Is Low-Frequency Electrical Stimulation a Tool for Recovery after a Water Rescue? A Cross-Over Study with Lifeguards
Abstract
:1. Introduction
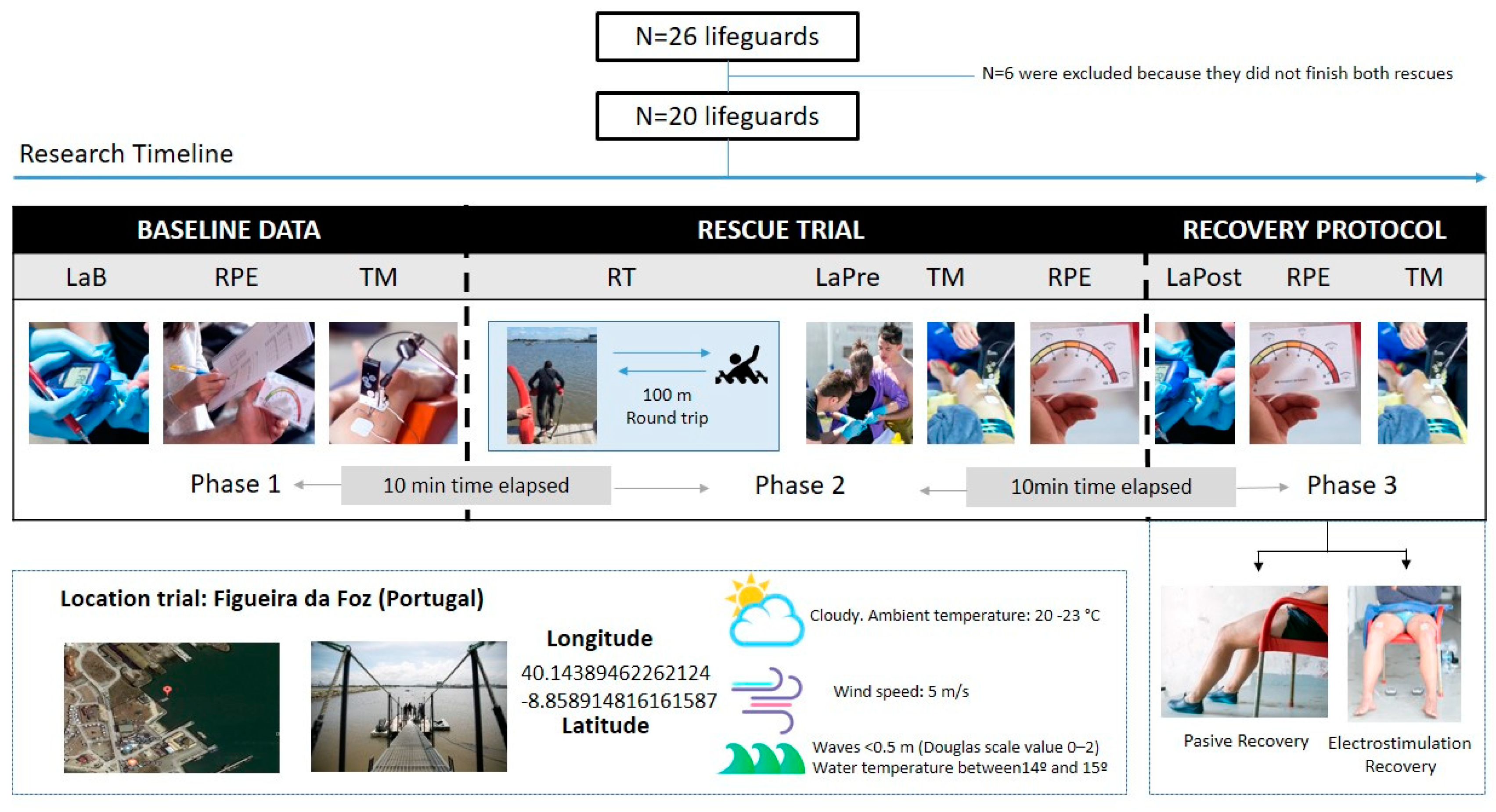
2. Materials and Methods
2.1. Experimental Design
2.2. Participants
2.3. Procedures
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prieto, J.A.; Nistal, P.; Méndez, D.; Abelairas-Gómez, C.; Barcala-Furelos, R. Impact of error self-perception of aerobic capacity in the safety and efficacy of the lifeguards. Int. J. Occup. Saf. Ergon. 2016, 22, 159–163. [Google Scholar] [CrossRef]
- Franklin, R.C.; Peden, A.E.; Hamilton, E.B.; Bisignano, C.; Castle, C.D.; Dingels, Z.V.; Hay, S.I.; Liu, Z.; Mokdad, A.H.; Roberts, N.L.; et al. The burden of unintentional drowning: Global, regional and national estimates of mortality from the Global Burden of Disease 2017 Study. Inj. Prev. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szpilman, D.; Bierens, J.; Handley, A.; Orlowski, J. Drowning. N. Engl. J. Med. 2012, 366, 2102–2110. [Google Scholar] [CrossRef] [PubMed]
- Abelairas-Gómez, C.; Barcala-Furelos, R.; Mecías-Calvo, M.; Rey-Eiras, E.; López-García, S.; Costas-Veiga, J.; Bores-Cerezal, A.; Palacios-Aguilar, J. Prehospital emergency medicine at the beach: What is the effect of fins and rescue tubes in lifesaving and cardiopulmonary resuscitation after rescue? Wilderness Environ. Med. 2017, 28, 176–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barcala-Furelos, R.; Szpilman, D.; Palacios-Aguilar, J.; Costas-Veiga, J.; Abelairas-Gómez, C.; Bores-Cerezal, A.; López-García, S.; Rodríguez-Núñez, A. Assessing the efficacy of rescue equipment in lifeguard resuscitation efforts for drowning. Am. J. Emerg. Med. 2016, 34, 480–485. [Google Scholar] [CrossRef]
- Kalén, A.; Pérez-Ferreirós, A.; Barcala-Furelos, R.; Fernández-Méndez, M.; Padrón-Cabo, A.; Prieto, J.A.; Ríos-Ave, A.; Abelairas-Gómez, C. How can lifeguards recover better? A cross-over study comparing resting, running, and foam rolling. Am. J. Emerg. Med. 2017, 35, 1887–1891. [Google Scholar] [CrossRef]
- Morgan, D.; Ozanne-Smith, J. Surf Lifeguard Rescues. Wilderness Environ. Med. 2013, 24, 285–290. [Google Scholar] [CrossRef] [Green Version]
- Neric, F.B.; Beam, W.C.; Brown, L.E.; Wiersma, L.D. Comparison of swim recovery and muscle stimulation on lactate removal after sprint swimming. J. Strength Cond. Res. 2009, 23, 2560–2567. [Google Scholar] [CrossRef] [Green Version]
