Abstract
We investigated if greenness and air pollution exposure in parents’ childhood affect offspring asthma and hay fever, and if effects were mediated through parental asthma, pregnancy greenness/pollution exposure, and offspring exposure. We analysed 1106 parents with 1949 offspring (mean age 35 and 6) from the Respiratory Health in Northern Europe, Spain and Australia (RHINESSA) generation study. Mean particulate matter (PM2.5 and PM10), nitrogen dioxide (NO2), black carbon (BC), ozone (O3) (µg/m3) and greenness (normalized difference vegetation index (NDVI)) were calculated for parents 0–18 years old and offspring 0–10 years old, and were categorised in tertiles. We performed logistic regression and mediation analyses for two-pollutant models (clustered by family and centre, stratified by parental lines, and adjusted for grandparental asthma and education). Maternal medium PM2.5 and PM10 exposure was associated with higher offspring asthma risk (odds ratio (OR) 2.23, 95%CI 1.32–3.78, OR 2.27, 95%CI 1.36–3.80), and paternal high BC exposure with lower asthma risk (OR 0.31, 95%CI 0.11–0.87). Hay fever risk increased for offspring of fathers with medium O3 exposure (OR 4.15, 95%CI 1.28–13.50) and mothers with high PM10 exposure (OR 2.66, 95%CI 1.19–5.91). The effect of maternal PM10 exposure on offspring asthma was direct, while for hay fever, it was mediated through exposures in pregnancy and offspring’s own exposures. Paternal O3 exposure had a direct effect on offspring hay fever. To conclude, parental exposure to air pollution appears to influence the risk of asthma and allergies in future offspring.
1. Introduction
Air pollution is a major risk factor for disease worldwide and is estimated to cause almost 500,000 premature annual deaths across Europe [1]. Studies have shown that long-term exposure to high levels of air pollution affects multiple organs in the human body, causing cardiovascular and respiratory diseases [2]. Regarding the development of asthma, some studies have found childhood exposure to air pollution to be a risk factor [3], while other studies did not reveal those effects [4]. Less is known regarding the intergenerational effects of exposure to lower levels of air pollution, e.g., levels below recommended limits from the European Union (EU) and the World Health Organisation (WHO) [5,6], on offspring asthma and hay fever.
Exposure to greenspace has, on the other hand, been associated with beneficial health effects such as reduced risk of mortality, diabetes, and high blood pressure [7]. However, effects of greenness on asthma and allergies are less clear [8,9,10,11]. Some studies have indicated decreased respiratory morbidity in adulthood due to living near green areas [7,12,13,14] while the effects of residential greenness on childhood allergic rhinitis and aeroallergen sensitization have depended on the region [15,16,17,18]. Access to green areas may decrease stress through rest, increase opportunities for physical activity and increase social interaction [19]. Furthermore, vegetation may remove pollutants such as ozone (O3), particulate matter (PM) and nitrogen dioxide (NO2) from the air and may reduce exposure to harmful noise [9,20]. Negative effects of greenness, on the other hand, may be explained by higher exposure to pollen triggering allergic responses [17].
Asthma and allergies may result from both genetic susceptibility and environmental exposures, and the importance of early life factors have been widely acknowledged [21,22,23]. Emerging research suggests that even preconception exposures may be of relevance, and that epigenetic mechanisms may be at play across generations [24]. Recent studies have found that father’s smoking and overweight onset in adolescence was associated with higher asthma risk in their future offspring [25,26,27], suggesting vulnerable time windows many years before conception of offspring. There are, however, no studies investigating such intergenerational effects of exposures to air pollution and greenness.
To address the knowledge gaps of these long-term effects of exposure to air pollution and greenness on asthma and hay fever, the aims of our study were to (1) explore the associations between parental childhood exposures of greenness and air pollution in relation to their future offspring asthma and allergies, in areas with relatively low air pollution and to (2) assess if the observed associations were direct or mediated by other factors.
2. Materials and Methods
2.1. Study Design and Population
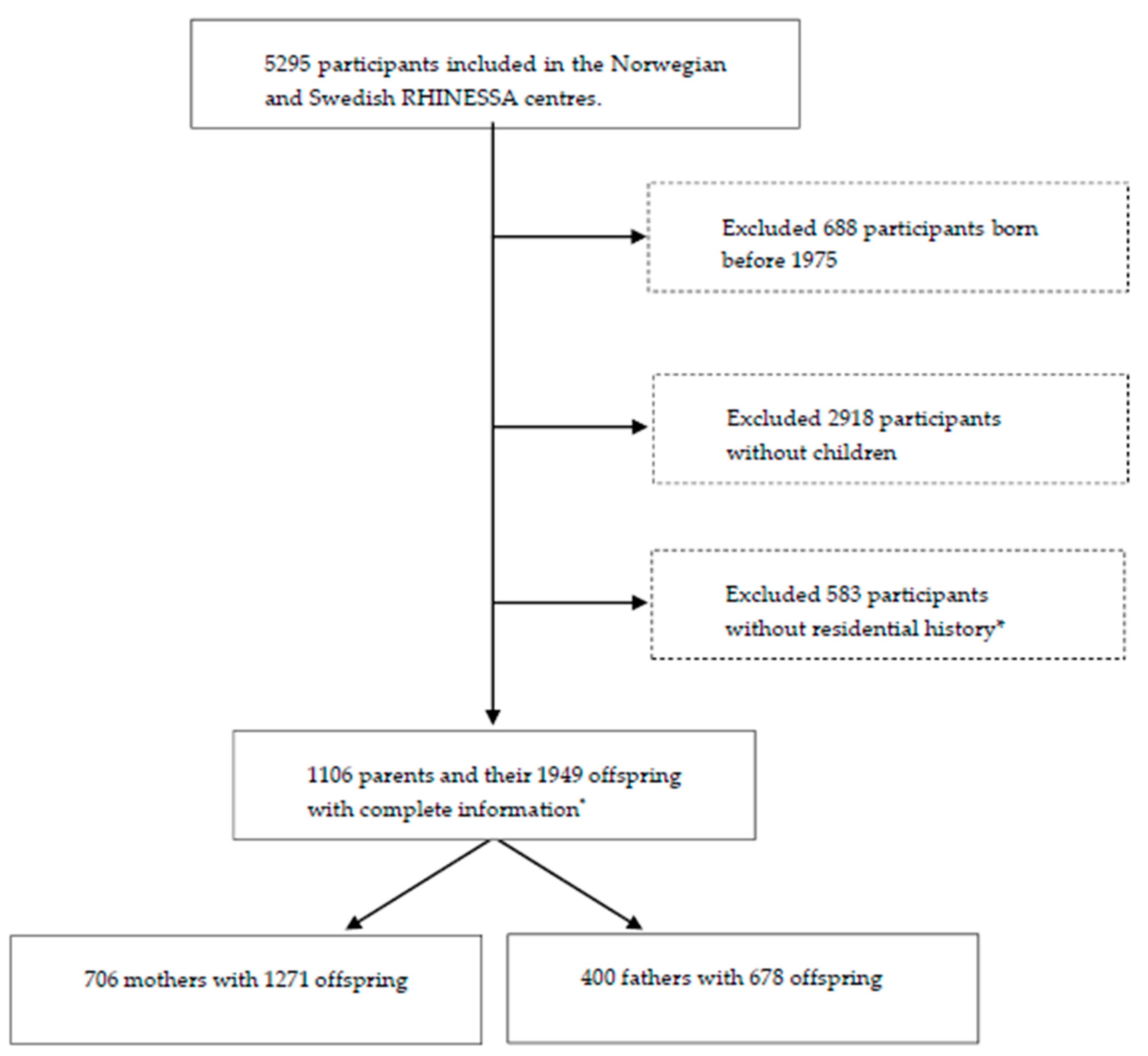
