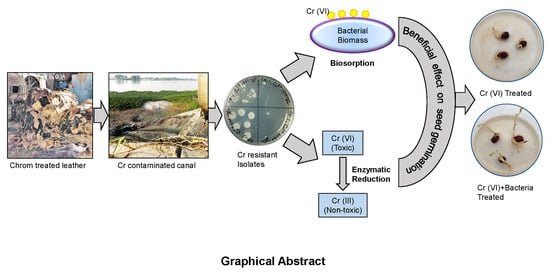
Bioremediation of Hexavalent Chromium by Chromium Resistant Bacteria Reduces Phytotoxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sampling Sites and Sample Collection
2.3. Screening of Potential Chromium Reducing Bacteria
2.4. Molecular Identification and Phylogeny Analysis of the Selected Isolate
2.5. Determination of Optimum Growth Condition of the Isolate
2.6. Resistance to Antibiotics and other Heavy Metals
2.7. Analysis of Reduction Capacity of Cr(VI) by the Isolate in the Culture Medium, Pond Water, and Tannery Effluents
2.8. Enzyme Assay
2.9. Batch Biosorption Experiments
2.9.1. Analysis of Cr(VI) and Measurement of Metal Uptake
2.9.2. Adsorption Isotherms
2.9.3. Kinetics Studies
2.10. Scanning Electron Microscopic (SEM) and Fourier Transform Infrared Spectrometric (FTIR) Analysis
2.11. Assessment of Toxicity of Cr(VI) on Chickpea Seed Germination
2.12. Statistical Analysis
3. Results and Discussion
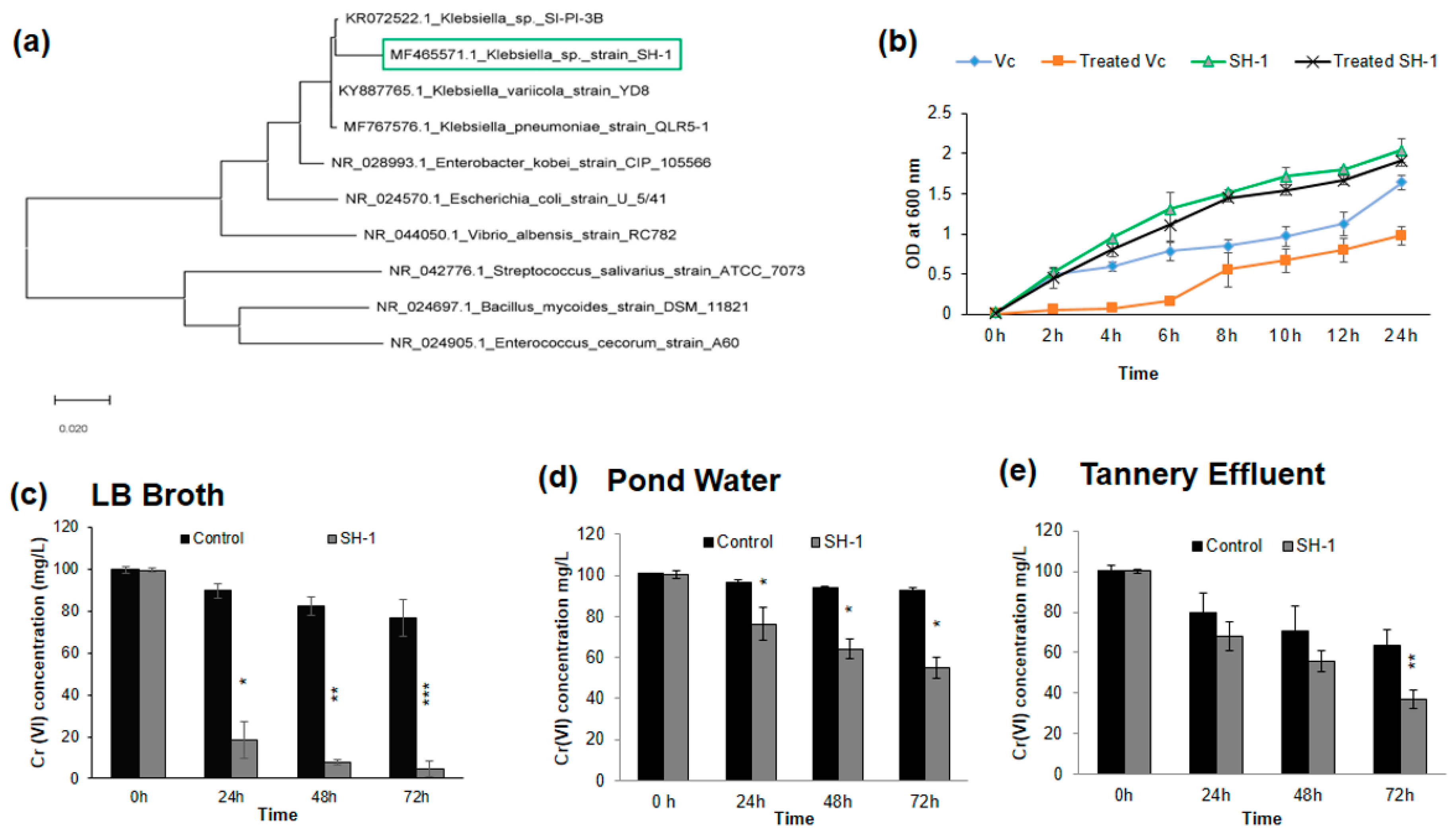
3.1. Identification of the Isolate by 16S rRNA Sequencing and Phylogeny Analysis
3.2. Effect of Chromium under Optimum Growth Conditions
3.3. Isolate Showed Different Chromium (VI) Reduction Capacity in Different Media
3.4. Isolates Showed Enhanced Chromate Reductase Activity in the Presence of Cr(VI)
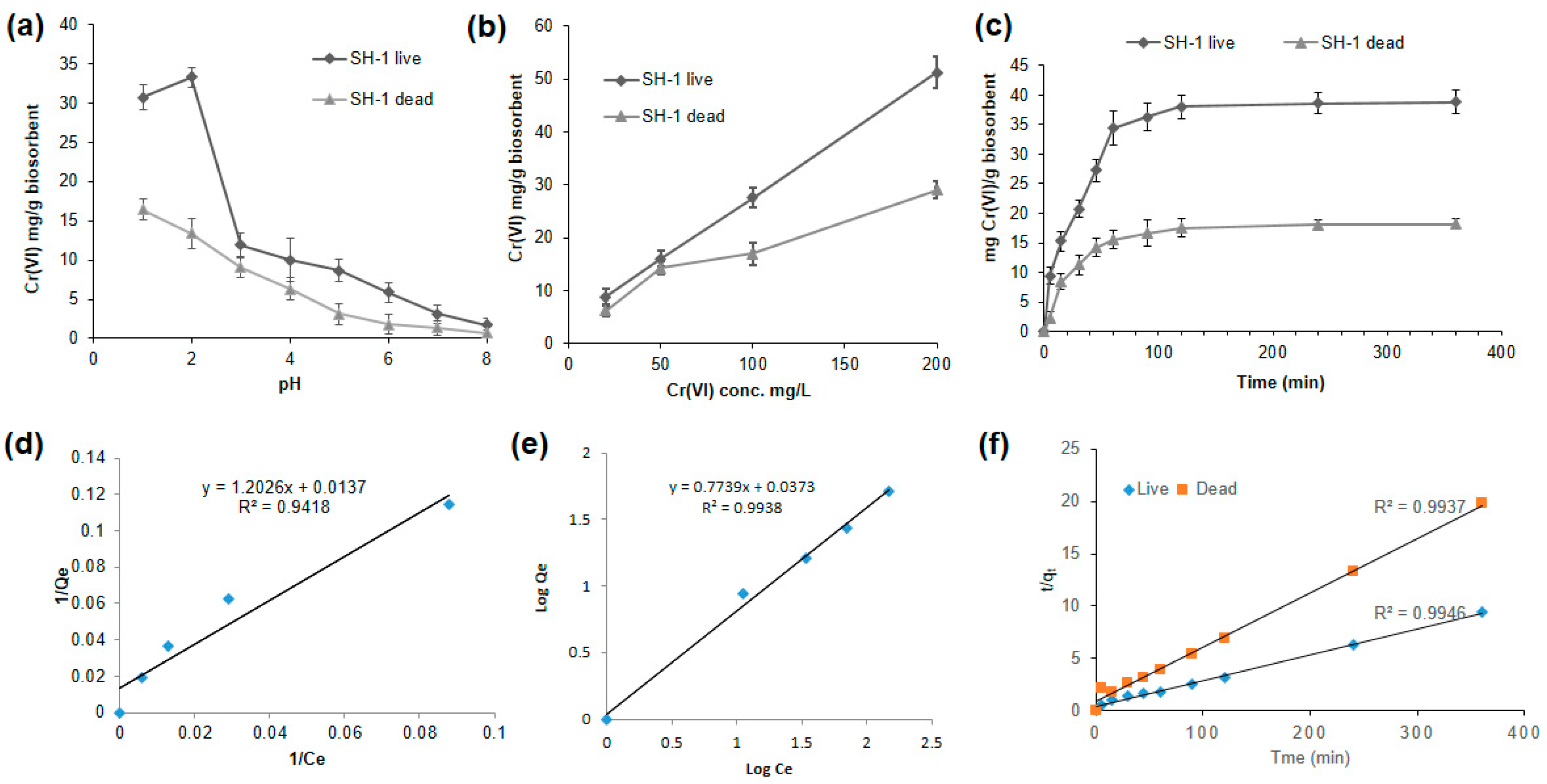
3.5. SH-1 Live Biomass Has Better Biosorption Capacity in Response to Different Conditions
3.5.1. Better Biosorption Found at Low pH
3.5.2. Biosorption Increased with the Increasing Initial Concentration of Cr(VI)
3.5.3. Effect of Time on Biosorption Process
3.5.4. Adsorption Isotherm
3.5.5. Kinetic Modeling
3.6. SEM and EDX Analysis
3.7. FTIR Analysis
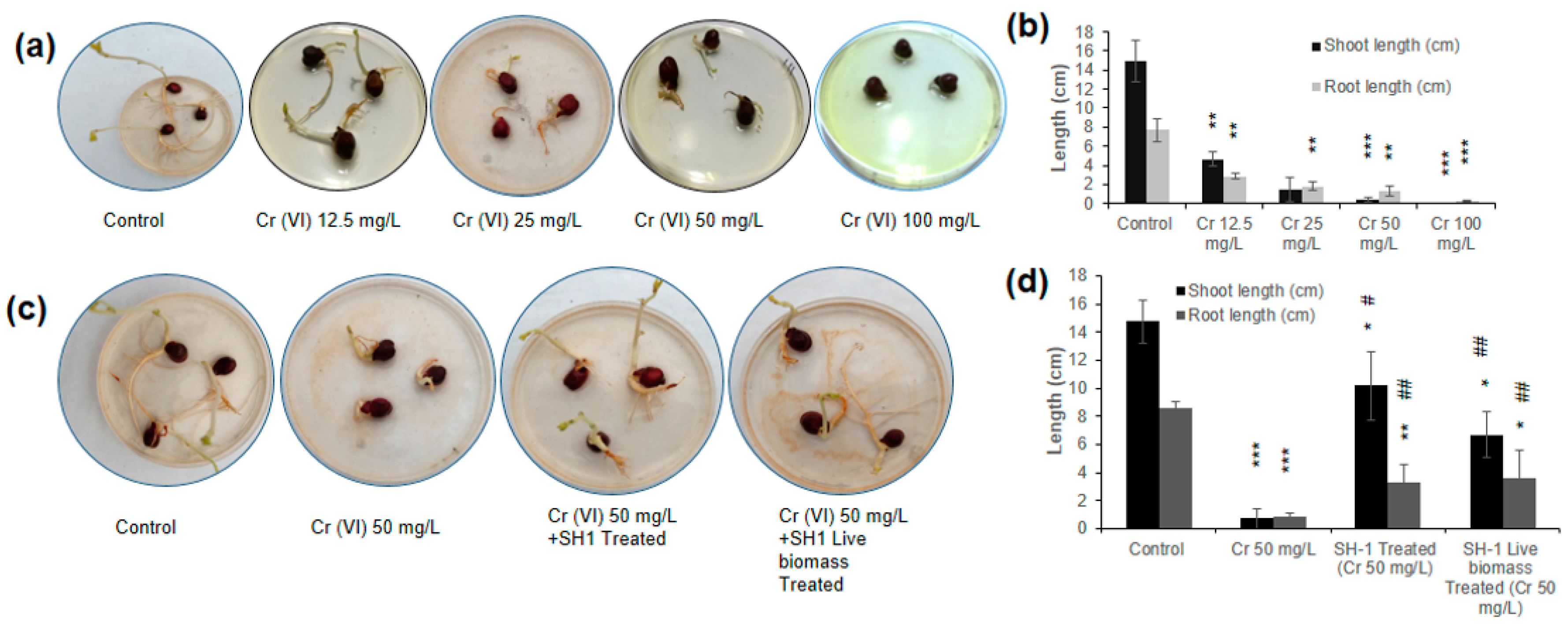
3.8. Phytotoxicity Assay Using Chickpea Seed Germination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saranraj, P.; Sujitha, D. Microbial bioremediation of chromium in tannery effluent: A review. Int. J. Microbiol. Res. 2013, 4, 305–320. [Google Scholar]
- Saha, G.C.; Ali, M.A. Groundwater contamination in Dhaka City from tannery waste. J. Civ. Eng. 2001, 29, 151–166. [Google Scholar]
- GracePavithra, K.; Jaikumar, V.; Kumar, P.S.; SundarRajan, P. A review on cleaner strategies for chromium industrial wastewater: Present research and future perspective. J. Clean. Prod. 2019, 228, 580–593. [Google Scholar] [CrossRef]
