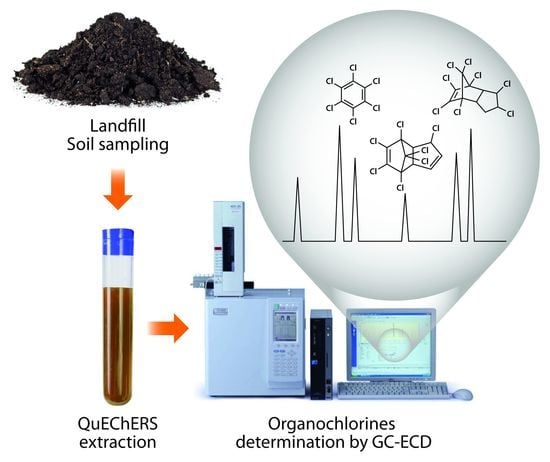
Determination of Organochlorines in Soil of a Suburban Area of São Paulo Brazil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
2.3. Standard Solutions
2.4. Soil Collection and Classification
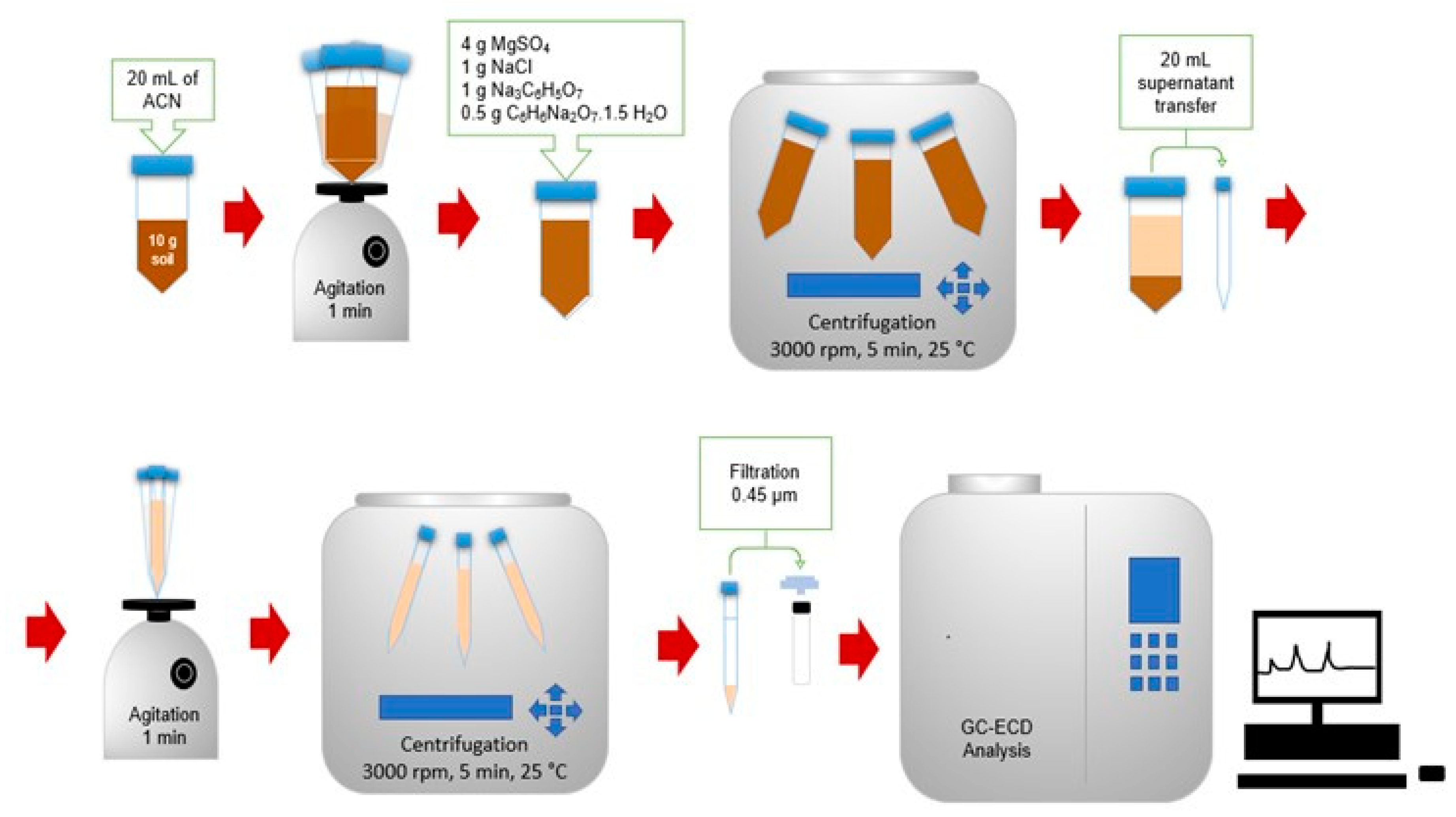
2.5. Extraction
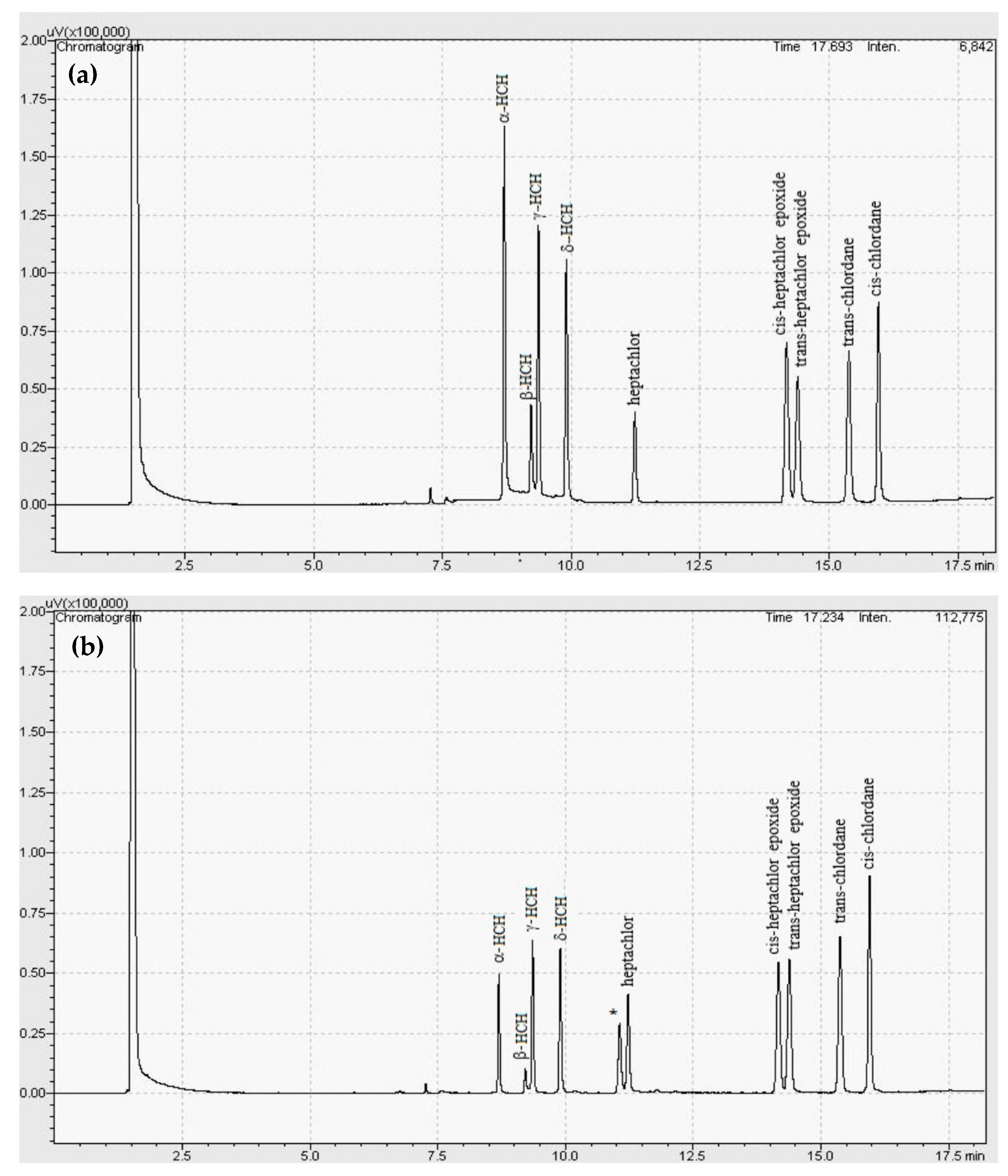
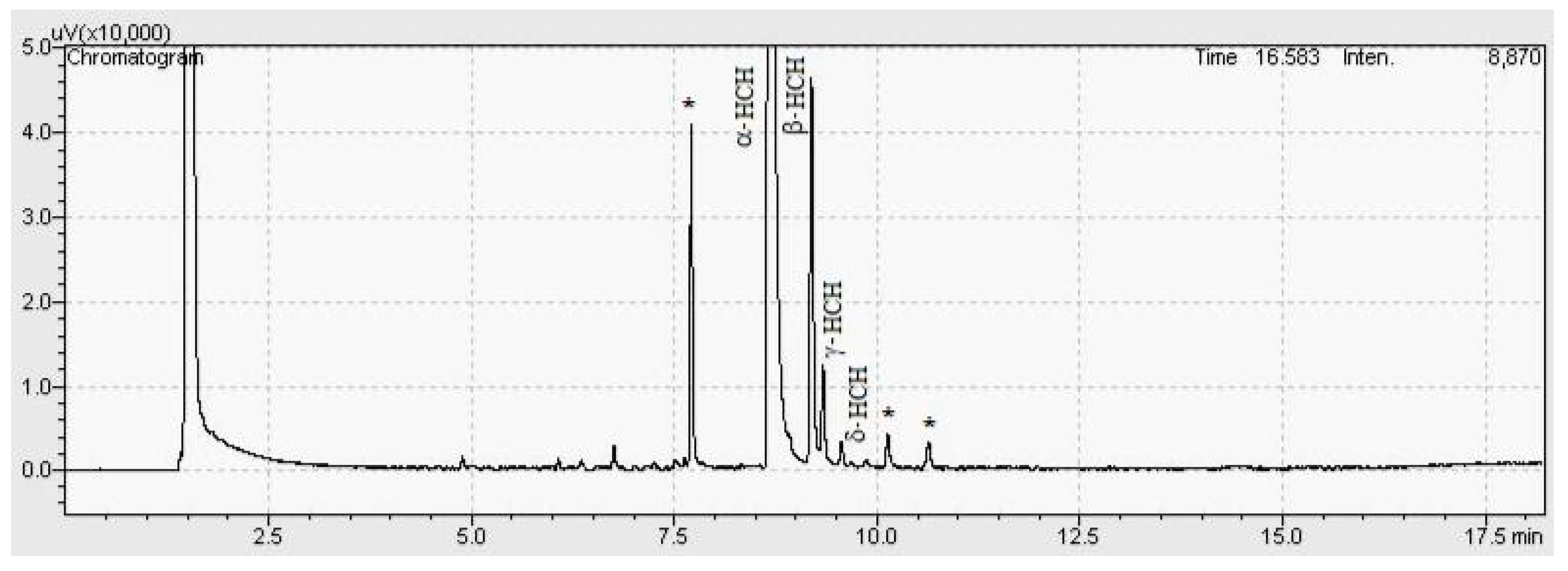
2.6. GC-ECD Technique
2.7. Quality Assurance
2.8. Statistical Analysis
3. Results
3.1. Method Development
3.2. Soil Classification
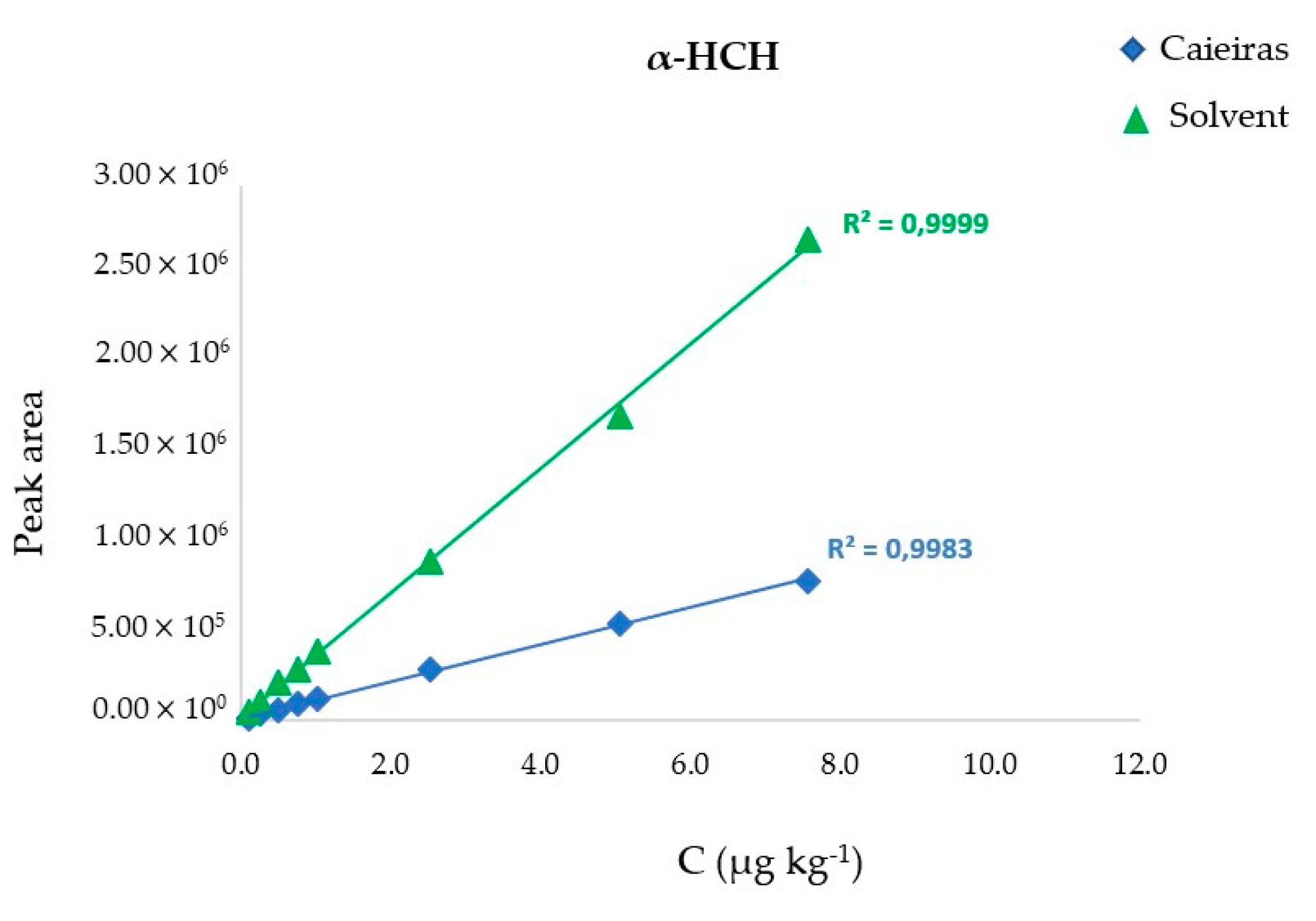
3.3. Validation
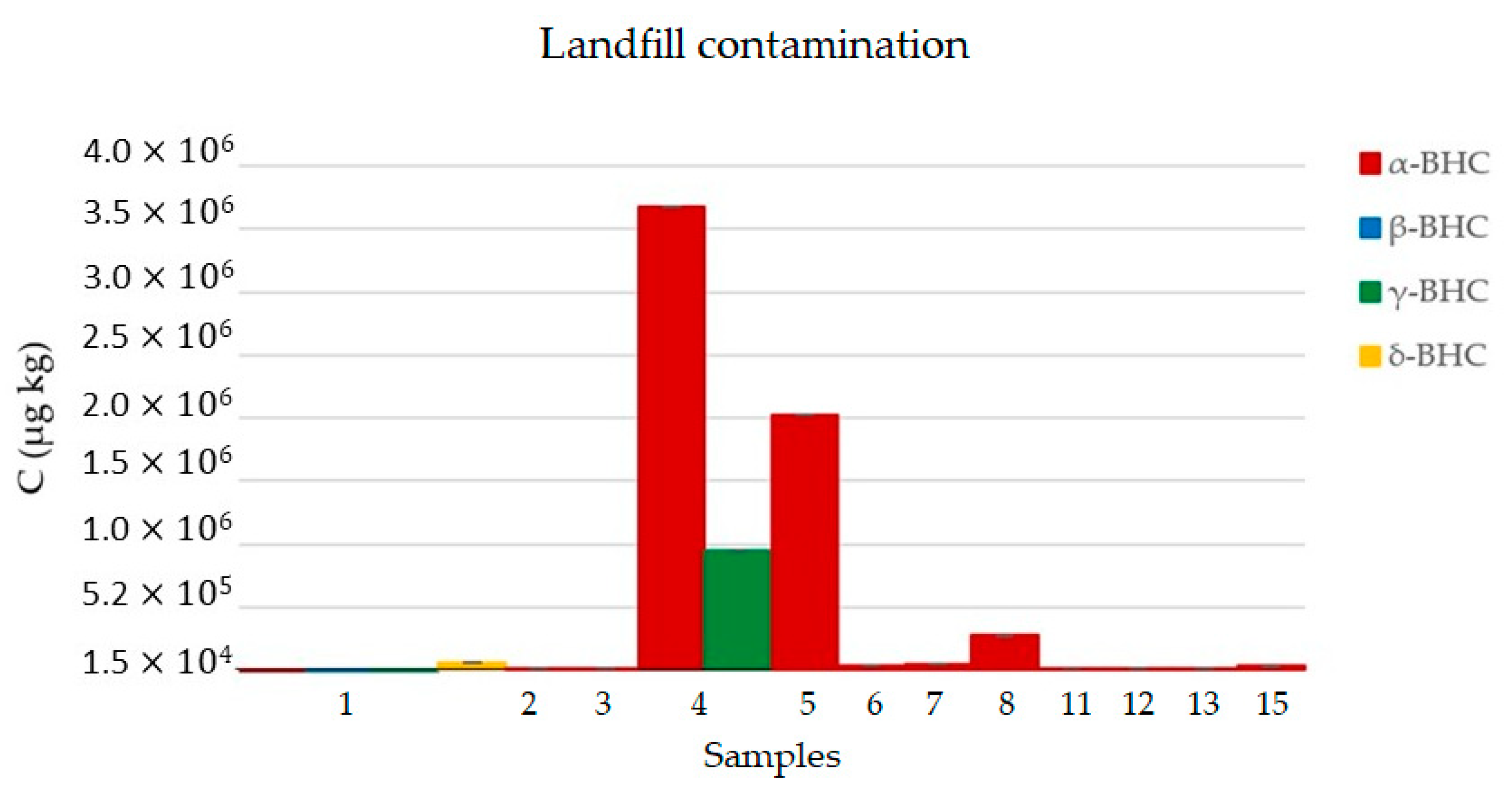
3.4. Sample Analysis
4. Discussion
4.1. Soil Classification
4.2. Method Performance and Validation
4.3. Soil Contamination Study
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheng, Z.; Dong, F.; Xu, J.; Liu, X.; Wu, X.; Chen, Z.; Pan, X.; Zheng, Y. Simultaneous determination of organophosphorus pesticides in fruits