We included participants born after 1975 as well as their offspring from centres with available pollution data and relatively low air pollution levels in the Respiratory Health in Northern Europe, Spain and Australia (RHINESSA) generation study, conducted in 2013–2015 [28,29]: Bergen (Norway); and Umea, Uppsala, and Gothenburg (Sweden), as shown in Figure 1. Individual residential address history was only available from 1975 onwards and participants born before that were therefore not included. The participants answered questionnaires regarding their lung health and provided information on their offspring asthma and allergies. The overall response rate was 40% in Norway and 44% in Sweden [28]. Informed consent was obtained from each participant, and the study was approved by regional committees of medical research ethics according to national legislations [30].
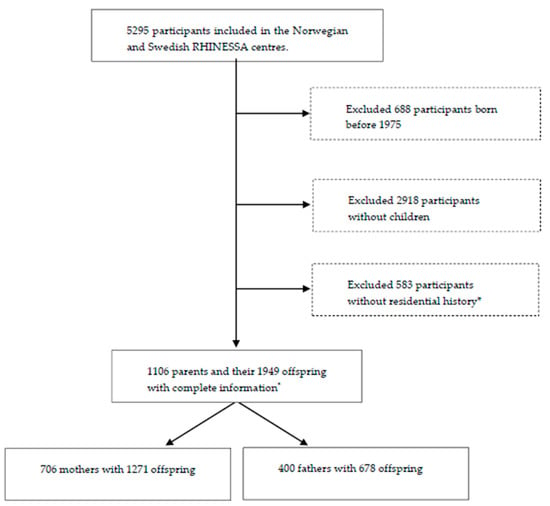
Figure 1.
Flowchart of the Respiratory Health in Northern Europe, Spain and Australia (RHINESSA) generation study population. * Lack of residential history due to lack of registered addresses in the population registries or lack of consent to address history retrieval.
2.2. Residential Address History
We retrieved the parents’ geocoded residential addresses from the Swedish and Norwegian national population registries for each year ranging from parents’ birth until the age of 18 years, as well as for offspring from birth until the age of 10 years.
2.3. Outcomes
The main outcomes in this study were offspring early-onset asthma and hay fever, defined as affirmative answers to the questions “For each of your biological children, please tick yes if they have had asthma before 10 years of age”, and “For each of your biological children please tick yes if they have had hay fever/rhinitis”, respectively.
2.4. Exposure Assessment
2.4.1. Air Pollution
We assigned annual mean concentrations (µg/m3) of 5 different air pollutants—NO2, PM2.5, PM10, black carbon (BC) and O3—to each participant based on their geocoded residential history. The exposures were assigned from air pollution rasters developed previously [31,32,33]. Annual mean PM10 exposures were extracted for 2005 to 2007 from surfaces (100 × 100 m) based on western Europe-wide hybrid land use regression (LUR) models [31]. Annual mean NO2, PM2.5 and O3 exposures and BC exposures for 2010 originate from similar hybrid LUR models [32,33]. An overview of the models used for the different pollutants can be found in the online supplement (Table S1).
We back-and-forth extrapolated the air pollution concentrations from the LUR models using the ratio method for each year from 1990 to 2015 following the procedure from the European Study of Cohorts for Air Pollution Effects (ESCAPE) project [34], that is based on the Danish Eulerian Hemispheric (DEHM) model [35]. For the years before 1990, we used 1990 estimates as proxies.
2.4.2. Greenness
Greenness was assessed using the normalized difference vegetation index (NDVI) [36], which refers to both structured and unstructured vegetation. NDVI estimates were derived from cloud free Landsat 4–5 TM and 8 OLI satellite images [37] (Table S2). NDVI values range from −1 to +1, with +1 indicating highly vegetated areas [38].
Satellite images were retrieved for every 5 years during the most vegetation rich months (May, June, July) (Table S3), and NDVI maps were calculated with mean NDVI in a circular 100 m, 300 m, 500 m and 1000 m buffer around each participant’s residential address. In the main analysis, we included the 300 m buffer, while the other buffer zones were included in sensitivity analysis (Tables S4 and S6a,b).
2.5. Time Windows for Exposures
We averaged mean annual exposures for the air pollutants and greenness across the period 0–18 years of age for parents and 0–10 years of age for offspring. Although desirable to estimate separate exposures for parents’ childhood and adolescence, stable residential patterns made this unfeasible (Table S7a–f).
2.6. Covariates and Mediators
To identify the minimal sufficient covariate adjustment set, we used a directed acyclic graph (DAG) (Figures S1–S4) [39,40]. To be considered as a confounder variable, the covariate had to be associated with both the exposure and the outcome and precede them both in time. Based on the DAG, we adjusted the multivariable analyses for grandparental education and grandparental asthma. Grandparental asthma was defined based on positive report by the parents on the question: “Have your biological parents ever had asthma?” with separate answer categories for “mother” and “father”. Grandparental education level was defined based on the question: “What was the highest level of education your mother/father has/had?”, with categories primary school, secondary school and college/university.