- Jobby, R.; Jha, P.; Yadav, A.K.; Desai, N. Biosorption and biotransformation of hexavalent chromium [Cr (VI)]: A comprehensive review. Chemosphere 2018, 207, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Ilias, M.; Rafiqullah, I.M.; Debnath, B.C.; Mannan, K.S.B.; Hoq, M.M. Isolation and characterization of chromium (VI)-reducing bacteria from tannery effluents. Indian J. Microbiol. 2011, 51, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Lofrano, G.; Meriç, S.; Zengin, G.E.; Orhon, D. Chemical and biological treatment technologies for leather tannery chemicals and wastewaters: A review. Sci. Total Environ. 2013, 461, 265–281. [Google Scholar] [CrossRef]
- Song, Z.; Williams, C.J.; Edyvean, R.G.J. Treatment of tannery wastewater by chemical coagulation. Desalination 2004, 164, 249–259. [Google Scholar] [CrossRef]
- Dogruel, S.; Genceli, E.A.; Babuna, F.G.; Orhon, D. An investigation on the optimal location of ozonation within biological treatment for a tannery wastewater. J. Chem. Technol. Biotechnol. Int. Res. Process. Environ. Clean Technol. 2006, 81, 1877–1885. [Google Scholar] [CrossRef]
- Islam, K.M.N.; Misbahuzzaman, K.; Majumder, A.K.; Chakrabarty, M. Efficiency of different coagulants combination for the treatment of tannery effluents: A case study of Bangladesh. Afr. J. Environ. Sci. Technol. 2011, 5, 409–419. [Google Scholar]
- Markiewicz, B.; Komorowicz, I.; Sajnóg, A.; Belter, M.; Barałkiewicz, D. Chromium and its speciation in water samples by HPLC/ICP-MS–technique establishing metrological traceability: A review since 2000. Talanta 2015, 132, 814–828. [Google Scholar] [CrossRef] [PubMed]
- Rosen, B.P. Transport and detoxification systems for transition metals, heavy metals and metalloids in eukaryotic and prokaryotic microbes. Comp. Biochem. Physiol. Part. Mol. Integr. Physiol. 2002, 133, 689–693. [Google Scholar] [CrossRef]
- Hasan, M.; Hosain, S.; Asaduzzaman, A.M.; Haque, M.A.; Roy, U.K. Prevalence of Health Diseases among Bangladeshi Tannery Workers and associated Risk factors with Workplace Investigation. J. Pollut. Eff. Control. 2016, 4, 1–3. [Google Scholar] [CrossRef]
- Franco, D.V.; Da Silva, L.M.; Jardim, W.F. Reduction of hexavalent chromium in soil and ground water using zero-valent iron under batch and semi-batch conditions. Water. Air Soil Pollut. 2009, 197, 49–60. [Google Scholar] [CrossRef]
- Ahluwalia, S.S.; Goyal, D. Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour. Technol. 2007, 98, 2243–2257. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Duan, G.; Liu, Y.; Zeng, G.; Li, X.; Liang, J.; Zhang, W. Simultaneous removal of hexavalent chromium and o-dichlorobenzene by isolated Serratia marcescens ZD-9. Biodegradation 2018, 29, 605–616. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, Y.; Lei, C.; Liu, P.; Gao, M. Chromate reduction by a chromate-resistant bacterium, Microbacterium sp. World J. Microbiol. Biotechnol. 2012, 28, 1585–1592. [Google Scholar] [CrossRef]
