Exposure to ZnO/TiO2 Nanoparticles Affects Health Outcomes in Cosmetics Salesclerks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sunscreen Selection and Analysis
2.2. Sample Preparation for sp-ICP-MS Analysis
2.3. sp-ICP-MS Method
2.4. Recruiting Participants
2.5. Sample Collection
2.6. Daily Exposure Dose and Cumulative Risk Calculation
2.7. Determining 8-Hydroxy-2′-Deoxyguanosine (8-OHdG)
2.8. Statistical Analysis
3. Results
3.1. Size and Concentration of TiO2 and ZnO NPs in Sunscreens
3.2. Study Population
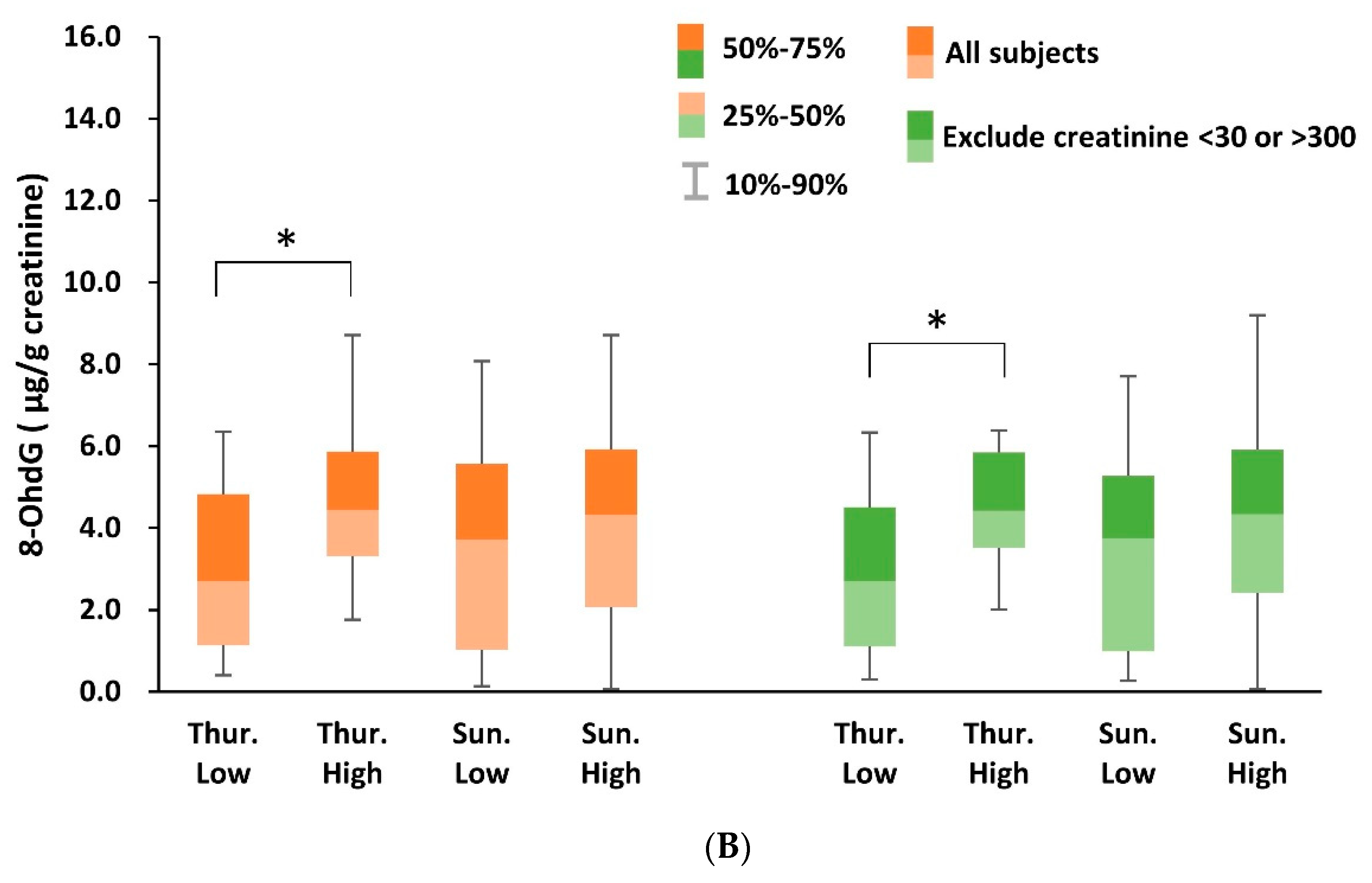
3.3. Urinary 8-OHdG Analysis in Nanocosmetics Salesclerks
4. Discussion
4.1. Findings
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NPs | nanoparticles |
| TiO2 | titanium dioxide |
| ZnO | zinc oxide |
| 8-OHdG | 8-hydroxy-2′-deoxyguanosin |
| UV | ultraviolet |
| sp-ICP-MS | single-particle inductively coupled plasma-mass spectrometry |
| ELISA | enzyme-linked immunosorbent assay |
References
- Thomas, T.; Thomas, K.; Sadrieh, N.; Savage, N.; Adair, P.; Bronaugh, R. Research strategies for safety evaluation of nanomaterials, part VII: Evaluating consumer exposure to nanoscale materials. Toxicol. Sci. 2006, 91, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Aro, H.; Dahms, G. Compatibility of micro-fine titanium dioxide with organic UV filters. Cosmet. Toilet. Manuf. Worldw. 2004, 115–118. [Google Scholar]
- Tyner, K.M.; Wokovich, A.M.; Godar, D.E.; Doub, W.H.; Sadrieh, N. The state of nano-sized titanium dioxide (TiO2) may affect sunscreen performance. Int. J. Cosmet. Sci. 2011, 33, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Wiesenthal, A.; Hunter, L.; Wang, S.; Wickliffe, J.; Wilkerson, M. Nanoparticles: Small and mighty. Int. J. Dermatol. 2011, 50, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique cellular interaction of silver nanoparticles: Size-dependent generation. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, M.; Jabeen, F.; Shabbir, S.; Asghar, M.S.; Khan, M.S.; Chaudhry, A.S. Toxicity of Nano-Titanium Dioxide (TiO2-NP) Through Various Routes of Exposure: A Review. Biol. Trace Elem. Res. 2016, 172, 1–36. [Google Scholar] [CrossRef]