- Babault, N.; Cometti, C.; Maffiuletti, N.A.; Deley, G. Does electrical stimulation enhance post-exercise performance recovery? Eur. J. Appl. Physiol. 2011, 111, 2501–2507. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, A.; Senin-Camargo, F.; Raposo-Vidal, I.; Chouza-Insua, M.; Rodríguez-Romero, B.; Jácome, M.A. Effects of transcutaneous electrical nerve stimulation via peroneal nerve or soleus muscle on venous flow: A randomized cross-over study in healthy subjects. Medicine (Baltim.) 2018, 97, e12084. [Google Scholar] [CrossRef]
- Johnson, M.I.; Paley, C.A.; Howe, T.E.; Sluka, K.A. Transcutaneous electrical nerve stimulation for acute pain. Cochrane Database Syst. Rev. 2015, 6, CD006142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, K.J.; Ravikumar, R.; Gaweesh, A.S.; Moore, H.M.; Lifsitz, A.D.; Lane, T.R.; Shalhoub, J.; Babber, A.; Davies, A.H. A Review of the evidence to support neuromuscular electrical stimulation in the prevention and management of venous disease. Adv. Exp. Med. Biol. 2017, 906, 377–386. [Google Scholar] [PubMed]
- Bahadori, S.; Immins, T.; Wainwright, T.W. The effect of calf neuromuscular electrical stimulation and intermittent pneumatic compression on thigh microcirculation. Microvasc. Res. 2017, 111, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Macgregor, L.J.; Hunter, A.M.; Orizio, C.; Fairweather, M.M.; Ditroilo, M. Assessment of skeletal muscle contractile properties by radial displacement: The case for Tensiomyography. Sports Med. 2018, 48, 1607–1620. [Google Scholar] [CrossRef] [Green Version]
- Martín-Rodríguez, S.; Loturco, I.; Hunter, A.M.; Rodríguez-Ruiz, D.; Munguía-Izquierdo, D. Reliability and measurement error of tensiomyography to assess mechanical muscle function: A systematic review. J. Strength Cond. Res. 2017, 31, 3524–3536. [Google Scholar] [CrossRef]
- Rey, E.; Corredoira, F.J.; Costa, P.B.; Pérez-Ferreirós, A.; Fernandez-Villarino, M.A. Acute effects of training load on contractile properties during a competitive microcycle in elite soccer players. Biol. Sport 2020, 37, 157–163. [Google Scholar] [CrossRef]
- Abelairas-Gómez, C.; Rey, E.; González-Salvado, V.; Mecías-Calvo, M.; Rodríguez-Ruiz, E.; Rodriguez-Nuñez, A. Acute muscle fatigue and CPR quality assisted by visual feedback devices: A randomized-crossover simulation trial. PLoS ONE 2018, 13, e0203576. [Google Scholar] [CrossRef]
- Carrasco, L.; Sañudo, B.; Hoyo, M.; Pradas, F.; Silva, M.E. Effectiveness of low-frequency vibration recovery method on blood lactate removal, muscle contractile properties and on time to exhaustion during cycling at VO2max power output. Eur. J. Appl. Physiol. 2011, 111, 2271–2279. [Google Scholar] [CrossRef] [Green Version]