- Campos, A.F.C.; de Oliveira, H.A.L.; da Silva, F.N.; da Silva, F.G.; Coppola, P.; Aquino, R.; Mezzi, A.; Depeyrot, J. Core-shell bimagnetic nanoadsorbents for hexavalent chromium removal from aqueous solutions. J. Hazard. Mater. 2019, 362, 82–91. [Google Scholar] [CrossRef]
- Thacker, U.; Parikh, R.; Shouche, Y.; Madamwar, D. Reduction of chromate by cell-free extract of Brucella sp. isolated from Cr (VI) contaminated sites. Bioresour. Technol. 2007, 98, 1541–1547. [Google Scholar] [CrossRef]
- da Rocha Junior, R.B.; Meira, H.M.; Almeida, D.G.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Application of a low-cost biosurfactant in heavy metal remediation processes. Biodegradation 2018, 30, 215–233. [Google Scholar] [CrossRef]
- Zouboulis, A.I.; Loukidou, M.X.; Matis, K.A. Biosorption of toxic metals from aqueous solutions by bacteria strains isolated from metal-polluted soils. Process. Biochem. 2004, 39, 909–916. [Google Scholar] [CrossRef]
- Zahoor, A.; Rehman, A. Isolation of Cr (VI) reducing bacteria from industrial effluents and their potential use in bioremediation of chromium containing wastewater. J. Environ. Sci. 2009, 21, 814–820. [Google Scholar] [CrossRef]
- Sun, W.; Xiao, E.; Krumins, V.; Häggblom, M.M.; Dong, Y.; Pu, Z.; Li, B.; Wang, Q.; Xiao, T.; Li, F. Rhizosphere microbial response to multiple metal (loid) s in different contaminated arable soils indicates crop-specific metal-microbe interactions. Appl. Environ. Microbiol. 2018, 84, e00701-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watts, M.P.; Khijniak, T.V.; Boothman, C.; Lloyd, J.R. Treatment of alkaline Cr (VI) contaminated leachate with an alkaliphilic metal-reducing bacterium. Appl. Environ. Microbiol. 2015, 81, 5511–5518. [Google Scholar] [CrossRef] [Green Version]
- Fernández, P.M.; Viñarta, S.C.; Bernal, A.R.; Cruz, E.L.; Figueroa, L.I.C. Bioremediation strategies for chromium removal: Current research, scale-up approach and future perspectives. Chemosphere 2018, 208, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Sturm, G.; Brunner, S.; Suvorova, E.; Dempwolff, F.; Reiner, J.; Graumann, P.; Bernier-Latmani, R.; Majzlan, J.; Gescher, J. Chromate resistance mechanisms in Leucobacter chromiiresistens. Appl. Environ. Microbiol. 2018, 84, e02208-18. [Google Scholar] [CrossRef] [Green Version]
- Francisco, R.; Alpoim, M.C.; Morais, P.V. Diversity of chromium-resistant and-reducing bacteria in a chromium-contaminated activated sludge. J. Appl. Microbiol. 2002, 92, 837–843. [Google Scholar] [CrossRef] [Green Version]