- Trouiller, B.; Reliene, R.; Westbrook, A.; Solaimani, P.; Schiestl, R.H. Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res. 2009, 69, 8784–8789. [Google Scholar] [CrossRef] [Green Version]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nano level. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Xia, T.; Nel, A.E. The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic. Biol. Med. 2008, 44, 1689–1699. [Google Scholar] [CrossRef] [Green Version]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Barregard, L.; Moller, P.; Henriksen, T.; Mistry, V.; Koppen, G.; Rossner, P., Jr.; Sram, R.J.; Weimann, A.; Poulsen, H.E.; Nataf, R.; et al. Human and methodological sources of variability in the measurement of urinary 8-oxo-7,8-dihydro-2’-deoxyguanosine. Antioxid. Redox Signal. 2013, 18, 2377–2391. [Google Scholar] [CrossRef] [PubMed]
- Zanolin, M.E.; Girardi, P.; Degan, P.; Rava, M.; Olivieri, M.; Di Gennaro, G.; Nicolis, M.; De Marco, R. Measurement of a urinary marker (8-hydroxydeoxy-guanosine, 8-ohdg) of DNA oxidative stress in epidemiological surveys: A pilot study. Int. J. Biol. Markers 2015, 30, e341–e345. [Google Scholar] [CrossRef] [PubMed]
- Hopf, N.B.; Bourgkard, E.; Demange, V.; Hulo, S.; Sauvain, J.J.; Levilly, R.; Jeandel, F.; Robert, A.; Guichard, Y.; Pralong, J.A.; et al. Early effect markers and exposure determinants of metalworking fluids among metal industry workers: Protocol for a field study. JMIR Res. Protoc. 2019, 8, e13744. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, E.; Poland, C.; Guseva Canu, I.; Prina-Mello, A. The role of biological monitoring in nano-safety. Nano Today 2015, 10, 274–277. [Google Scholar] [CrossRef]
- Dunford, R.; Salinaro, A.; Cai, L.; Serpone, N.; Horikoshi, S.; Hidaka, H.; Knowland, J. Chemical oxidation and DNA damage catalysed by inorganic sunscreen ingredients. FEBS Lett. 1997, 418, 87–90. [Google Scholar] [CrossRef] [Green Version]