In addition, parental asthma, offspring’s own pollution/greenness exposures and pollution/greenness exposures during pregnancy (defined as birth year and the preceding year) were included as potential mediators based on a priori hypothesis that they may lie in the pathway between parental air pollution/greenness exposures and offspring asthma/allergies.
2.7. Statistical Analyses
All statistical analyses were performed using Stata version 16.0.
Descriptive analyses were stratified by parental sex.
We performed multilevel logistic regression analyses to investigate associations between air pollutants and greenness categorized in tertiles (low, medium and high exposures; see definition for all categories in Table S5), and early-onset asthma and hay fever as binary outcomes. The analyses were complete case analyses, clustered by family (to account for siblings) and study centre, and stratified by parental sex. All models were adjusted for O3 and NDVI (300 m buffer), except for the O3 model which was adjusted for NO2 and NDVI (300 m buffer) and the NDVI model which was adjusted for O3 and NO2. All models were also adjusted for grandparental education and grandparental asthma.
As sensitivity analyses, we fitted regression models separately for each country (Table S8a,b) and for parents born after 1985 (Table S9a,b). p-values < 0.05 were considered statistically significant.
Correlation analyses were performed for all exposures to decide which pollutants to include in the same models (Tables S10a,b and S11a–f).
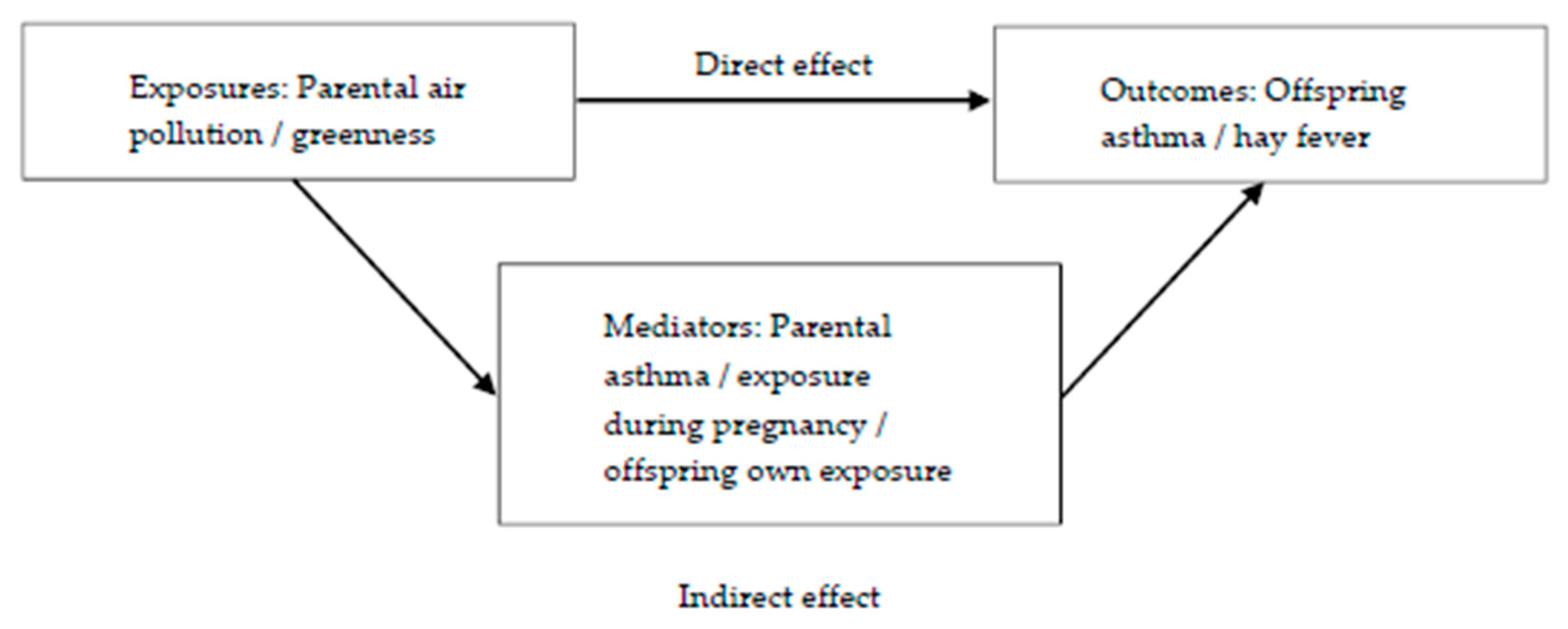
Mediation analyses were performed to decompose the total effects of parental exposures to greenness and each air pollutant on offspring’s outcomes into their direct and indirect (mediated) effects (Figure 2). Parental asthma, offspring’s exposure during pregnancy and offspring’s own exposure were all evaluated as potential mediators. In order to be a mediator, the exposure must be associated with the mediator and the mediator must be associated with the outcome. Mediation tests showed that offspring’s own exposure and exposure during pregnancy were potential mediators between maternal pollution exposure (PM10) and both offspring’s outcomes. For the paternal line, exposure during pregnancy (O3) was a potential mediator between paternal O3 exposure and offspring hay fever.
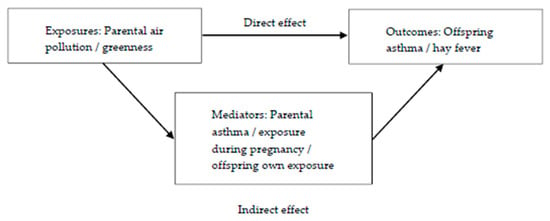
Figure 2.
Mediation models for the effects of parental exposures (air pollution/greenness) on offspring’s outcomes (asthma or hay fever).
The mediation analyses were conducted using ldecomp in Stata, a simple counterfactual mediation method that requires a categorical main exposure variable and a binary outcome, and allows any distribution of the mediator [41,42]. We used bootstrapping (1000 iterations) to obtain the 95% confidence interval (95%CI).
3. Results
The parents were on average 35 years old, and there were more mothers than fathers in the study population (Table 1). More mothers had asthma and hay fever compared to the fathers. The majority of the parents were never-smokers.

Table 1.
Study population characteristics. N = 706 mothers and 400 fathers and their 1949 offspring.