- Megharaj, M.; Avudainayagam, S.; Naidu, R. Toxicity of hexavalent chromium and its reduction by bacteria isolated from soil contaminated with tannery waste. Curr. Microbiol. 2003, 47, 51–54. [Google Scholar] [CrossRef]
- Campos, V.L.; Moraga, R.; Yánez, J.; Zaror, C.A.; Mondaca, M.A. Chromate reduction by Serratia marcescens isolated from tannery effluent. Bull. Environ. Contam. Toxicol. 2005, 75, 400–406. [Google Scholar] [CrossRef]
- Elangovan, R.; Abhipsa, S.; Rohit, B.; Ligy, P.; Chandraraj, K. Reduction of Cr (VI) by a Bacillus sp. Biotechnol. Lett. 2006, 28, 247–252. [Google Scholar] [CrossRef]
- Viamajala, S.; Smith, W.A.; Sani, R.K.; Apel, W.A.; Petersen, J.N.; Neal, A.L.; Roberto, F.F.; Newby, D.T.; Peyton, B.M. Isolation and characterization of Cr (VI) reducing Cellulomonas spp. from subsurface soils: Implications for long-term chromate reduction. Bioresour. Technol. 2007, 98, 612–622. [Google Scholar] [CrossRef]
- Camargo, F.A.O.; Bento, F.M.; Okeke, B.C.; Frankenberger, W.T. Chromate reduction by chromium-resistant bacteria isolated from soils contaminated with dichromate. J. Environ. Qual. 2003, 32, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; He, M.; Wang, G. Removal of toxic chromate using free and immobilized Cr (VI)-reducing bacterial cells of Intrasporangium sp. Q5-1. World J. Microbiol. Biotechnol. 2009, 25, 1579–1587. [Google Scholar] [CrossRef]
- Bopp, L.H.; Chakrabarty, A.M.; Ehrlich, H.L. Chromate resistance plasmid in Pseudomonas fluorescens. J. Bacteriol. 1983, 155, 1105–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.-C.; Mori, T.; Komori, K.; Sasatsu, M.; Toda, K.; Ohtake, H. Isolation and characterization of an Enterobacter cloacae strain that reduces hexavalent chromium under anaerobic conditions. Appl. Environ. Microbiol. 1989, 55, 1665–1669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, H.; Wang, Y. Characterization of enzymatic reduction of hexavalent chromium by Escherichia coli ATCC 33456. Appl. Environ. Microbiol. 1993, 59, 3771–3777. [Google Scholar] [CrossRef] [Green Version]
- Nancharaiah, Y.V.; Dodge, C.; Venugopalan, V.P.; Narasimhan, S.V.; Francis, A.J. Immobilization of Cr (VI) and its reduction to Cr (III) phosphate by granular biofilms comprising a mixture of microbes. Appl. Environ. Microbiol. 2010, 76, 2433–2438. [Google Scholar] [CrossRef] [Green Version]
- Monachese, M.; Burton, J.P.; Reid, G. Bioremediation and tolerance of humans to heavy metals through microbial processes: A potential role for probiotics? Appl. Environ. Microbiol. 2012, 78, 6397–6404. [Google Scholar] [CrossRef] [Green Version]