- Hidaka, H.; Horikoshi, S.; Serpone, N.; Knowland, J. In vitro photochemical damage to DNA, RNA and their bases by an inorganic sunscreen agent on exposure to UVA and UVB radiation. J. Photochem. Photobiol. A Chem. 1997, 111, 205–213. [Google Scholar] [CrossRef]
- Oberdorster, G.; Oberdorster, E.; Oberdorster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Friends of the Earth. Nanomaterials, Sunscreens, and Cosmetics: Small Ingredients, Big Risks. 2006. Available online: http://emergingtech.foe.org.au/wp-content/uploads/2014/06/nano-cosmetics-report-2MB.pdf (accessed on 1 September 2019).
- Jansen, R.; Osterwalder, U.; Wang, S.Q.; Burnett, M.; Lim, H.W. Photoprotection: Part II. Sunscreen: Development, efficacy, and controversies. J. Am. Acad. Dermatol. 2013, 69, 867.e1–867.e14. [Google Scholar] [CrossRef]
- Gulson, B.; McCall, M.; Korsch, M.; Gomez, L.; Casey, P.; Oytam, Y.; Taylor, A.; McCulloch, M.; Trotter, J.; Kinsley, L.; et al. Small amounts of zinc from zinc oxide particles in sunscreens applied outdoors are absorbed through human skin. Toxicol. Sci. 2010, 118, 140–149. [Google Scholar] [CrossRef] [Green Version]
- James, S.A.; Feltis, B.N.; de Jonge, M.D.; Sridhar, M.; Kimpton, J.A.; Altissimo, M.; Mayo, S.; Zheng, C.; Hastings, A.; Howard, D.L.; et al. Quantification of ZnO nanoparticle uptake, distribution, and dissolution within individual human macrophages. ACS Nano 2013, 7, 10621–10635. [Google Scholar] [CrossRef]
- Tran, D.T.; Salmon, R. Potential photocarcinogenic effects of nanoparticle sunscreens. Australas. J. Dermatol. 2011, 52, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pace, H.E.; Rogers, N.J.; Jarolimek, C.; Coleman, V.A.; Higgins, C.P.; Ranville, J.F. Determining transport efficiency for the purpose of counting and sizing nanoparticles via single particle inductively coupled plasma mass spectrometry. Anal. Chem. 2011, 83, 9361–9369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DOH. Compilation of Exposure Factors; Department of Health, Executive Yuan: Taipei, Taiwan, 2008.