Mean air pollution exposures in the parents’ childhood were lowest in Umea and highest in Gothenburg (NO2 14.0 and 38.0 µg/m3, PM2.5 10.3 and 24.4 µg/m3, PM10 16.5 and 28.6 µg/m3, BC 0.09 and 1.09 µg/m3), except for O3, which was lowest in Bergen (62.7 µg/m3) and highest in Umea (68.4 µg/m3) (Table S12). Only annual mean values for PM2.5 and PM10 exceeded WHO recommendations in some centres (PM2.5 for parents 0–18 years old in Umea, Uppsala and Bergen; parents 0–18 years old and offspring 0–10 years old in Gothenburg; PM10 for parents 0–18 years old in Uppsala and Gothenburg). No annual mean exposures exceeded the recommended EU-values (Table S12).
The correlations between PM2.5, PM10, NO2 and BC were medium to strong, while O3 showed weaker correlation with the other pollutants (Table S10a,b).
Maternal medium PM2.5 and PM10 exposure was associated with a higher risk of offspring early-onset asthma when compared to low exposure (Table 2). Maternal high PM10 exposure was associated with a higher risk of hay fever in offspring. Paternal medium O3 exposure increased the risk of offspring hay fever, while paternal high BC exposure reduced the risk of offspring early-onset asthma when compared to low exposure. NO2 and NDVI were not associated with any outcomes, neither in the maternal nor the paternal line.

Table 2.
Univariable and multivariable analyses: associations between paternal (N = 400) and maternal (N = 706) exposure to air pollutants and NDVI and offspring (N = 1949) early-onset asthma (a) and hay fever (b) in the RHINESSA generation study.
Sensitivity analyses revealed protective associations of paternal high NDVI exposure (1000 m) for offspring early-onset asthma (Table S6b). Sensitivity analyses stratified by country and for parents born after 1985 gave roughly the same patterns, but with some variations due to low numbers (Tables S8a,b and S9a,b).
Maternal PM10 exposure had a direct effect on offspring early-onset asthma (Table 3) and an indirect effect on offspring hay fever (mediated by offspring’s own exposure and by exposure during pregnancy) (Table 3). Paternal O3 exposure was associated with increased odds for offspring hay fever through a direct and total effect, and was not mediated by O3 exposure during pregnancy.

Table 3.
Mediation analysis of the association between parental exposure and offspring early-onset asthma and hay fever (outcome) through exposure during pregnancy and offspring own exposure (potential mediators).
4. Discussion
Exposure to PM2.5 and PM10 in mothers’ childhood was associated with higher risk of offspring early-onset asthma, and exposure to PM10 was associated with higher risk of offspring hay fever. Fathers’ exposure to O3 was associated with more offspring hay fever, while fathers’ BC exposure was associated with less offspring early-onset asthma. NO2 and NDVI were not significantly associated with any of offspring’s outcomes in neither the maternal nor paternal line, although a protective NDVI association was suggested with a larger buffer zone. The association between maternal exposure to PM10 and offspring early-onset asthma was a direct effect, while the effect on offspring hay fever was indirect, mediated by exposures during pregnancy and offspring’s own childhood. The association between paternal O3 exposure and offspring hay fever was direct and not mediated by other factors.
To the best of our knowledge, this is the first study to investigate the associations between individual exposures to air pollution and greenness during childhood of one generation on lung health and allergy in the second generation. In previous literature, the focus on the parents’ role in offspring’s health revolves around maternal factors and in particular exposures during pregnancy. Recent studies associating prenatal air pollution exposure in mothers with childhood asthma show how maternal environmental exposure just before and during pregnancy is critical for fetal lung development and future respiratory health [43,44,45]. Our results expand and elaborate on this, suggesting that also exposures as far back in time as the childhood of the parents may play an important role in offspring health.
A possible explanation for our findings is that potential epigenetic processes can be induced in response to environmental exposures and influence disease risk also in the next generation [46]. Even air pollution levels that are below recommended limit values may through such epigenetic processes have a potential harmful effect on the respiratory health of future offspring. While we found clearer signals in the maternal than in the paternal line, previous studies have identified associations between paternal exposures and offspring asthma. One study discovered an association between paternal smoking prior to conception and offspring non-allergic early-onset asthma, while other studies found associations between smoking and overweight onset in adolescent boys and increased risk of asthma in the next generation [25,27,47]. A similar pattern was observed in our study were fathers’ exposure to O3 was associated with higher risk of offspring hay fever. However, we also found a seemingly protective association between paternal BC exposure and offspring early-onset asthma. The estimates in the paternal line should be interpreted with caution due to low number of fathers in our analysis, but this is nevertheless a surprising result that should be investigated further. Ideally, information on both parents should be included in the same analyses to give a complete picture of the possible epigenetic processes. Unfortunately, we only had information on one parent and his/her offspring in our study, and not on entire family units. Analyses of offspring with both parents in a long timeframe should be emphasized in future research.
Our study revealed few associations between exposure to greenness and early-onset asthma or hay fever in offspring. This may be because we do not have data on the time spent in green spaces. In the sensitivity analyses performed for wider NDVI buffer zones, we observed protective associations for offspring early-onset asthma after parental exposure to high levels of NDVI. For offspring hay fever, NDVI exposure was on the contrary associated with increased risk. The latter is in line with existing literature, and is possibly due to pollen exposure triggering allergic disease [17,48].
A noteworthy feature in our study was that medium parental exposure levels were associated with significantly increased risk for offspring asthma and hay fever, despite the fact that these levels are quite low—even high exposure levels in our study were in fact well beyond the international recommended limit values. It appears that there were no clear dose–response relation between parental air pollution exposures and offspring disease risk—for offspring asthma, the risk was actually highest for those whose parents were medium exposed. This may be related to the importance of the exposure time window and the epigenetic processes discussed above. In a study by Svanes et al. [25], the age of smoking onset in the parents was an important risk factor for asthma in their offspring, even after adjustment for the number of cigarettes they had smoked before conception. Moreover, a recent epigenome-wide association study showed associations between pre-conception paternal smoking and DNA methylation characteristics in adult and adolescent offspring—independent of the amount smoked [49]. Our findings suggest that the same patterns may be present for air pollution exposures as for smoking exposures. Given the low levels of exposures, these results suggest the need for re-evaluation of the recommended limit values.
Associations between air pollutants are complex, and one could hypothesize that there are interactive effects at play. The focus of the present study on inter-generational effects of relatively low air pollution exposure is however still in its early days. There is a need to establish evidence that there are certain basic associations before moving on to disentangle whether these exposures depend on interactions and/or which pollutants are of most importance with regard to respiratory health in the next generation. The exploration of interactive inter-generational effects of air pollution components on lung health would be a valuable next step for future studies.