- Apha, A. WPCF, Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1995. [Google Scholar]
- Carlos, F.S.; Giovanella, P.; Bavaresco, J.; de Souza Borges, C.; de Oliveira Camargo, F.A. A comparison of microbial bioaugmentation and biostimulation for hexavalent chromium removal from wastewater. Water Air Soil Pollut. 2016, 227, 175. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Shakoori, A.R.; Makhdoom, M.; Haq, R.U. Hexavalent chromium reduction by a dichromate-resistant gram-positive bacterium isolated from effluents of tanneries. Appl. Microbiol. Biotechnol. 2000, 53, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Camargo, F.A.O.; Okeke, B.C.; Bento, F.M.; Frankenberger, W.T. In vitro reduction of hexavalent chromium by a cell-free extract of Bacillus sp. ES 29 stimulated by Cu 2+. Appl. Microbiol. Biotechnol. 2003, 62, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, G.; Ceyhan, N.; Ozturk, T.; Akirmak, F.; Cosar, T. Biosorption of chromium (VI), cadmium (II) and copper (II) by Pantoea sp. TEM18. Chem. Eng. J. 2004, 102, 249–253. [Google Scholar] [CrossRef]
- Li, H.; Lin, Y.; Guan, W.; Chang, J.; Xu, L.; Guo, J.; Wei, G. Biosorption of Zn (II) by live and dead cells of Streptomyces ciscaucasicus strain CCNWHX 72-14. J. Hazard. Mater. 2010, 179, 151–159. [Google Scholar] [CrossRef]
- Khodaverdiloo, H.; Samadi, A. Batch equilibrium study on sorption, desorption, and immobilisation of cadmium in some semi-arid zone soils as affected by soil properties. Soil Res. 2011, 49, 444–454. [Google Scholar] [CrossRef]
- Vanderborght, B.M.; Van Grieken, R.E. Enrichment of trace metals in water by adsorption on activated carbon. Anal. Chem. 1977, 49, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Dada, A.O.; Olalekan, A.P.; Olatunya, A.M.; Dada, O. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR J. Appl. Chem. 2012, 3, 38–45. [Google Scholar]
- Hokkanen, S.; Bhatnagar, A.; Repo, E.; Lou, S.; Sillanpää, M. Calcium hydroxyapatite microfibrillated cellulose composite as a potential adsorbent for the removal of Cr (VI) from aqueous solution. Chem. Eng. J. 2016, 283, 445–452. [Google Scholar] [CrossRef]
- Mohan, S.V.; Karthikeyan, J. Removal of lignin and tannin colour from aqueous solution by adsorption onto activated charcoal. Environ. Pollut. 1997, 97, 183–187. [Google Scholar] [CrossRef]
- Goldberg, S. Equations and models describing adsorption processes in soils. Chem. Process. Soils 2005, 489–517. [Google Scholar]