and vegetables using atmospheric pressure gas chromatography quadrupole-time-of-flight mass spectrometry. J. Chromatogr. A 2016, 1435, 115. [Google Scholar] [CrossRef]
- Flores, A.V.; Ribeiro, J.N.; Neves, A.A.; Queiroz, E.L.R. Organoclorados: Um problema de saúde pública. Rev. Ambiente Sociedade (ANPPAS). 2004, VII, 114–124. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Smith, A. Distribution of organochlorine pesticides in soils from South Korea. Chemosphere 2001, 43, 137. [Google Scholar] [CrossRef]
- Saleh, M.; Kamel, A.; Ragab, A.; El-Baroty, G.; El-Sebae, A.K. Regional distribution of organochlorine insecticide residues in human milk from Egypt. J. Environ. Sci. Health. 1996, B31, 241. [Google Scholar] [CrossRef]
- Silva, A.M.F.; Pavesi, T.; Rosa, A.C.S.; Santos, T.P.; Tabalipa, M.M.; Lemes, V.R.R.; Alves, S.R.; Sarcinelli, P.N.M. Organochlorines and polychlorinated biphenyl environmental pollution in south coast of Rio De Janeiro state. Pollut. Bull. 2016, 108, 325. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, L.; Shi, S.; Zhou, L.; Zhang, T.; Huang, Y. Organochlorine pesticides contamination in surface soils from two pesticide factories in Southeast China. Chemosphere 2009, 77, 628. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Ohtake, M.; Kunisue, T.; Tanabe, S. High levels of organochlorines in mothers’ milk from Chennai (Madras) city, India. Chemosphere 2007, 68, 928. [Google Scholar] [CrossRef]
- Caldas, S.S.; Bolzan, C.M.; Cerqueira, M.B.; Tomasini, D.; Furlong, E.B.; Fagundes, C.; Primel, E.G.J. Evaluation of a Modified QuEChERS Extraction of Multiple Classes of Pesticides from a Rice Paddy Soil by LC-APCI-MS/MS. Agric. Food Chem. 2011, 59, 11918–11926. [Google Scholar] [CrossRef]
- Fernandes, V.C.; Domingues, V.F.; Delerue-Matos, C. Multiresidue pesticides analysis in soils using modified QuEChERS with disposable pipette extraction and dispersive solid-phase extraction. J. Sep. Sci. 2013, 36, 376–382. [Google Scholar] [CrossRef]
- Correia-Sá, L.; Fernandes, V.C.; Calhau, C.; Domingues, V.F.; Delerue-Matos, C. Optimization of QuEChERS method for the analysis of organochlorine pesticides in soils with diverse organic matter. J. Sep. Sci. 2012, 35, 1521–1530. [Google Scholar] [CrossRef]
- Ministry of the Environment. National Implementation Plan Brazil: Convention Stockholm; MMA: Distrito Federal, Brasília, 2015.
- Stockholm Convention—Protection Human Health and the Environment from Persistent Organic Pollutants. Available online: http://chm.pops.int/TheConvention/Overview/TextoftheConvention/tabid/2232/Default.aspx (accessed on 25 October 2019).
- Companhia Ambiental do Estado de São Paulo—CETESB. Contaminated areas in São Paulo State. Available online: http://cetesb.sp.gov.br/ (accessed on 27 October 2019).
