 |
| TG area | 3.21 [−1.66–8.09] | 0.194 | −2.77 [−6.06–0.52] | 0.098 | −0.45 [−5.04–4.14] | 0.848 | −0.28 [−0.52–(−0.04)] | 0.024 | 0.60 [−0.67–1.88] | 0.352 | |
| Total of 4 areas | 0.36 [−2.85–3.57] | 0.826 | −1.54 [−3.66–0.57] | 0.152 | 1.19 [−1.81–4.18] | 0.437 | −0.12 [−0.31–0.07] | 0.224 | 0.11 [−0.57–0.79] | 0.746 | |
| Fish Consumption—Yes (at Least 1–2 Times a Week) vs. No | |||||||||||
| iAs% | p | MMA% | p | DMA% | p | PMI | p | SMI | p | ||
| AV area | SMD [95%CI] | 5.37 [0.13–10.61] | 0.045 | 0.45 [−2.94–3.84] | 0.792 | −5.82 [−10.75–(−0.90)] | 0.021 | −0.05 [−0.42–0.33] | 0.811 | −0.44 [−0.92–0.04] | 0.073 |
| TG area | 1.65 [−4.14–7.44] | 0.573 | −3.61 [−7.49–0.26] | 0.067 | 1.96 [−3.46–7.38] | 0.475 | −0.24 [−0.52–0.05] | 0.107 | 0.53 [−0.98–2.04] | 0.491 | |
| Total of 4 areas | 3.31 [−0.61–7.23] | 0.098 | −1.64 [−4.24–0.96] | 0.217 | −1.67 [−5.35–2.00] | 0.371 | −0.14 [−0.38–0.09] | 0.237 | 0.04 [−0.79–0.87] | 0.921 | |
| Pasta and Cereals Consumption—Yes (at Least 1–2 Times a Week) vs. No | |||||||||||
| iAs% | p | MMA% | p | DMA% | p | PMI | p | SMI | p | ||
| AV area | SMD [95%CI] | −1.63 [−26.00–22.73] | 0.895 | 1.51 [−14.00–17.03] | 0.847 | 0.12 [−22.91–23.14] | 0.992 | 0.35 [−1.38–2.08] | 0.691 | 0.122 [−2.10–2.35] | 0.913 |
| TG area | 5.38 [−14.59–25.34] | 0.595 | −9.80 [−23.24–3.62] | 0.151 | 4.43 [−14.27–23.13] | 0.640 | −0.47 [−1.47–0.53] | 0.354 | 1.92 [−3.28–7.12] | 0.467 | |
| Total of 4 areas | 2.55 [−12.68–17.78] | 0.742 | −5.66 [−15.72–4.39] | 0.268 | 3.11 [−11.11–17.34] | 0.667 | −0.17 [−1.08–0.74] | 0.717 | 1.14 [−2.06–4.35] | 0.483 | |
| AS3MT—Mutated Allele vs. Non Mutated Allele | |||||||||||
| iAs% | p | MMA% | p | DMA% | p | PMI | p | SMI | p | ||
| AV area | SMD [95%CI] | −1.47 [−6.22–3.28] | 0.542 | −1.66 [−4.68–1.35] | 0.278 | 3.13 [−1.33–7.59] | 0.168 | −0.13 [−0.47–0.20] | 0.449 | 0.38 [−0.05–0.81] | 0.081 |
| TG area | 2.99 [−2.15–8.13] | 0.252 | −0.95 [−4.45–2.54] | 0.591 | −2.04 [−6.86–2.79] | 0.405 | 0.02 [−0.24–0.28] | 0.866 | −0.06 [−1.40–1.29] | 0.935 | |
| Total of 4 areas | 1.03 [−2.48–4.55] | 0.563 | −1.38 [−3.70–0.94] | 0.243 | 0.34 [−2.94–3.63] | 0.836 | −0.06 [−0.27–0.15] | 0.595 | 0.20 [−0.54–0.94] | 0.599 | |
| GSTT—Wildtype Carrier vs. No Wildtype Carrier | |||||||||||
| iAs% | p | MMA% | p | DMA% | p | PMI | p | SMI | p | ||
| AV area | SMD [95%CI] | −3.17 [−8.54–2.20] | 0.246 | −0.13 [−3.57–3.31] | 0.942 | 3.29 [−1.78–8.37] | 0.201 | 0.06 [−0.33–0.44] | 0.773 | 0.03 [−0.46–0.52] | 0.909 |
| TG area | −3.48 [−9.00–2.04] | 0.214 | 5.35 [1.71–9.00] | 0.004 | −1.87 [−7.06–3.31] | 0.476 | 0.25 [−0.03–0.52] | 0.077 | −0.75 [−2.19–0.69] | 0.303 | |
| Total of 4 areas | −3.56 [−7.42–0.27] | 0.068 | 3.10 [0.57–5.63] | 0.017 | 0.47 [−3.14–4.09] | 0.797 | 0.18 [−0.05–0.40] | 0.132 | 0.50 [−1.31–0.32] | 0.231 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bustaffa, E.; Gorini, F.; Bianchi, F.; Minichilli, F. Factors Affecting Arsenic Methylation in Contaminated Italian Areas. Int. J. Environ. Res. Public Health 2020, 17, 5226. https://doi.org/10.3390/ijerph17145226
Bustaffa E, Gorini F, Bianchi F, Minichilli F. Factors Affecting Arsenic Methylation in Contaminated Italian Areas. International Journal of Environmental Research and Public Health. 2020; 17(14):5226. https://doi.org/10.3390/ijerph17145226
Chicago/Turabian StyleBustaffa, Elisa, Francesca Gorini, Fabrizio Bianchi, and Fabrizio Minichilli. 2020. "Factors Affecting Arsenic Methylation in Contaminated Italian Areas" International Journal of Environmental Research and Public Health 17, no. 14: 5226. https://doi.org/10.3390/ijerph17145226
APA StyleBustaffa, E., Gorini, F., Bianchi, F., & Minichilli, F. (2020). Factors Affecting Arsenic Methylation in Contaminated Italian Areas. International Journal of Environmental Research and Public Health, 17(14), 5226. https://doi.org/10.3390/ijerph17145226