- Voudrias, E.; Fytianos, K.; Bozani, E. Sorption–desorption isotherms of dyes from aqueous solutions and wastewaters with different sorbent materials. Glob. Nest Int. J. 2002, 4, 75–83. [Google Scholar]
- Febrianto, J.; Kosasih, A.N.; Sunarso, J.; Ju, Y.-H.; Indraswati, N.; Ismadji, S. Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: A summary of recent studies. J. Hazard. Mater. 2009, 162, 616–645. [Google Scholar] [CrossRef] [PubMed]
- Demiral, H.; Demiral, I.; Tümsek, F.; Karabacakoğlu, B. Adsorption of chromium (VI) from aqueous solution by activated carbon derived from olive bagasse and applicability of different adsorption models. Chem. Eng. J. 2008, 144, 188–196. [Google Scholar] [CrossRef]
- François, F.; Lombard, C.; Guigner, J.-M.; Soreau, P.; Brian-Jaisson, F.; Martino, G.; Vandervennet, M.; Garcia, D.; Molinier, A.-L.; Pignol, D. Isolation and characterization of environmental bacteria capable of extracellular biosorption of mercury. Appl. Environ. Microbiol. 2012, 78, 1097–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Adholeya, A. Hexavalent chromium reduction in tannery effluent by bacterial species isolated from tannery effluent contaminated soil. J. Environ. Sci. Technol. 2012, 5, 142–154. [Google Scholar] [CrossRef] [Green Version]
- Abou-Shanab, R.A.I.; Van Berkum, P.; Angle, J.S. Heavy metal resistance and genotypic analysis of metal resistance genes in gram-positive and gram-negative bacteria present in Ni-rich serpentine soil and in the rhizosphere of Alyssum murale. Chemosphere 2007, 68, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-T.; Xiao, C. Factors affecting hexavalent chromium reduction in pure cultures of bacteria. Water Res. 1995, 29, 2467–2474. [Google Scholar] [CrossRef]
- Ozdemir, G.; Ozturk, T.; Ceyhan, N.; Isler, R.; Cosar, T. Heavy metal biosorption by biomass of Ochrobactrum anthropi producing exopolysaccharide in activated sludge. Bioresour. Technol. 2003, 90, 71–74. [Google Scholar] [CrossRef]
- Gupta, V.K.; Shrivastava, A.K.; Jain, N. Biosorption of chromium (VI) from aqueous solutions by green algae Spirogyra species. Water Res. 2001, 35, 4079–4085. [Google Scholar] [CrossRef]
- Dadrasnia, A.; Chuan Wei, K.S.; Shahsavari, N.; Azirun, M.S.; Ismail, S. Biosorption potential of Bacillus salmalaya strain 139SI for removal of Cr (VI) from aqueous solution. Int. J. Environ. Res. Public Health 2015, 12, 15321–15338. [Google Scholar] [CrossRef]
- Saha, B.; Orvig, C. Biosorbents for hexavalent chromium elimination from industrial and municipal effluents. Coord. Chem. Rev. 2010, 254, 2959–2972. [Google Scholar] [CrossRef]