- Smijs, T.G.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, P.J.; Huang, S.C.; Chen, Y.P.; Chiueh, L.C.; Shih, D.Y. Analysis of titanium dioxide and zinc oxide nanoparticles in cosmetics. J. Food Drug Anal. 2015, 23, 587–594. [Google Scholar] [CrossRef] [Green Version]
- Wokovich, A.; Tyner, K.; Doub, W.; Sadrieh, N.; Buhse, L.F. Particle size determination of sunscreens formulated with various forms of titanium dioxide. Drug Dev. Ind. Pharm. 2009, 35, 1180–1189. [Google Scholar] [CrossRef]
- Graille, M.; Wild, P.; Sauvain, J.J.; Hemmendinger, M.; Canu, I.G.; Hopf, N. Urinary 8-OHdG as a Biomarker for Oxidative Stress: A Systematic Literature Review and Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 3743. [Google Scholar] [CrossRef]
- Jacob, K.D.; Noren Hooten, N.; Trzeciak, A.R.; Evans, M.K. Markers of oxidant stress that are clinically relevant in aging and age-related disease. Mech. Ageing Dev. 2013, 134, 139–157. [Google Scholar] [CrossRef] [Green Version]
- Mizoue, T.; Kasai, H.; Kubo, T.; Tokunaga, S. Leanness, smoking, and enhanced oxidative DNA damage. Cancer Epidemiol. Biomark. Prev. 2006, 15, 582–585. [Google Scholar] [CrossRef] [Green Version]
- Loft, S.; Vistisen, K.; Ewertz, M.; Tjonneland, A.; Overvad, K.; Poulsen, H.E. Oxidative DNA damage estimated by 8-hydroxydeoxyguanosine excretion in humans: Influence of smoking, gender and body mass index. Carcinogenesis 1992, 13, 2241–2247. [Google Scholar] [CrossRef]
- Kasai, H.; Iwamoto-Tanaka, N.; Miyamoto, T.; Kawanami, K.; Kawanami, S.; Kido, R.; Ikeda, M. Life style and urinary 8-hydroxydeoxyguanosine, a marker of oxidative DNA damage: Effects of exercise, working conditions, meat intake, body mass index, and smoking. Jpn. J. Cancer Res. 2001, 92, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Godschalk, R.W.; Feldker, D.E.; Borm, P.J.; Wouters, E.F.; van Schooten, F.J. Body mass index modulates aromatic DNA adduct levels and their persistence in smokers. Cancer Epidemiol. Biomark. Prev. 2002, 11, 790–793. [Google Scholar]
- Martorano, L.M.; Stork, C.J.; Li, Y.V. UV irradiation-induced zinc dissociation from commercial zinc oxide sunscreen and its action in human epidermal keratinocytes. J. Cosmet. Dermatol. 2010, 9, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Setyawati, M.I.; Tay, C.Y.; Leong, D.T. Mechanistic Investigation of the Biological Effects of SiO2, TiO2, and ZnO Nanoparticles on Intestinal Cells. Small 2015, 11, 3458–3468. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, I.; Barone, F.; Zijno, A.; Bizzarri, L.; Russo, M.T.; Pozzi, R.; Franchini, F.; Giudetti, G.; Uboldi, C.; Ponti, J.; et al. Comparative study of ZnO and TiO2 nanoparticles: Physicochemical characterisation and toxicological effects on human colon carcinoma cells. Nanotoxicology 2013, 7, 1361–1372. [Google Scholar] [CrossRef]