- Anastassiades, M.; Lehotay, S.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the Determination of Pesticide Residues in Produce. J. AOAC Int. 2003, 86, 412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Cao, P.; Liu, R. A multi-residue method for fast determination of pesticides in tea by ultraperformance liquid chromatography–electrospray tandem mass spectrometry combined with modified QuEChERS sample preparation procedure. Food Chem. 2011, 125, 1406. [Google Scholar] [CrossRef]
- Cieslik, E.; Sodowska-Rociek, A.; Ruiz, J.M.M.; Surma-Zadora, M. Evaluation of QuEChERS method for the determination of organochlorine pesticide residues in selected group of fruits. Food Chem. 2011, 125, 773. [Google Scholar] [CrossRef]
- Kolberg, D.I.; Prestes, O.D.; Adaime, M.B.; Zanella, R. Development of a fast multiresidue method for the determination of pesticides in dry samples (wheat grains. flour and bran) using QuEChERS based method and GC-MS. Food Chem. 2011, 125, 1436–1442. [Google Scholar] [CrossRef]
- Wilkowska, A.; Biziuk, M. Determination of pesticide residues in food matrices using the QuEChERS methodology. Food Chem. 2011, 125, 803. [Google Scholar] [CrossRef]
- Pinto, C.G.; Laespada, M.E.F.; Martin, S.H.; Ferreira, A.M.C.; Pavón, J.L.P.; Cordero, B.M. Simplified QuEChERS approach for the extraction of chlorinated compounds from soil samples. Talanta 2010, 81, 385–391. [Google Scholar] [CrossRef]
- Stunnings, G.; Bigwood, T. The development and validation of a multiclass liquid chromatography tandem mass spectrometry (LC-MS/MS) procedure for the determination of veterinary drug residues in animal tissue using a QuEChERS (Quick. Easy. Cheap. Effective. Rugged and Safe) approach. T. Anal. Chim. Acta 2009, 637, 1–2. [Google Scholar] [CrossRef]
- Koesukwiwat, U.; Lehotay, S.J.; Miao, S.; Leepipatpiboon, N. High throughput analysis of 150 pesticides in fruits and vegetables using QuEChERS and low-pressure gas chromatography—time-of-flight mass spectrometry. J. Chromatogr. A 2010, 1217, 6692–6703. [Google Scholar] [CrossRef]
- Lehotay, S.J.; Son, K.A.; Kwon, H.; Koesukwiwat, U.; Fu, W.; Mastovska, K.; Hoh, E.; Leepipatpiboon, N. Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in fruits and vegetables. J. Chromatogr. A 2010, 1217, 2548. [Google Scholar] [CrossRef]
- Shi, C.; Gui, W.; Chen, J.; Zhu, G. Determination of oxadiagyl residues in environmental samples and rice samples. Environ. Contam. Toxicol. 2010, 84, 236–239. [Google Scholar] [CrossRef]
- Quinete, N.S.; Oliveira, E.S.; Fernandes, D.R.; Avelar, A.S.; Santelli, R.E. Assessment of organochlorine pesticide residues in Atlantic Rain Forest fragments. Rio de Janeiro. Brazil. Environ. Pollut. 2011, 159, 3604–3612. [Google Scholar] [CrossRef] [PubMed]
- Drożdżyński, D.; Kowalska, J. Rapid analysis of organic farming insecticides in soil and produce using ultra-performance liquid chromatography/tandem mass spectrometry. J. Anal. Bioanal. Chem. 2009, 394, 2241–2247. [Google Scholar] [CrossRef]
- Magnusson, B.; Örnemark, U. (Eds.) Eurachem Guide: The Fitness for Purpose of Analytical Methods – A Laboratory Guide to Method Validation and Related Topics, (2nd ed. 2014). ISBN 978-91-87461-59-0. Available online: http://www.eurachem.org (accessed on 13 July 2020).
- International Standardization Organization (ISO 5725-4). Accuracy (Trueness and Precision) of Measurement Methods and Results—Part 4: Basic Methods for the Determination of the Trueness of a Standard Measurement Method; ISO: Geneva, Switzerland, 2020. [Google Scholar]
- National Institute of Metrology. Quality and Technology (INMETRO). General Coordination of Accreditation—DOC-CGCRE-008. 2011. Available online: http://www.inmetro.gov.br/Sidoq/Arquivos/Cgcre/DOQ/DOQ-Cgcre-8_04.pdf (accessed on 10 July 2020).
- EPA (Environmental Protection Agency). Regional Screening Level (RSL) for Chemical Contaminants May. 2020. Available online: https://semspub.epa.gov/work/HQ/199922.pdf (accessed on 13 May 2020).
- CETESB—GTS (Environmental Sanitation Technology Company). Soil Sampling Guidance. 2011. Available online: http://arquivos.ana.gov.br/institucional/sge/CEDOC/Catalogo/2012/GuiaNacionalDeColeta.pdf (accessed on 10 July 2020).