- Rey, E.; Lago-Peñas, C.; Lago-Ballesteros, J.; Casáis, L. The effect of recovery strategies on contractile properties using tensiomyography and perceived muscle soreness in professional soccer players. J. Strength Cond. Res. 2012, 26, 3081–3088. [Google Scholar] [CrossRef]
- Mur Gimeno, E.; Campa, F.; Badicu, G.; Castizo-Olier, J.; Palomera-Fanegas, E.; Sebio-Garcia, R. Changes in muscle contractile properties after cold-or warm-water immersion using tensiomyography: A cross-over randomised trial. Sensors 2020, 20, 3193. [Google Scholar] [CrossRef]
- García-Manso, J.M.; Rodríguez-Matoso, D.; Rodríguez-Ruiz, D.; Sarmiento, S.; De Saa, Y.; Calderón, J. Effect of cold-water immersion on skeletal muscle contractile properties in soccer players. Am. J. Phys. Med. Rehab. 2011, 90, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Claesson, A.; Karlsson, T.; Thorén, A.B.; Herlitz, J. Delay and performance of cardiopulmonary resuscitation in surf lifeguards after simulated cardiac arrest due to drowning. Am. J. Emerg. Med. 2011, 29, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- García-García, O.; Cuba-Dorado, A.; Fernández-Redondo, D.; López-Chicharro, J. Neuromuscular parameters predict the performance in an incremental cycling test. Int. J. Sports Med. 2018, 39, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.J.; Moyna, N.M.; Sward, K.L.; Millich, N.B.; Goss, F.L.; Thompson, P.D. Gender comparison of RPE at absolute and relative physiological criteria. Med. Sci. Sports Exerc. 2000, 32, 2120–2129. [Google Scholar] [CrossRef] [PubMed]
- Marion, K.; Guillaume, G.; Pascale, C.; Charlie, B.; Anton, S. Muscle activity during fin swimming. Procedia Eng. 2010, 2, 3029–3034. [Google Scholar] [CrossRef] [Green Version]
- Lattier, G.; Millet, G.Y.; Martin, A.; Martin, V. Fatigue and recovery after high-intensity exercise. Part II: Recovery interventions. Int. J. Sports Med. 2004, 25, 509–515. [Google Scholar] [CrossRef]
- Martin, V.; Millet, G.Y.; Lattier, G.; Perrod, L. Effects of recovery modes after knee extensor muscles eccentric contractions. Med. Sci. Sports Exerc. 2004, 36, 1907–1915. [Google Scholar] [CrossRef]
- Vanderthommen, M.; Makrof, S.; Demoulin, C. Comparison of active and electrostimulated recovery strategies after fatiguing exercise. J. Sports Sci. Med. 2010, 9, 164–169. [Google Scholar]