- Yu, K.N.; Chang, S.H.; Park, S.J.; Lim, J.; Lee, J.; Yoon, T.J.; Kim, J.S.; Cho, M.H. Titanium Dioxide Nanoparticles Induce Endoplasmic Reticulum Stress-Mediated Autophagic Cell Death via Mitochondria-Associated Endoplasmic Reticulum Membrane Disruption in Normal Lung Cells. PLoS ONE 2015, 10, e0131208. [Google Scholar] [CrossRef] [Green Version]
- Crosera, M.; Prodi, A.; Mauro, M.; Pelin, M.; Florio, C.; Bellomo, F.; Adami, G.; Apostoli, P.; De Palma, G.; Bovenzi, M.; et al. Titanium Dioxide Nanoparticle Penetration into the Skin and Effects on HaCaT Cells. Int. J. Environ. Res. Public Health 2015, 12, 9282–9297. [Google Scholar] [CrossRef]
- Rancan, F.; Nazemi, B.; Rautenberg, S.; Ryll, M.; Hadam, S.; Gao, Q.; Hackbarth, S.; Haag, S.F.; Graf, C.; Ruhl, E.; et al. Ultraviolet radiation and nanoparticle induced intracellular free radicals generation measured in human keratinocytes by electron paramagnetic resonance spectroscopy. Skin Res. Technol. 2014, 20, 182–193. [Google Scholar] [CrossRef]
- Gui, S.; Li, B.; Zhao, X.; Sheng, L.; Hong, J.; Yu, X.; Sang, X.; Sun, Q.; Ze, Y.; Wang, L.; et al. Renal injury and Nrf2 modulation in mouse kidney following chronic exposure to TiO2 nanoparticles. J. Agric. Food Chem. 2013, 61, 8959–8968. [Google Scholar] [CrossRef]
- Shukla, R.K.; Kumar, A.; Vallabani, N.V.; Pandey, A.K.; Dhawan, A. Titanium dioxide nanoparticle-induced oxidative stress triggers DNA damage and hepatic injury in mice. Nanomedicine 2014, 9, 1423–1434. [Google Scholar] [CrossRef] [Green Version]
- Sobek, A.; Bejgarn, S.; Ruden, C.; Molander, L.; Breitholtz, M. In the shadow of the Cosmetic Directive—Inconsistencies in EU environmental hazard classification requirements for UV-filters. Sci. Total Environ. 2013, 461, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Minno, A.; Turnu, L.; Porro, B.; Squellerio, I.; Cavalca, V.; Tremoli, E.; Di Minno, M.N. 8-hydroxy-2-deoxyguanosine levels and cardiovascular disease: A systematic review and meta-analysis of the literature. Antioxid. Redox Signal. 2016, 24, 548–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neofytou, E.; Tzortzaki, E.G.; Chatziantoniou, A.; Siafakas, N.M. DNA damage due to oxidative stress in chronic obstructive pulmonary disease (copd). Int. J. Mol. Sci. 2012, 13, 16853–16864. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Sample ID | SPF | Geo-Mean Size (nm) | TiO2 # Concentration (#/g Product) | Mode Size (nm) | Fraction of Nanosized TiO2 1 (%) | TiO2 Weight Percentage 2 (%) | TiO2 Labeling |
|---|---|---|---|---|---|---|---|
| #001 | 50+ | 98.8 | 6.04 × 109 | 55.6 | 64 | 0.01 | No |