We focused on residential air pollution exposures, but exposures can be substantially higher when commuting, compared to being at home. Children spend only around 40–50% of their time at home [50]. However, in most Scandinavian cities, it is common to live in close proximity of the children’s school or kindergarten and it is therefore likely that the true everyday air pollution and greenness exposures are similar to the residential exposure levels.
Associations between maternal PM10 childhood exposure and offspring hay fever were mediated by offspring’s own exposure and by maternal pregnancy exposure, while the effect was direct and unmediated with regard to early-onset asthma in offspring. These findings may suggest that asthma risk is susceptible for an epigenetic transmission across generations, while risk for hay fever is more likely triggered by own exposures. This could in turn imply a different transmission susceptibility for allergic asthma and non-allergic asthma. Unfortunately, we could not distinguish between allergic and non-allergic asthma in our study.
Correlation analyses revealed a strong correlation between pregnancy exposure and offspring childhood exposure, but weaker correlation between parental childhood exposures and pregnancy/offspring childhood exposures. Many parents moved to other areas with other levels of exposures after they grew up, and then settled in the same area during pregnancy and upbringing of children. This was also illustrated by the mediation analyses, where the effects of maternal childhood exposures to pollutants on offspring hay fever were mediated in the same manner by exposure during pregnancy and offspring’s own childhood exposure.
There are several strengths of this study. The RHINESSA generation study was designed to study respiratory health across generations with detailed information on mothers and fathers and their offspring, making it possible to investigate different susceptibility time windows for developing disease. The detailed address history was collected for each participant, together with the standardized exposure assessment of numerous air pollutants. The extrapolation formulas from the LUR models enabled us to estimate concentrations for specific areas and time points by integrating data on topography, road network, traffic information and land use within geographic information systems, resulting in accurate exposure calculations also for unmonitored locations and years. Although we do not know the precise accuracy of our selected study centres, previous validation studies from the ESCAPE project have shown that the model has satisfactory accuracy, with 68 to 71% explained variance for the PM variables and 82% explained variance for NO2 [32,51].
Another strength is the mediation analysis to disentangle the effects of the exposures on the outcomes into the direct and indirect (mediated through offspring’s own exposure and maternal exposure during pregnancy) components, and the use of DAGs to avoid over-adjustment in our analyses and to identify the possible mediators.
Some limitations should be acknowledged. First, population-based studies are vulnerable to bias. The response rate in RHINESSA was fairly low (around 40%). However, compared to the general population in the same age range, the RHINESSA population did not differ substantially when looking at demographic distributions (e.g., sex, smoking habits, educational level and asthma status) [28]. Additionally, recall bias is a challenge in many population-based studies. However, we do not suspect this in our study—partly due to exposure data being objectively registered based on residential address histories from the Norwegian and Swedish population registries, and with air pollution exposures being modelled by land-use regression models and greenness exposures being assigned through satellite images. Furthermore, the outcomes (offspring asthma and offspring hay fever) were not dependent on the parents’ memory far back in time since their offspring were still young (mean age of 6 years). Second, in the current study, we tested numerous exposures for associations with the outcomes. This multiple testing can increase the possibility of more false positive findings due to type 1 error [52]. However, due to a relatively low sample size, we believe instead that there may be an under-estimation rather than over-estimation of the associations in our analyses. Thirdly, the use of back extrapolation methods in the air pollution assignments may be a weakness for assignments before 1990 since the 1990 estimates were used as a proxy for these years. Air pollutants (except for O3) had a large variation over time, which may cause error in the earliest years. However, in additional analyses where we excluded all parents born before 1985, the patterns remained roughly unchanged, with pollutants as asthma risk factors in the maternal line but not in the paternal line. Lastly, the information on included parents was self-reported through questionnaires, and information about children and grandparents was parent-reported, thus, posing a potential information bias. However, validation studies carried out in RHINESSA showed a minimal risk of bias for asthma, smoking status, body silhouettes and overweight status reported across generations [28,53,54].
5. Conclusions
In conclusion, this study found that air pollution exposure in a mother’s childhood appeared to be a risk factor for early-onset asthma and hay fever in her future offspring. The observed effect of maternal exposures on asthma was direct, while the effect on hay fever was partly mediated through both offspring’s own exposure and exposure during pregnancy. Results regarding fathers were inconclusive and should be investigated further. Furthermore, future research with larger study populations are needed to fully understand the intergenerational effects of air pollution and greenness on offspring asthma and hay fever. However, our results suggest that the current air pollution limit values may be too high and that the long-term effects of exposure to air pollution may have harmful effects even across generations.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-4601/17/16/5828/s1, Table S1. Overview of the models used to calculate air pollution exposures. Table S2. Landsat images used for NDVI calculations. Table S3. NDVI assignment to addresses. Table S4. Mean annual average exposure (range) for NDVI buffer zones per center for parental exposure (0–18 years) and offspring’s exposure (0–10 years). Table S5. Low, medium and high exposure categories for air pollutants for the time windows: parents (0–18 years) and offspring (0–10 years). Table S6. Associations of paternal (N = 400) and maternal (N = 706) exposure to additional buffer zones of NDVI with offspring (N = 1949) early-onset asthma (Table S6a) and hay fever (Table S6b) in the RHINESSA generation study. Table S7a–f. Correlation coefficients for the exposure time windows: parent (0–10 years) and parent (10–18 years) for each exposure. Table S8. Analyses stratified per country (Swedish centers versus Bergen): Associations between paternal (N = 400) and maternal (N = 706) exposure to air pollution and NDVI (300 m) and offspring (N = 1949) early-onset asthma (Table S8a) and hay fever (Table S8b) in the RHINESSA generation study. Table S9. Analyses for parents born after 1985: associations of paternal (N = 73) and maternal (N = 154) exposure to air pollutants and NDVI with offspring (N = 309) early-onset asthma (Table S9a) and hay fever (Table S9b) in the RHINESSA generation study. Table S10. Correlation coefficients for the included air pollutants and NDVI: parental exposure (S10a) and offspring exposure (S10b). Table S11a–f. Correlation coefficients for the exposure time windows: parent (0–18 years), pregnancy and offspring (0–10 years) per exposure. Table S12. Mean annual average exposure (range) for air pollutants and NDVI (300 m) per center for parent exposure (0–18 years) and offspring exposure (0–10 years). Figure S1. Directed acyclic graph for parental air pollution exposure and offspring’s early-onset asthma. Figure S2. Directed acyclic graph for parental greenness exposure and offspring’s early-onset asthma. Figure S3. Directed acyclic graph for parental air pollution exposure and offspring’s hay fever. Figure S4. Directed acyclic graph for parental greenness exposure and offspring’s hay fever.