- Bieuzen, F.; Borne, R.; Toussaint, J.F.; Hausswirth, C. Positive effect of specific low-frequency electrical stimulation during short-term recovery on subsequent high-intensity exercise. Appl. Physiol. Nutr. Metab. 2014, 39, 202–210. [Google Scholar] [CrossRef]
- Finberg, M.; Braham, R.; Goodman, C.; Gregory, P.; Peeling, P. Effects of electrostimulation therapy on recovery from acute team-sport activity. Int. J. Sports Physiol. Perform. 2013, 8, 293–299. [Google Scholar] [CrossRef]
- Taylor, T.; West, D.J.; Howatson, G.; Jones, C.; Bracken, R.M.; Love, T.D.; Cook, C.J.; Swift, E.; Baker, J.S.; Kilduff, L.P. The impact of neuromuscular electrical stimulation on recovery after intensive, muscle damaging, maximal speed training in professional team sports players. J. Sci. Med. Sport 2015, 18, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Malone, J.K.; Blake, C.; Caulfield, B.M. Neuromuscular electrical stimulation during recovery from exercise: A systematic review. J. Strength Cond. Res. 2014, 28, 2478–2506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malone, J.K.; Coughlan, G.F.; Crowe, L.; Gissane, G.C.; Caulfield, B. The physiological effects of low-intensity neuromuscular electrical stimulation (NMES) on short-term recovery from supra-maximal exercise bouts in male triathletes. Eur. J. Appl. Physiol. 2012, 112, 2421–2432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucker, A.; Maass, A.; Bain, D.; Chen, L.H.; Azzam, M.; Dawson, H.; Johnston, A. Augmentation of venous, arterial and microvascular blood supply in the leg by isometric neuromuscular stimulation via the peroneal nerve. Int. J. Angiol. Off. Publ. Int. Coll. Angiol. Inc. 2010, 19, e31–e37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prieto Saborit, J.A.; del Valle Soto, M.; González Díez, V.; Montoliu Sanclement, M.Á.; Nistal Hernández, P.; Egocheaga Rodríguez, J.; Santos Rodríguez, L. Physiological response of beach lifeguards in a rescue simulation with surf. Ergonomics 2010, 53, 1140–1150. [Google Scholar] [CrossRef] [PubMed]
| TMG Variables * | Data before Trial | ||||||||
| M | Sd | ||||||||
| TC | 26.24 | 3.81 | |||||||
| DM | 7.61 | 2.71 | |||||||
| Variables ** | PR | ES | Test of Difference 1 | Equivalence Test 2 | |||||
| M | Sd | M | Sd | T | p-Value | Cohen’s d | t | p-Value | |
| Lactate | 2.58 | 1.32 | 2.64 | 0.997 | −0.115 | 0.909 | −0.0258 | 2.12 | 0.024 |
| RPE overall | 2.05 | 1.50 | 1.77 | 1.88 | 0.370 | 0.715 | 0.0808 | −1.92 | 0.035 |
| RPE legs | 2.23 | 1.85 | 2.33 | 2.31 | −0.145 | 0.886 | −0.0325 | 2.09 | 0.025 |