| #002 | 30 | 85.2 | 1.13 × 1013 | 62.3 | 77 | 3.17 | Yes |
| #003 | 40 | 75.1 | 2.00 × 1013 | 60.3 | 91 | 2.40 | Yes |
| #004 | 0 | 268.3 | 4.91 × 1012 | 111.0 | 0 | 30.07 | Yes |
| #005 | 50+ | 91.8 | 1.50 × 1012 | 61.3 | 66 | 0.62 | Yes |
| #006 | 15 | 72.8 | 1.02 × 1012 | 53.7 | 86 | 0.16 | Yes (1.23%) |
| #007 | 50+ | 71.8 | 1.25 × 1011 | 52.7 | 84 | 0.03 | No |
| #008 | 50 | 74.6 | 7.86 × 1011 | 58.0 | 85 | 0.10 | Yes |
| #009 | 50 | 75.6 | 1.06 × 1011 | 52.8 | 80 | 0.03 | Yes |
| #010 | 19 | 71.4 | 2.23 × 1012 | 54.0 | 89 | 0.55 | Yes |
| #011 | 50+ | 86.9 | 2.15 × 1011 | 65.6 | 78 | 0.06 | Yes |
| #012 | 50 | 81.8 | 7.00 × 1012 | 66.0 | 85 | 1.23 | Yes |
| #013 | 50+ | 101.5 | 1.83 × 107 | 66.9 | 65 | 0.00 | No |
| #014 | 50 | 79.6 | 7.34 × 1012 | 63.2 | 85 | 1.23 | Yes |
| #015 | 15 | 107.8 | 2.54 × 1012 | 63.7 | 57 | 2.84 | Yes |
| #016 | 50 | 94.1 | 8.39 × 1012 | 56.6 | 61 | 3.06 | Yes |
| #017 | 24 | 82.2 | 1.76 × 1012 | 62.6 | 80 | 0.37 | Yes |
| #018 | 50+ | 106.4 | 3.60 × 1010 | 64.1 | 54 | 0.03 | No |
| #019 | 20 | 74.3 | 5.09 × 1012 | 56.1 | 87 | 1.21 | Yes |
| #020 | 25 | 112.0 | 1.14 × 1012 | 68.0 | 50 | 1.24 | Yes |
| Sample ID | SPF | Geo-Mean Size (Nm) | Zno # Concentration (#/G Product) | Mode Size (Nm) | Fraction of Nanosized Zno 1 (%) | Zno Weight Percentage 2 (%) | Zno Labelled Labeling |
|---|---|---|---|---|---|---|---|
| #001 | 50+ | 57.7 | 3.66 × 107 | 51.6 | 95 | 0.00 | No |
| #002 | 30 | 102.3 | 4.51 × 1011 | 83.0 | 53 | 0.22 | No |
| #003 | 40 | 66.2 | 2.22 × 107 | 67.1 | 94 | 0.00 | No |
| #004 | 0 | 61.7 | 1.64 × 108 | 62.4 | 100 | 0.00 | No |
| #005 | 50+ | 98.2 | 2.36 × 1012 | 72.1 | 30 | 1.72 | Yes (12.53%) |
| #006 | 15 | 69.7 | 4.29 × 107 | 59.4 | 88 | 0.00 | No |
| #007 | 50+ | 136.0 | 9.19 × 1012 | 91.5 | 21 | 9.14 | Yes (9.45%) |
| #008 | 50 | 55.6 | 1.72 × 108 | 48.5 | 98 | 0.00 | No |
| #009 | 50 | 67.0 | 1.37 × 107 | 50.2 | 89 | 0.00 | No |
| #010 | 19 | 65.2 | 5.42 × 107 | 55.3 | 93 | 0.00 | No |
| #011 | 50+ | 101.0 | 6.16 × 1011 | 96.1 | 39 | 0.37 | Yes (7.36%) |
| #012 | 50 | 99.1 | 1.27 × 107 | 80.5 | 60 | 0.00 | No |
| #013 | 50+ | 144.4 | 6.98 × 1011 | 103.3 | 16 | 1.22 | Yes (9.59%) |
| #014 | 50 | 92.2 | 1.68 × 108 | 59.0 | 69 | 0.00 | No |
| #015 | 15 | 67.7 | 1.51 × 109 | 57.3 | 97 | 0.00 | No |
| #016 | 50 | 88.7 | 1.51 × 108 | 73.4 | 79 | 0.00 | No |
| #017 | 24 | 96.0 | 1.48 × 108 | 78.9 | 74 | 0.00 | No |
| #018 | 50+ | 91.6 | 3.02 × 106 | 60.2 | 62 | 0.00 | No |
| #019 | 20 | 77.7 | 1.32 × 108 | 72.4 | 86 | 0.00 | No |
| #020 | 25 | 106.3 | 4.34 × 1012 | 76.4 | 42 | 2.47 | No |
| Cosmetics Salesclerks (n = 40) | Clothing Salesclerks (n = 24) | pa,b | |
|---|---|---|---|