- Brazilian Company of Agriculture Research (EMBRAPA). Guidance of Methods for Soil Analysis, 2nd ed.; EMBRAPA CNPS: Rio de Janeiro, RJ, Brazil, 1997. [Google Scholar]
- Brazilian Association of Technical Rules (ABNT). Soil—Determination of Organic Matter Content by Burning at 440 °C (NBR 13600); Brazilian Association of Technical Standards: Rio de Janeiro, Brazil, 1996. [Google Scholar]
- Prestes, O.D.; Friggi, C.A.; Adaime, M.B.; Zanella, R. QuEChERS—Um método moderno de preparo de amostra para determinação multirresíduo de pesticidas em alimentos por métodos cromatográficos acoplados à espectrometria de massas. Quím. Nova. 2009, 32, 1620–1634. [Google Scholar] [CrossRef]
- Rissato, S.R.; Galhiane, M.S.; Ximenes, V.F.; Andrade, R.M.B.; Talamoni, J.L.B.; Libânio, M.; Almeida, M.V.; Benhard, M.A.; Cavalari, A.A. Organochlorine pesticides and polychlorinated biphenyls in soil and water samples in the Northeastern part of São Paulo State. Brazil. Chemosphere 2006, 65, 1949–1958. [Google Scholar] [CrossRef]
- Barron, M.G.; Ashurova, Z.J.; Mukhamadcho, A.K.; Avloev, H.K.; Khaidarov, K.K.; Jamshedov, J.N.; Rahmatullova, O.S.; Atolikshoeva, S.S.; Mamadshova, S.S. Residues of organochlorine pesticides in surface soil and raw foods from rural areas of the Republic of Tajikistan. Environ. Pollut. 2017, 224, 494–502. [Google Scholar] [CrossRef]
- Wong, F.; Robson, M.; Diamond, M.L.; Harrad, S.; Truong, J. Concentration and chiral signatures of POPs in soils and sediments: A comparative urban versus rural study in Canada and UK. Chemosphere. 2009, 74, 404–411. [Google Scholar] [CrossRef] [PubMed]

| Compound | Residential Soil (µg kg−1) | Industrial Soil (µg kg−1) |
|---|---|---|
| α-HCH | 86.0 | 360.0 |
| β-HCH | 300.0 | 1.3 × 103 |
| γ-HCH | 570.0 | 2.5 × 103 |
| δ-HCH | - | - |
| Heptachlor | 130.0 | 630.0 |
| Cis/trans-heptachlor | 7.0 | 330.0 |
| Cis/trans-Chlordane | 1.77 × 103 | 7.70 × 103 |
| N | South | West | N | South | West | N | South | West |
|---|---|---|---|---|---|---|---|---|
| 1 | 23°20′438″ | 46°49′13″ | 6 | 23°20′437″ | 46°49′991″ | 11 | 23°20′491″ | 46°49′0.87″ |
| 2 | 23°20′434″ | 46°49′132″ | 7 | 23°20′441″ | 46°49′441″ | 12 | 23°20′445″ | 46°49′105″ |
| 3 | 23°20′418″ | 46°49′3″ | 8 | 23°20′442″ | 46°49′977″ | 13 | 23°20′435″ | 46°49′113″ |
| 4 | 23°20′422″ | 46°49′3″ | 9 | 23°20′465″ | 46°49′0.28″ | 14 | 23°20′387″ | 46°49′0.56″ |
| 5 | 23°20′403″ | 46°49′988″ | 10 | 23°20′470″ | 46°49′470″ | 15 | 23°20′398″ | 46°49′0.20″ |
| Compound | Linear Range (µg kg−1) | Solvent | Matrix | ||
|---|---|---|---|---|---|
| Linear Regression Equation | r2 | Linear Regression Equation | r2 | ||
| α-HCH | 101–1010 | y = 3.72 × 105x + 9.86 × 103 | 0.998 | y = 1.21 × 105x − 1.651 | 0.999 |
| β-HCH | 103–1030 | y = 91,966x – 65,633 | 0.999 | y = 25,827x − 277.1 | 0.999 |
| γ-HCH | 102–1020 | y = 35,554x + 874.03 | 0.997 | y = 16,445x − 9228 | 0.997 |
| δ-HCH | 100–1000 | y = 278,039x + 10,590 | 0.997 | y = 18,069x −2448 | 0.999 |
| Heptachlor | 101–1010 | y = 129,344x − 1946 | 0.999 | y = 12,245x − 197.7 | 0.999 |
| Cis-heptachlor epoxide | 111–1114 | y = 312,320x − 6795.2 | 0.998 | y = 25,022x − 2840 | 0.997 |
| Trans-heptachlor epoxide | 105–1050 | y = 232,739x − 2407 | 0.998 | y = 271,470x − 1393 | 0.998 |
| Cis-chlordane | 116–1160 | y = 286,709x + 1111.2 | 0.999 | y = 290,550x − 1090 | 0.999 |
| Trans-chlordane | 108–1080 | y = 260,109x − 2265 | 0.997 | y = 33,889x − 4037 | 0.992 |
| C (µg kg−1) | 0.10 | 0.25 | 0.50 | 0.75 | 1.00 | 2.50 | 5.00 | 10.00 |
|---|---|---|---|---|---|---|---|---|
| α-HCH | ||||||||
| s2 (ACN) | 3.82 × 106 | 1.65 × 107 | 4.28 × 107 | 3.41 × 108 | 1.64 × 109 | 3.19 × 109 | 7.93 × 1010 | 4.77 × 101 |
| s2 (C) | 3.56 × 105 | 2.89 × 106 | 3.82 × 106 | 2.04 × 107 | 4.24 × 107 | 2.37 × 107 | 2.10 × 108 | 7.51 × 108 |
| Fcalc | 0.09 | 0.18 | 0.09 | 0.06 | 0.03 | 0.01 | 0.00 | 0.02 |
| tcalc | 40.16 | 47.22 | 58.89 | 26.76 | 17.28 | 28.27 | 10.93 | 23.08 |
| β-HCH | ||||||||
| s2 (ACN) | 1.42 × 106 | 2.92 × 107 | 1.33 × 107 | 4.09 × 107 | 1.99 × 108 | 1.94 × 108 | 6.41 × 109 | 5.15 × 109 |