Author Contributions
Conceptualization, I.N.K., C.S. and A.J.; Formal analysis, I.N.K.; Investigation, I.M., J.H.C., O.H. and K.d.H.; Methodology, I.N.K., C.S. and A.J.; Supervision, T.H., C.S. and A.J.; Writing—original draft, I.N.K.; Writing—review and editing, I.N.K., I.M., S.A., R.J.B., L.B., J.H.C., B.F., T.H., J.H., O.H., G.H., M.H., K.d.H., C.J., A.M. (Andrei Malinovschi), A.M. (Alessandro Marcon), T.S., C.S. and A.J. All authors made equal contributions and have read and agreed to the published version of the manuscript.
Funding
The funding for this research is as follows: Ingrid Nordeide Kuiper received a PhD grant from Western Norway Regional Health Authorities (grant No 912011). The assessment of greenness was funded by the European Union’s Horizon 2020 research and innovation program as part of the ALEC (Ageing Lungs in European Cohorts study, Grant Agreement No. 633212). Co-ordination of the RHINESSA study and field work in Norwegian and Swedish study centres received funding from the Research Council of Norway (Grants No. 274767, 214123, 228174, 230827), the Bergen Medical Research Foundation, the Western Norwegian Regional Health Authorities (Grants No. 912011, 911892 and 911631), the World University Network, the Norwegian Asthma and Allergy Association, the Swedish Heart and Lung Foundation and the Swedish Asthma and Allergy Association [55].
Conflicts of Interest
The second author, Iana Markevych, is an assistant guest editor of the Special Issue “Environmental Exposures and Health–Mechanisms and Their Contingencies in a Developmental Perspective” of IJERPH. The co-author, Joachim Heinrich, is a co-editor of IJERPH. All other authors declare that they have no conflicts of interest.
References
- Air Quality in Europe—2015 Report by the European Environment Agency (EEA). Available online: https://www.eea.europa.eu/publications/air-quality-in-europe-2015 (accessed on 11 August 2020).
- Lelieveld, J.; Klingmuller, K.; Pozzer, A.; Poschl, U.; Fnais, M.; Daiber, A.; Munzel, T. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur. Heart J. 2019, 40, 1590–1596. [Google Scholar] [CrossRef]
- Khreis, H.; Kelly, C.; Tate, J.; Parslow, R.; Lucas, K.; Nieuwenhuijsen, M. Exposure to traffic-related air pollution and risk of development of childhood asthma: A systematic review and meta-analysis. Environ. Int. 2017, 100, 1–31. [Google Scholar] [CrossRef]
- Molter, A.; Simpson, A.; Berdel, D.; Brunekreef, B.; Custovic, A.; Cyrys, J.; de Jongste, J.; de Vocht, F.; Fuertes, E.; Gehring, U.; et al. A multicentre study of air pollution exposure and childhood asthma prevalence: The ESCAPE project. Eur. Respir. J. 2015, 45, 610–624. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Air Quality Guidelines for Particulate Matter, Ozone, Nitrogen Dioxide and Sulphur Dioxide. Global Update 2005. Available online: https://www.who.int/airpollution/publications/aqg2005/en/ (accessed on 11 August 2020).
- Air Quality Standards under the Air Quality Directive from the European Environment Agency (EEA, 2016). Available online: https://www.eea.europa.eu/data-and-maps/figures/air-quality-standards-under-the (accessed on 11 August 2020).
- Twohig-Bennett, C.; Jones, A. The health benefits of the great outdoors: A systematic review and meta-analysis of greenspace exposure and health outcomes. Environ. Res. 2018, 166, 628–637. [Google Scholar] [CrossRef]
- Lambert, K.A.; Bowatte, G.; Tham, R.; Lodge, C.; Prendergast, L.; Heinrich, J.; Abramson, M.J.; Dharmage, S.C.; Erbas, B. Residential greenness and allergic respiratory diseases in children and adolescents—A systematic review and meta-analysis. Environ. Res. 2017, 159, 212–221. [Google Scholar] [CrossRef]
- Eisenman, T.S.; Churkina, G.; Jariwala, S.P.; Kumar, P.; Lovasi, G.S.; Pataki, D.E.; Weinberger, K.R.; Whitlow, T.H. Urban trees, air quality, and asthma: An interdisciplinary review. Landsc. Urban Plan. 2019, 187, 47–59. [Google Scholar] [CrossRef]
- Lambert, K.A.; Bowatte, G.; Tham, R.; Lodge, C.J.; Prendergast, L.A.; Heinrich, J.; Abramson, M.J.; Dharmage, S.C.; Erbas, B. Greenspace and Atopic Sensitization in Children and Adolescents—A Systematic Review. Int. J. Environ. Res. Public Health 2018, 15, 2539. [Google Scholar] [CrossRef]
- Squillacioti, G.; Bellisario, V.; Levra, S.; Piccioni, P.; Bono, R. Greenness Availability and Respiratory Health in a Population of Urbanised Children in North-Western Italy. Int. J. Environ. Res. Public Health 2019, 17, 108. [Google Scholar] [CrossRef]
- Vienneau, D.; de Hoogh, K.; Faeh, D.; Kaufmann, M.; Wunderli, J.M.; Roosli, M. More than clean air and tranquillity: Residential green is independently associated with decreasing mortality. Environ. Int. 2017, 108, 176–184. [Google Scholar] [CrossRef]
- Gascon, M.; Triguero-Mas, M.; Martinez, D.; Dadvand, P.; Rojas-Rueda, D.; Plasencia, A.; Nieuwenhuijsen, M.J. Residential green spaces and mortality: A systematic review. Environ. Int. 2016, 86, 60–67. [Google Scholar] [CrossRef]
- James, P.; Hart, J.E.; Banay, R.F.; Laden, F. Exposure to Greenness and Mortality in a Nationwide Prospective Cohort Study of Women. Environ. Health Perspect. 2016, 124, 1344–1352. [Google Scholar] [CrossRef]
- Fong, K.C.; Hart, J.E.; James, P. A Review of Epidemiologic Studies on Greenness and Health: Updated Literature Through 2017. Curr. Environ. Health Rep. 2018, 5, 77–87. [Google Scholar] [CrossRef]