- Oves, M.; Khan, M.S.; Zaidi, A. Biosorption of heavy metals by Bacillus thuringiensis strain OSM29 originating from industrial effluent contaminated north Indian soil. Saudi J. Biol. Sci. 2013, 20, 121–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kratochvil, D.; Pimentel, P.; Volesky, B. Removal of trivalent and hexavalent chromium by seaweed biosorbent. Environ. Sci. Technol. 1998, 32, 2693–2698. [Google Scholar] [CrossRef]
- Park, D.; Yun, Y.-S.; Kim, J.Y.; Park, J.M. How to study Cr (VI) biosorption: Use of fermentation waste for detoxifying Cr (VI) in aqueous solution. Chem. Eng. J. 2008, 136, 173–179. [Google Scholar] [CrossRef]
- Wierzba, S. Biosorption of copper (II) by live and dead cells of Yarrowia lipolytica. Ecol. Chem. Eng. A 2013, 20, 875–883. [Google Scholar]
- Kumar, P.S.; Kirthika, K. Equilibrium and kinetic study of adsorption of nickel from aqueous solution onto bael tree leaf powder. J. Eng. Sci. Technol. 2009, 4, 351–363. [Google Scholar]
- van Niftrik, L.; Geerts, W.J.C.; van Donselaar, E.G.; Humbel, B.M.; Yakushevska, A.; Verkleij, A.J.; Jetten, M.S.M.; Strous, M. Combined structural and chemical analysis of the anammoxosome: A membrane-bounded intracytoplasmic compartment in anammox bacteria. J. Struct. Biol. 2008, 161, 401–410. [Google Scholar] [CrossRef]
- Mungasavalli, D.P.; Viraraghavan, T.; Jin, Y.-C. Biosorption of chromium from aqueous solutions by pretreated Aspergillus niger: Batch and column studies. Colloids Surf. Physicochem. Eng. Asp. 2007, 301, 214–223. [Google Scholar] [CrossRef]
- Bharagava, R.N.; Mishra, S. Hexavalent chromium reduction potential of Cellulosimicrobium sp. isolated from common effluent treatment plant of tannery industries. Ecotoxicol. Environ. Saf. 2018, 147, 102–109. [Google Scholar] [CrossRef]
- Yee, N.; Benning, L.G.; Phoenix, V.R.; Ferris, F.G. Characterization of metal− cyanobacteria sorption reactions: A combined macroscopic and infrared spectroscopic investigation. Environ. Sci. Technol. 2004, 38, 775–782. [Google Scholar] [CrossRef]
- Şahin, Y.; Öztürk, A. Biosorption of chromium (VI) ions from aqueous solution by the bacterium Bacillus thuringiensis. Process. Biochem. 2005, 40, 1895–1901. [Google Scholar] [CrossRef]
- Ali, N.A.; Ater, M.; Sunahara, G.I.; Robidoux, P.Y. Phytotoxicity and bioaccumulation of copper and chromium using barley (Hordeum vulgare L.) in spiked artificial and natural forest soils. Ecotoxicol. Environ. Saf. 2004, 57, 363–374. [Google Scholar] [CrossRef]