| s2 (C) | 3.38 × 104 | 9.19 × 105 | 1.05 × 106 | 1.51 × 106 | 1.70 × 106 | 7.06 × 106 | 2.36 × 107 | 6.34 × 107 |
| Fcalc | 0.02 | 0.03 | 0.08 | 0.04 | 0.01 | 0.04 | 0.00 | 0.01 |
| Tcalc | 16.50 | 7.80 | 25.13 | 19.33 | 13.06 | 31.04 | 11.53 | 22.38 |
| γ-HCH | ||||||||
| s2 (ACN) | 1.89 × 107 | 7.94 × 107 | 8.76 × 107 | 2.48 × 108 | 9.62 × 108 | 1.78 × 109 | 5.34 × 1010 | 3.47 × 1010 |
| s2 (C) | 9.80 × 105 | 4.36 × 107 | 8.40 × 106 | 5.17 × 107 | 4.07 × 107 | 6.91 × 107 | 2.89 × 108 | 1.32 × 109 |
| Fcalc | 0.05 | 0.55 | 0.10 | 0.21 | 0.04 | 0.04 | 0.01 | 0.04 |
| Tcalc | 15.32 | 9.53 | 22.83 | 13.50 | 11.27 | 19.39 | 7.45 | 16.60 |
| δ-HCH | ||||||||
| s2 (ACN) | 4.32 × 106 | 2.31 × 107 | 2.42 × 107 | 1.81 × 108 | 9.63 × 108 | 2.90 × 109 | 5.96 × 1010 | 3.88 × 1010 |
| s2 (C) | 2.91 × 105 | 1.28 × 107 | 1.35 × 107 | 8.69 × 107 | 8.46 × 107 | 1.97 × 107 | 4.53 × 108 | 1.18 × 109 |
| Fcalc | 0.07 | 0.55 | 0.56 | 0.48 | 0.09 | 0.01 | 0.01 | 0.03 |
| Tcalc | 19.52 | 17.45 | 29.76 | 12.99 | 9.73 | 15.07 | 6.97 | 16.08 |
| Heptachlor | ||||||||
| s2 (ACN) | 3.73 × 105 | 1.95 × 105 | 6.45 × 106 | 2.82 × 107 | 1.40 × 108 | 5.22 × 108 | 1.33 × 1010 | 9.21 × 109 |
| s2 (C) | 3.55 × 105 | 5.03 × 106 | 7.46 × 106 | 2.30 × 107 | 5.92 × 107 | 2.22 × 107 | 2.25 × 108 | 4.36 × 108 |
| Fcalc | 0.95 | 25.85 | 1.16 | 0.82 | 0.42 | 0.04 | 0.02 | 0.05 |
| tcalc | 6.00 | 6.77 | 7.36 | 6.70 | 2.27 | 0.87 | 1.43 | 6.53 |
| Cis-heptachlor epoxide | ||||||||
| s2 (ACN) | 2.27 × 106 | 9.37 × 106 | 6.48 × 107 | 8.10 × 107 | 1.14 × 109 | 1.88 × 109 | 5.09 × 1010 | 3.21 × 1010 |
| s2 (C) | 7.89 × 105 | 1.04 × 107 | 1.03 × 107 | 7.23 × 107 | 1.41 × 108 | 5.21 × 107 | 9.18 × 108 | 2.49 × 109 |
| Fcalc | 0.35 | 1.12 | 0.16 | 0.89 | 0.12 | 0.03 | 0.02 | 0.08 |
| Tcalc | 7.43 | 5.38 | 8.83 | 4.94 | 4.09 | 8.44 | 4.45 | 11.71 |
| Trans-heptachlor epoxide | ||||||||
| s2 (ACN) | 1.14 × 106 | 4.71 × 106 | 5.26 × 107 | 4.71 × 107 | 8.10 × 108 | 1.30 × 109 | 3.75 × 1010 | 2.36 × 1010 |
| s2 (C) | 6.63 × 105 | 1.52 × 107 | 9.52 × 106 | 9.10 × 107 | 1.58 × 108 | 6.44 × 107 | 1.15 × 109 | 2.59 × 109 |
| Fcalc | 0.58 | 3.23 | 0.18 | 1.93 | 0.20 | 0.05 | 0.03 | 0.11 |
| Tcalc | 8.05 | 8.93 | 7.02 | 10.03 | 2.51 | 3.34 | 0.64 | 5.30 |
| Cis-chlordane | ||||||||
| s2 (ACN) | 1.54 × 106 | 5.88 × 106 | 3.93 × 107 | 1.10 × 108 | 1.24 × 109 | 1.96 × 109 | 6.11 × 1010 | 2.98 × 101 |
| s2 (C) | 3.04 × 105 | 2.16 × 107 | 2.62 × 107 | 2.27 × 108 | 3.69 × 108 | 2.28 × 108 | 2.82 × 109 | 8.45 × 109 |
| Fcalc | 0.20 | 3.67 | 0.67 | 2.07 | 0.30 | 0.12 | 0.05 | 0.28 |
| tcalc | 0.44 | 10.42 | 11.22 | 9.29 | 2.22 | 3.12 | 0.35 | 6.56 |
| Trans-chlordane | ||||||||
| s2 (ACN) | 1.50 × 106 | 9.31 × 106 | 4.42 × 107 | 7.21 × 107 | 1.14 × 109 | 2.28 × 109 | 7.13 × 1010 | 4.44 × 1010 |
| s2 (C) | 3.67 × 105 | 2.08 × 107 | 2.41 × 107 | 1.54 × 108 | 2.27 × 108 | 1.59 × 108 | 2.16 × 109 | 5.08 × 109 |
| Fcalc | 0.25 | 2.23 | 0.54 | 2.14 | 0.20 | 0.07 | 0.03 | 0.11 |
| tcalc | 7.07 | 6.49 | 5.91 | 6.90 | 1.42 | 0.56 | 1.51 | 7.64 |
| Compound | C1 (µg kg−1) | VC1 (%) | C2 (µg kg−1) | VC2 (%) | C3 (µg kg−1) | VC3 (%) |
|---|---|---|---|---|---|---|
| α-HCH | 253 | 4.15 | 505 | 2.20 | 1010 | 2.66 |
| β-HCH | 258 | 5.64 | 515 | 4.19 | 1030 | 0.68 |
| γ-HCH | 255 | 1.35 | 510 | 2.53 | 1020 | 0.32 |
| δ-HCH | 250 | 5.08 | 500 | 0.54 | 1000 | 3.82 |
| Heptachlor | 253 | 2.76 | 505 | 1.77 | 1010 | 3.62 |
| Cis-heptachlor epoxide | 278 | 1.91 | 557 | 1.36 | 1110 | 1.70 |
| Trans-heptachlor epoxide | 263 | 1.11 | 525 | 0.92 | 1050 | 1.95 |
| Cis-chlordane | 270 | 2.11 | 540 | 0.56 | 1080 | 1.48 |
| Trans-chlordane | 290 | 1.98 | 580 | 1.85 | 1160 | 1.95 |
| Compounds | µg mL−1 | r | R | ||
|---|---|---|---|---|---|
| 0.101 | 0.0048 | 0.0134 | 0.0018 | 0.0051 | |
| α-HCH | 0.505 | 0.0157 | 0.0439 | 0.0096 | 0.0269 |
| 2.525 | 0.0391 | 0.1094 | 0.0218 | 0.0609 | |
| 0.103 | 0.773 | 5.150 | 0.0020 | 0.0056 | |
| β-HCH | 0.0071 | 0.0472 | 0.1869 | 0.0188 | 0.0526 |
| 0.0198 | 0.1323 | 0.5234 | 0.0879 | 0.2460 | |
| 0.102 | 0.765 | 5.100 | 0.0052 | 0.0144 | |
| γ-HCH | 0.0071 | 0.0472 | 0.1869 | 0.0273 | 0.0763 |