- Dadvand, P.; Villanueva, C.M.; Font-Ribera, L.; Martinez, D.; Basagana, X.; Belmonte, J.; Vrijheid, M.; Grazuleviciene, R.; Kogevinas, M.; Nieuwenhuijsen, M.J. Risks and benefits of green spaces for children: A cross-sectional study of associations with sedentary behavior, obesity, asthma, and allergy. Environ. Health Perspect. 2014, 122, 1329–1335. [Google Scholar] [CrossRef]
- Fuertes, E.; Markevych, I.; von Berg, A.; Bauer, C.P.; Berdel, D.; Koletzko, S.; Sugiri, D.; Heinrich, J. Greenness and allergies: Evidence of differential associations in two areas in Germany. J. Epidemiol. Community Health 2014, 68, 787–790. [Google Scholar] [CrossRef]
- Fuertes, E.; Markevych, I.; Bowatte, G.; Gruzieva, O.; Gehring, U.; Becker, A.; Berdel, D.; von Berg, A.; Bergstrom, A.; Brauer, M.; et al. Residential greenness is differentially associated with childhood allergic rhinitis and aeroallergen sensitization in seven birth cohorts. Allergy 2016, 71, 1461–1471. [Google Scholar] [CrossRef]
- Markevych, I.; Schoierer, J.; Hartig, T.; Chudnovsky, A.; Hystad, P.; Dzhambov, A.M.; de Vries, S.; Triguero-Mas, M.; Brauer, M.; Nieuwenhuijsen, M.J.; et al. Exploring pathways linking greenspace to health: Theoretical and methodological guidance. Environ. Res. 2017, 158, 301–317. [Google Scholar] [CrossRef]
- James, P.; Banay, R.F.; Hart, J.E.; Laden, F. A Review of the Health Benefits of Greenness. Curr. Epidemiol. Rep. 2015, 2, 131–142. [Google Scholar] [CrossRef]
- Svanes, C.; Omenaas, E.; Jarvis, D.; Chinn, S.; Gulsvik, A.; Burney, P. Parental smoking in childhood and adult obstructive lung disease: Results from the European Community Respiratory Health Survey. Thorax 2004, 59, 295–302. [Google Scholar] [CrossRef]
- Dratva, J.; Zemp, E.; Dharmage, S.C.; Accordini, S.; Burdet, L.; Gislason, T.; Heinrich, J.; Janson, C.; Jarvis, D.; de Marco, R.; et al. Early Life Origins of Lung Ageing: Early Life Exposures and Lung Function Decline in Adulthood in Two European Cohorts Aged 28–73 Years. PLoS ONE 2016, 11, e0145127. [Google Scholar] [CrossRef]
- de Marco, R.; Pattaro, C.; Locatelli, F.; Svanes, C. Influence of early life exposures on incidence and remission of asthma throughout life. J. Allergy Clin. Immunol. 2004, 113, 845–852. [Google Scholar] [CrossRef]
- Morkve Knudsen, T.; Rezwan, F.I.; Jiang, Y.; Karmaus, W.; Svanes, C.; Holloway, J.W. Transgenerational and intergenerational epigenetic inheritance in allergic diseases. J. Allergy Clin. Immunol. 2018, 142, 765–772. [Google Scholar] [CrossRef]
- Svanes, C.; Koplin, J.; Skulstad, S.M.; Johannessen, A.; Bertelsen, R.J.; Benediktsdottir, B.; Braback, L.; Elie Carsin, A.; Dharmage, S.; Dratva, J.; et al. Father’s environment before conception and asthma risk in his children: A multi-generation analysis of the Respiratory Health In Northern Europe study. Int. J. Epidemiol. 2017, 46, 235–245. [Google Scholar] [CrossRef]
- Accordini, S.; Calciano, L.; Johannessen, A.; Portas, L.; Benediktsdottir, B.; Bertelsen, R.J.; Braback, L.; Carsin, A.E.; Dharmage, S.C.; Dratva, J.; et al. A three-generation study on the association of tobacco smoking with asthma. Int. J. Epidemiol. 2018, 47, 1106–1117. [Google Scholar] [CrossRef]
- Johannessen, A.; Lonnebotn, M.; Calciano, L.; Benediktsdottir, B.; Bertelsen, R.J.; Braback, L.; Dharmage, S.; Franklin, K.A.; Gislason, T.; Holm, M.; et al. Being overweight in childhood, puberty, or early adulthood: Changing asthma risk in the next generation? J. Allergy Clin. Immunol. 2019. [Google Scholar] [CrossRef]
- Kuiper, I.N.; Svanes, C.; Benediktsdottir, B.; Bertelsen, R.J.; Braback, L.; Dharmage, S.C.; Holm, M.; Janson, C.; Jogi, R.; Malinovschi, A.; et al. Agreement in reporting of asthma by parents or offspring—The RHINESSA generation study. BMC Pulm. Med. 2018, 18, 122. [Google Scholar] [CrossRef]
- RHINESSA Generation Study Homepage. Available online: www.rhinessa.net (accessed on 11 August 2020).
- Overview of Ethics Committees and Approval Numbers of RHINESSA Centers. Available online: https://helse-bergen.no/seksjon/RHINESSA/Documents/Ethic%20Committees%20list.pdf (accessed on 11 August 2020).
- Vienneau, D.; de Hoogh, K.; Bechle, M.J.; Beelen, R.; van Donkelaar, A.; Martin, R.V.; Millet, D.B.; Hoek, G.; Marshall, J.D. Western European land use regression incorporating satellite- and ground-based measurements of NO2 and PM10. Environ. Sci. Technol. 2013, 47, 13555–13564. [Google Scholar] [CrossRef]
- de Hoogh, K.; Gulliver, J.; Donkelaar, A.V.; Martin, R.V.; Marshall, J.D.; Bechle, M.J.; Cesaroni, G.; Pradas, M.C.; Dedele, A.; Eeftens, M.; et al. Development of West-European PM2.5 and NO2 land use regression models incorporating satellite-derived and chemical transport modelling data. Environ. Res. 2016, 151, 1–10. [Google Scholar] [CrossRef]
- de Hoogh, K.; Chen, J.; Gulliver, J.; Hoffmann, B.; Hertel, O.; Ketzel, M.; Bauwelinck, M.; van Donkelaar, A.; Hvidtfeldt, U.A.; Katsouyanni, K.; et al. Spatial PM2.5, NO2, O3 and BC models for Western Europe—Evaluation of spatiotemporal stability. Environ. Int. 2018, 120, 81–92. [Google Scholar] [CrossRef]
- Procedure for Back-Extrapolation, Manual by the ESCAPE Project. Available online: http://www.escapeproject.eu/manuals/Procedure_for_extrapolation_back_in_time.pdf (accessed on 11 August 2020).