| RPE arms | 1.91 | 1.51 | 2.10 | 2.12 | −0.493 | 0.628 | −0.1102 | 1.74 | 0.049 |
| RPE ventilatory | 1.18 | 1.14 | 1.29 | 1.82 | −0.260 | 0.798 | −0.0581 | 1.98 | 0.031 |
| PR | ES | Anova | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Pre-Recovery Protocol | Post-Recovery Protocol | Pre-Recovery Protocol | Post-Recovery Protocol | Recovery | Time | Time × Recovery | |||||||||
| M | Sd | M | Sd | % diff | M | Sd | M | Sd | % diff | p-Value | ES | p-Value | ES | p-Value | ES | |
| Lactate | 9.89 | 2.63 | 6.27 | 3.69 | −36.6 | 10.9 | 3.06 | 4.77 | 1.86 | −56.2 | 0.342 | 0.050 | <0.001 | 0.757 | 0.020 | 0.267 |
| RPE overall | 7.59 | 1.71 | 3.57 | 2.40 | −53.0 | 7.24 | 1.18 | 2.7 | 1.53 | −62.7 | 0.017 | 0.278 | <0.001 | 0.849 | 0.091 | 0.151 |
| RPE legs | 7.27 | 1.83 | 3.71 | 2.43 | −49.0 | 7.05 | 1.72 | 2.65 | 1.66 | −62.4 | 0.017 | 0.279 | <0.001 | 0.836 | 0.053 | 0.192 |
| RPE arms | 6.18 | 1.68 | 3.29 | 1.79 | −46.8 | 5.62 | 1.91 | 2.30 | 1.84 | −59.1 | 0.011 | 0.306 | <0.001 | 0.763 | 0.444 | 0.033 |
| RPE ventilatory | 5.64 | 2.40 | 3.00 | 2.76 | −46.8 | 5.71 | 2.35 | 2.0 | 1.45 | −65.0 | 0.081 | 0.160 | <0.001 | 0.607 | 0.152 | 0.111 |
| TC | 27.5 | 3.31 | 27.8 | 3.76 | 1.1 | 27.3 | 2.97 | 28.1 | 4.82 | 2.9 | 0.943 | 0.000 | 0.441 | 0.033 | 0.537 | 0.021 |
| DM | 6.73 | 2.19 | 7.22 | 2.57 | 7.3 | 7.02 | 2.60 | 6.75 | 2.64 | −4.0 | 0.740 | 0.006 | 0.672 | 0.010 | 0.087 | 0.154 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barcala-Furelos, R.; González-Represas, A.; Rey, E.; Martínez-Rodríguez, A.; Kalén, A.; Marques, O.; Rama, L. Is Low-Frequency Electrical Stimulation a Tool for Recovery after a Water Rescue? A Cross-Over Study with Lifeguards. Int. J. Environ. Res. Public Health 2020, 17, 5854. https://doi.org/10.3390/ijerph17165854
Barcala-Furelos R, González-Represas A, Rey E, Martínez-Rodríguez A, Kalén A, Marques O, Rama L. Is Low-Frequency Electrical Stimulation a Tool for Recovery after a Water Rescue? A Cross-Over Study with Lifeguards. International Journal of Environmental Research and Public Health. 2020; 17(16):5854. https://doi.org/10.3390/ijerph17165854
Chicago/Turabian StyleBarcala-Furelos, Roberto, Alicia González-Represas, Ezequiel Rey, Alicia Martínez-Rodríguez, Anton Kalén, Olga Marques, and Luís Rama. 2020. "Is Low-Frequency Electrical Stimulation a Tool for Recovery after a Water Rescue? A Cross-Over Study with Lifeguards" International Journal of Environmental Research and Public Health 17, no. 16: 5854. https://doi.org/10.3390/ijerph17165854