- Kumar, V.; Dwivedi, S.K. Hexavalent chromium stress response, reduction capability and bioremediation potential of Trichoderma sp. isolated from electroplating wastewater. Ecotoxicol. Environ. Saf. 2019, 185, 109734. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Dwivedi, S.K. Hexavalent chromium reduction ability and bioremediation potential of Aspergillus flavus CR500 isolated from electroplating wastewater. Chemosphere 2019, 237, 124567. [Google Scholar] [CrossRef] [PubMed]


| Protein Sample | Growth Condition | |||
|---|---|---|---|---|
| Medium Minus Cr (VI) | Medium Plus 10 mg/L Cr (VI) | |||
| % Reduction a | Reductase Activity, U/mg Protein b | % Reduction a | Reductase Activity, U/mg Protein b | |
| SH-1 | 52 ± 1.52 | 0.098 ± 0.005 | 72.2 ± 0.93 | 0.124 ± 0. 01 |
| V. cholerae | 3.5 ± 0.8 | 0.009 ± 0.0005 | 8 ± 0.42 | 0.022 ± 0.004 |
| Langmuir Isotherm | Freundlich Isotherm | ||||||
|---|---|---|---|---|---|---|---|
| Qo (mg/g) | KL (L/mg) | RL | R2 | 1/n | n | Kf (mg1 −1/n g −1 L1/n) | R2 |
| 72.99 | 0.01 | 0.23 | 0.94 | 0.77 | 1.30 | 1.08 | 0.99 |
| Element | Mass (%) | Atom (%) |
|---|---|---|
| C K | 57.59 | 75.87 |
| O K | 18.87 | 18.66 |
| S K | 2.01 | 0.99 |
| Cl K | 3.80 | 1.70 |
| Cr K | 6.02 | 1.83 |
| Pt M | 11.72 | 0.95 |
| Total | 100.00 | 100.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossan, S.; Hossain, S.; Islam, M.R.; Kabir, M.H.; Ali, S.; Islam, M.S.; Imran, K.M.; Moniruzzaman, M.; Mou, T.J.; Parvez, A.K.; et al. Bioremediation of Hexavalent Chromium by Chromium Resistant Bacteria Reduces Phytotoxicity. Int. J. Environ. Res. Public Health 2020, 17, 6013. https://doi.org/10.3390/ijerph17176013
Hossan S, Hossain S, Islam MR, Kabir MH, Ali S, Islam MS, Imran KM, Moniruzzaman M, Mou TJ, Parvez AK, et al. Bioremediation of Hexavalent Chromium by Chromium Resistant Bacteria Reduces Phytotoxicity. International Journal of Environmental Research and Public Health. 2020; 17(17):6013. https://doi.org/10.3390/ijerph17176013
Chicago/Turabian StyleHossan, Shanewaz, Saddam Hossain, Mohammad Rafiqul Islam, Mir Himayet Kabir, Sobur Ali, Md Shafiqul Islam, Khan Mohammad Imran, M. Moniruzzaman, Taslin Jahan Mou, Anowar Khasru Parvez, and et al. 2020. "Bioremediation of Hexavalent Chromium by Chromium Resistant Bacteria Reduces Phytotoxicity" International Journal of Environmental Research and Public Health 17, no. 17: 6013. https://doi.org/10.3390/ijerph17176013
APA StyleHossan, S., Hossain, S., Islam, M. R., Kabir, M. H., Ali, S., Islam, M. S., Imran, K. M., Moniruzzaman, M., Mou, T. J., Parvez, A. K., & Mahmud, Z. H. (2020). Bioremediation of Hexavalent Chromium by Chromium Resistant Bacteria Reduces Phytotoxicity. International Journal of Environmental Research and Public Health, 17(17), 6013. https://doi.org/10.3390/ijerph17176013