- Christensen, J.H. The Danish eulerian hemispheric model—A three-dimensional air pollution model used for the arctic. Atmos. Environ. 1997, 31, 4169–4191. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Satellite images, United States Geological Survey. Available online: http://earthexplorer.usgs.gov (accessed on 11 August 2020).
- Weier, J.H.; Herring, D. Measuring Vegeation (NDVI & EVI). Available online: https://earthobservatory.nasa.gov/features/MeasuringVegetation (accessed on 11 August 2020).
- Greenland, S.; Pearl, J.; Robins, J.M. Causal diagrams for epidemiologic research. Epidemiology 1999, 10, 37–48. [Google Scholar]
- DAGitty, a browser based environment to draw and analyze causal diagrams. Available online: www.dagitty.net (accessed on 11 August 2020).
- Buis, M.L. Direct and indirect effects in a logit model. Stata J. 2010, 10, 11–29. [Google Scholar]
- Hayes, A.F.; Rockwood, N.J. Regression-based statistical mediation and moderation analysis in clinical research: Observations, recommendations, and implementation. Behav. Res. Ther. 2017, 98, 39–57. [Google Scholar] [CrossRef]
- Lee, A.; Leon Hsu, H.H.; Mathilda Chiu, Y.H.; Bose, S.; Rosa, M.J.; Kloog, I.; Wilson, A.; Schwartz, J.; Cohen, S.; Coull, B.A.; et al. Prenatal fine particulate exposure and early childhood asthma: Effect of maternal stress and fetal sex. J. Allergy Clin. Immunol. 2018, 141, 1880–1886. [Google Scholar] [CrossRef]
- Hsu, H.H.; Chiu, Y.H.; Coull, B.A.; Kloog, I.; Schwartz, J.; Lee, A.; Wright, R.O.; Wright, R.J. Prenatal Particulate Air Pollution and Asthma Onset in Urban Children. Identifying Sensitive Windows and Sex Differences. Am. J. Respir. Crit. Care Med. 2015, 192, 1052–1059. [Google Scholar] [CrossRef]
- Zhou, C.; Baiz, N.; Zhang, T.; Banerjee, S.; Annesi-Maesano, I. Modifiable exposures to air pollutants related to asthma phenotypes in the first year of life in children of the EDEN mother-child cohort study. BMC Public Health 2013, 13, 506. [Google Scholar] [CrossRef]
- Arshad, S.H.; Karmaus, W.; Zhang, H.; Holloway, J.W. Multigenerational cohorts in patients with asthma and allergy. J. Allergy Clin. Immunol. 2017, 139, 415–421. [Google Scholar] [CrossRef]
- Northstone, K.; Golding, J.; Davey Smith, G.; Miller, L.L.; Pembrey, M. Prepubertal start of father’s smoking and increased body fat in his sons: Further characterisation of paternal transgenerational responses. Eur. J. Hum. Genet. 2014, 22, 1382–1386. [Google Scholar] [CrossRef]
- Parmes, E.; Pesce, G.; Sabel, C.E.; Baldacci, S.; Bono, R.; Brescianini, S.; D’Ippolito, C.; Hanke, W.; Horvat, M.; Liedes, H.; et al. Influence of residential land cover on childhood allergic and respiratory symptoms and diseases: Evidence from 9 European cohorts. Environ. Res. 2019, 108953. [Google Scholar] [CrossRef]
- Mørkve Knudsen, G.T.; Rezwan, F.I.; Johannessen, A.; Skulstad, S.M.; Bertelsen, R.J.; Real, F.G.; Krauss-Etschmann, S.; Patil, V.; Jarvis, D.; Arshad, S.H.; et al. Epigenome-wide association of father’s smoking with offspring DNA methylation: A hypothesis-generating study. Environ. Epigenet. 2019, 5. [Google Scholar] [CrossRef]
- Khreis, H.; Nieuwenhuijsen, M.J. Traffic-Related Air Pollution and Childhood Asthma: Recent Advances and Remaining Gaps in the Exposure Assessment Methods. Int. J. Environ. Res. Public Health 2017, 14, 312. [Google Scholar] [CrossRef]
- Beelen, R.; Hoek, G.; Vienneau, D.; Eeftens, M.; Dimakopoulou, K.; Pedeli, X.; Tsai, M.-Y.; Künzli, N.; Schikowski, T.; Marcon, A.; et al. Development of NO2 and NOx land use regression models for estimating air pollution exposure in 36 study areas in Europe—The ESCAPE project. Atmos. Environ. 2013, 72, 10–23. [Google Scholar] [CrossRef]
- Patel, C.J.; Ioannidis, J.P.A. Placing epidemiological results in the context of multiplicity and typical correlations of exposures. J. Epidemiol. Community Health 2014, 68, 1096–1100. [Google Scholar] [CrossRef]
- Pape, K.; Svanes, C.; Malinovschi, A.; Benediktsdottir, B.; Lodge, C.; Janson, C.; Moratalla, J.; Sánchez-Ramos, J.L.; Bråbäck, L.; Holm, M.; et al. Agreement of offspring-reported parental smoking status: The RHINESSA generation study. BMC Public Health 2019, 19, 94. [Google Scholar] [CrossRef]
- Lonnebotn, M.; Svanes, C.; Igland, J.; Franklin, K.A.; Accordini, S.; Benediktsdottir, B.; Bentouhami, H.; Blanco, J.A.G.; Bono, R.; Corsico, A.; et al. Body silhouettes as a tool to reflect obesity in the past. PLoS ONE 2018, 13, e0195697. [Google Scholar] [CrossRef]
- Overview Funding RHINESSA. Available online: https://helse-bergen.no/seksjon/RHINESSA/Documents/Funding%20RHINESSA.pdf (accessed on 11 August 2020).
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).