| Age (years) c | 27.3 (20–47) | 42.2 (23–54) | <0.001 |
| Weight (kg) d | 54.4 (6.56) | 60.6 (10.1) | 0.004 |
| Height (cm) d | 161.8 (5.27) | 161.5 (6.62) | 0.812 |
| BMI | 20.7 (2.0) | 23.2 (3.28) | <0.001 |
| Marital status e | |||
| Unmarried/Divorced | 29 (72.5) | 11 (45.9) | 0.033 |
| Married | 11 (27.5) | 13 (54.2) | |
| Educational level | |||
| High school | 15 (37.5) | 17 (70.9) | 0.069 |
| Tech school | 4 (10.0) | 2 (8.3) | |
| Bachelor’s degree | 21 (52.5) | 5 (20.8) | |
| Monthly income (NT$) | |||
| <24,999 | 11 (27.5) | 9 (37.5) | 0.048 |
| 25,000~29,999 | 5 (12.5) | 9 (37.5) | |
| 30,000~34,999 | 15 (37.5) | 5 (20.8) | |
| 35,000~39,999 | 5 (12.5) | 1 (4.2) | |
| 40,000~44,999 | 4 (10.0) | 0 (0.0) | |
| Seniority (years) | 4.72 (5.70) | 11.9 (7.70) | <0.001 |
| Working time (day/month) | 20.5 (2.80) | 21.3 (2.63) | 0.262 |
| Weekday working time (h/day) | 8.59 (0.53) | 7.96 (2.13) | 0.078 |
| Weekend working time (h/day) | 8.70 (0.71) | 8.52 (2.03) | 0.611 |
| Exposed to Tobacco Smoke Exposure | |||
| Active | 7 (17.5) | 2 (8.3) | 0.264 |
| passive | 13 (32.5) | 10 (41.7) | 0.317 |
| Alcohol drinker f | 5 (12.5) | 0 (0.0) | 0.086 |
| Tea drinker f | 26 (65.0) | 13 (54.2) | 0.275 |
| Coffee drinker f | 15 (37.5) | 14 (58.3) | 0.087 |
| Exposure Index b | ZnO NPs | TiO2 NPs | ZnO and TiO2 NPs |
|---|---|---|---|
| Urinary 8-OHdG (ng/mL) c | β = −0.047 (−0.272, 0.177) | β = 0.383 ** (0.176, 0.589) | β = 0.308 ** (0.106, 0.510) |
| Urinary 8-OHdG (μg/g creatinine) c | β = −0.087 (−0.600, 0.426) | β = 0.649 ** (0.167, 1.131) | β = 0.486 * (0.017, 0.954) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-C.; Lin, Y.-H.; Hou, W.-C.; Li, M.-H.; Chang, J.-W. Exposure to ZnO/TiO2 Nanoparticles Affects Health Outcomes in Cosmetics Salesclerks. Int. J. Environ. Res. Public Health 2020, 17, 6088. https://doi.org/10.3390/ijerph17176088
Lee C-C, Lin Y-H, Hou W-C, Li M-H, Chang J-W. Exposure to ZnO/TiO2 Nanoparticles Affects Health Outcomes in Cosmetics Salesclerks. International Journal of Environmental Research and Public Health. 2020; 17(17):6088. https://doi.org/10.3390/ijerph17176088
Chicago/Turabian StyleLee, Ching-Chang, Yi-Hsin Lin, Wen-Che Hou, Meng-Han Li, and Jung-Wei Chang. 2020. "Exposure to ZnO/TiO2 Nanoparticles Affects Health Outcomes in Cosmetics Salesclerks" International Journal of Environmental Research and Public Health 17, no. 17: 6088. https://doi.org/10.3390/ijerph17176088
APA StyleLee, C.-C., Lin, Y.-H., Hou, W.-C., Li, M.-H., & Chang, J.-W. (2020). Exposure to ZnO/TiO2 Nanoparticles Affects Health Outcomes in Cosmetics Salesclerks. International Journal of Environmental Research and Public Health, 17(17), 6088. https://doi.org/10.3390/ijerph17176088