| 0.0198 | 0.1323 | 0.5234 | 0.0295 | 0.0826 | |
| 0.100 | 0.750 | 5.000 | 0.0010 | 0.0028 | |
| δ-HCH | 0.0071 | 0.0472 | 0.1869 | 0.0215 | 0.0603 |
| 0.0198 | 0.1323 | 0.5234 | 0.0321 | 0.0899 | |
| 0.101 | 0.758 | 5.050 | 0.0017 | 0.0047 | |
| Heptachlor | 0.0071 | 0.0472 | 0.1869 | 0.0188 | 0.0526 |
| 0.0198 | 0.1323 | 0.5234 | 0.0310 | 0.0867 | |
| 0.111 | 0.835 | 5.568 | 0.0019 | 0.0054 | |
| Cis-heptachlor epoxide | 0.0071 | 0.0472 | 0.1869 | 0.0107 | 0.0299 |
| 0.0198 | 0.1323 | 0.5234 | 0.0299 | 0.0836 | |
| 0.105 | 0.788 | 5.251 | 0.0019 | 0.0052 | |
| Trans-heptachlor epoxide | 0.0071 | 0.0472 | 0.1869 | 0.0112 | 0.0313 |
| 0.0198 | 0.1323 | 0.5234 | 0.0307 | 0.0861 | |
| 0.108 | 0.270 | 0.540 | 0.0019 | 0.0052 | |
| Cis-chlordane | 0.0071 | 0.0472 | 0.1869 | 0.0112 | 0.0313 |
| 0.0198 | 0.1323 | 0.5234 | 0.0307 | 0.0861 | |
| 0.116 | 0.870 | 5.799 | 0.0009 | 0.0027 | |
| Trans-chlordane | 0.0071 | 0.0472 | 0.1869 | 0.0132 | 0.0369 |
| 0.0198 | 0.1323 | 0.5234 | 0.0388 | 0.1085 |
| Compound | C (µg kg−1) | Z | Matrix (µg kg−1) | ACN (µg kg−1) | Rec1 | SD | Rec2 | SD | ||
|---|---|---|---|---|---|---|---|---|---|---|
| LD | LQ | LD | LQ | (%) | (%) | (%) | (%) | |||
| α-HCH | 10.0 | 0.015 | 1.20 | 12.60 | 1.30 | 11.60 | 92.1 | 1.0 | 44.2 | 1.9 |
| β-HCH | 96.0 | 1.409 | 1.70 | 12.00 | 3.20 | 13.10 | 101.1 | 3.9 | 84.9 | 4.1 |
| γ-HCH | 233.0 | 0. 699 | 1.50 | 11.60 | 3.80 | 11.70 | 88.6 | 0.7 | 211.7 | 7.9 |
| δ-HCH | 101.0 | 0.188 | 0.80 | 11.60 | 1.90 | 12.30 | 77.4 | 4.7 | 69.3 | 1.1 |
| Heptachlor | 99.0 | 0.991 | 1.00 | 10.80 | 1.20 | 12.40 | 100.5 | 1.2 | 65.9 | 2.3 |
| Cis-heptachlor epoxide | 574.0 | 1.818 | 0.90 | 11.50 | 1.20 | 12.60 | 103.2 | 2.6 | 83.4 | 2.9 |
| Trans-heptachlor epoxide | 111.0 | 1.447 | 0.30 | 11.00 | 1.10 | 13.10 | 84.5 | 4.2 | 71.3 | 4.4 |
| Cis-chlordane | 281.0 | 1.487 | 0.40 | 7.90 | 1.10 | 12.30 | 105.0 | 4.0 | 84.4 | 3.0 |
| Trans-chlordane | 289.0 | 0.069 | 0.50 | 10.90 | 1.10 | 14.30 | 102.4 | 4.6 | 84.0 | 3.5 |
| n | α-HCH | β-HCH | γ-HCH | δ-HCH |
|---|---|---|---|---|
| C (µg kg−1) | ||||
| 1 | 3.91 × 103 ± 0.18 | 6.23 × 103 ± 0.33 | 1.61 × 103 ± 0.07 | 7.68 × 104 ± 5.92 |
| 2 | 2.81 × 104 ± 9.56 | <LQ | <LQ | <LQ |
| 3 | 2.55 × 104 ± 3.28 | <LQ | <LQ | <LQ |
| 4 | 3.69 × 106 ± 0.17 | 9.7 × 105 ± 0.05 | <LQ | <LQ |
| 5 | 2.04 × 106 ± 0.08 | <LQ | <LQ | <LQ |
| 6 | 5.67 × 104 ± 4.26 | <LQ | <LQ | <LQ |
| 7 | 66.010 ± 4.01 | <LQ | <LQ | <LQ |
| 8 | 2.9 × 105 ± 0.01 | <LQ | <LQ | <LQ |
| 9 | <LQ | <LQ | <LQ | <LQ |
| 10 | <LQ | <LQ | <LQ | <LQ |
| 11 | 1.75 × 104 ± 1.25 | <LQ | <LQ | <LQ |
| 12 | 3.0 × 104 ± 1.84 | <LQ | <LQ | <LQ |
| 13 | 2.55 × 104 ± 1.15 | <LQ | <LQ | <LQ |
| 14 | <LQ | <LQ | <LQ | <LQ |
| 15 | 4.73 × 104 ± 4.73 | <LQ | <LQ | <LQ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
R. O. Varca, J.P.; A. J. Martins, E.; H. C. Varca, G.; L. Romano, R.; T. Lebre, D.; E. O. Lainetti, P.; V. Bustillos, J.O. Determination of Organochlorines in Soil of a Suburban Area of São Paulo Brazil. Int. J. Environ. Res. Public Health 2020, 17, 5666. https://doi.org/10.3390/ijerph17165666
R. O. Varca JP, A. J. Martins E, H. C. Varca G, L. Romano R, T. Lebre D, E. O. Lainetti P, V. Bustillos JO. Determination of Organochlorines in Soil of a Suburban Area of São Paulo Brazil. International Journal of Environmental Research and Public Health. 2020; 17(16):5666. https://doi.org/10.3390/ijerph17165666
Chicago/Turabian StyleR. O. Varca, Justine P., Elâine A. J. Martins, Gustavo H. C. Varca, Renato L. Romano, Daniel T. Lebre, Paulo E. O. Lainetti, and José O. V. Bustillos. 2020. "Determination of Organochlorines in Soil of a Suburban Area of São Paulo Brazil" International Journal of Environmental Research and Public Health 17, no. 16: 5666. https://doi.org/10.3390/ijerph17165666
APA StyleR. O. Varca, J. P., A. J. Martins, E., H. C. Varca, G., L. Romano, R., T. Lebre, D., E. O. Lainetti, P., & V. Bustillos, J. O. (2020). Determination of Organochlorines in Soil of a Suburban Area of São Paulo Brazil. International Journal of Environmental Research and Public Health, 17(16), 5666. https://doi.org/10.3390/ijerph17165666