Predictors of Urinary Pyrethroid and Organophosphate Compound Concentrations among Healthy Pregnant Women in New York
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethical Statement
2.3. Urinary Concentrations of Organophosphate and Pyrethroid Pesticide Metabolites
2.4. Assessment of Predictor Variables
2.5. Statistical Analysis
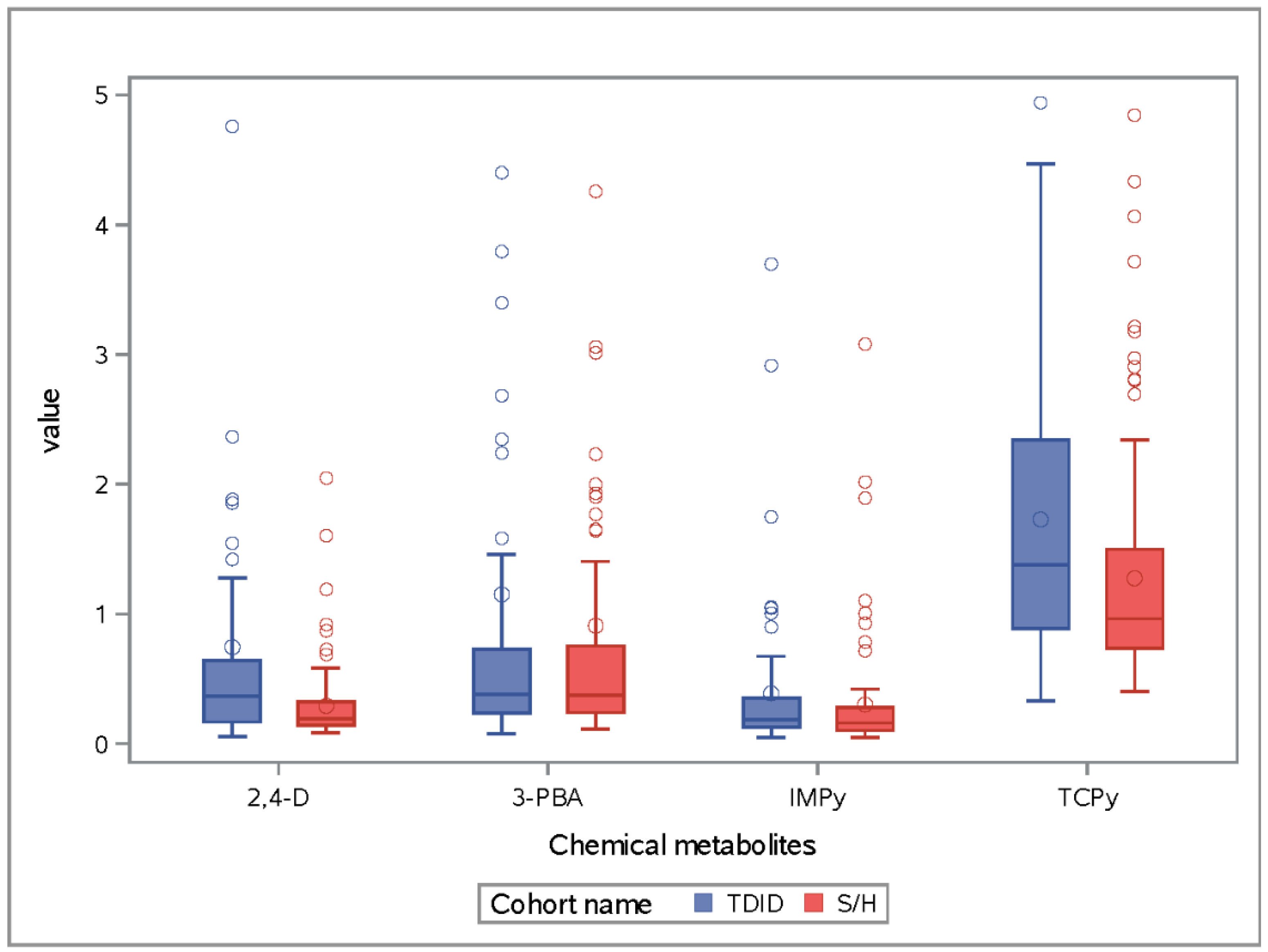
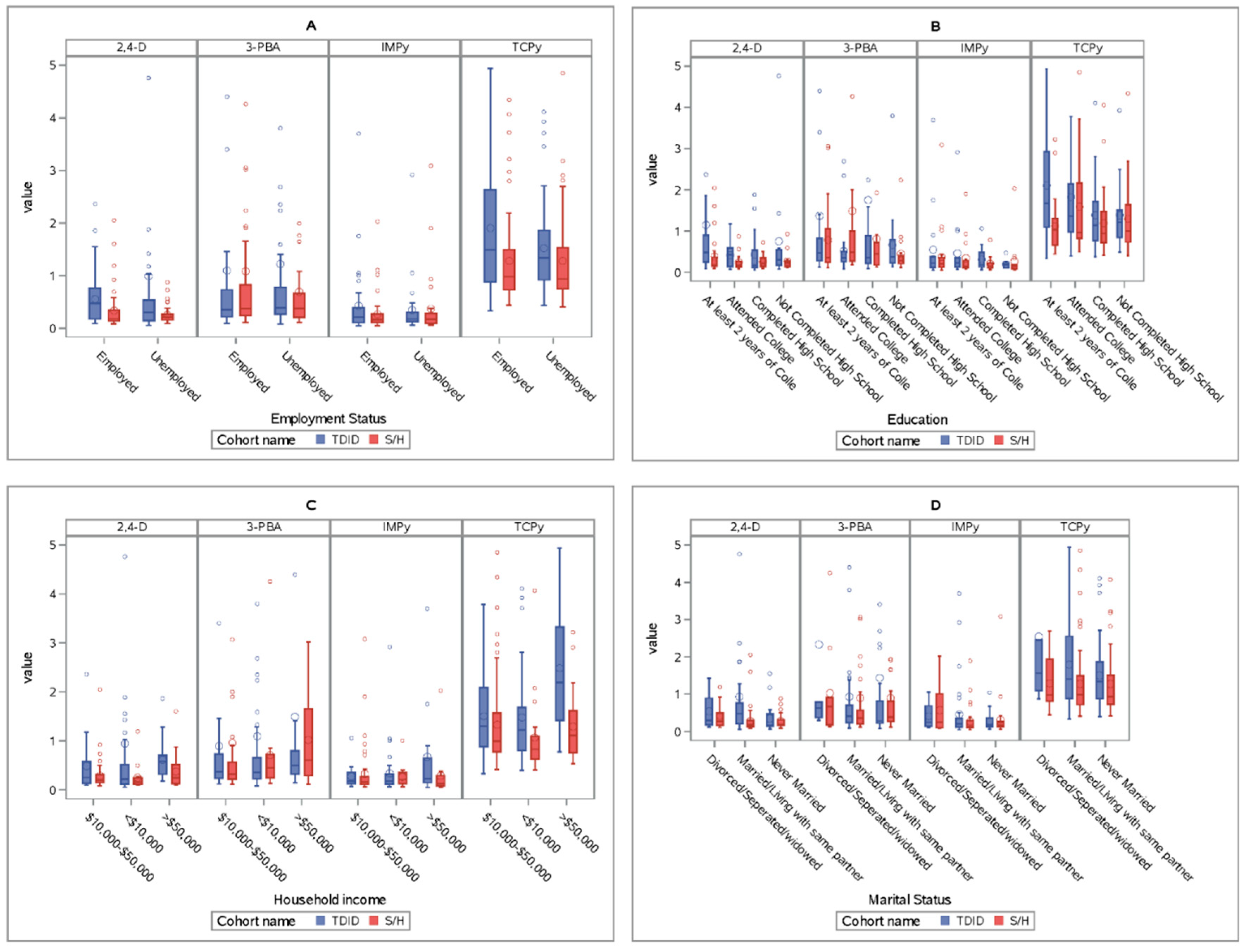
3. Results
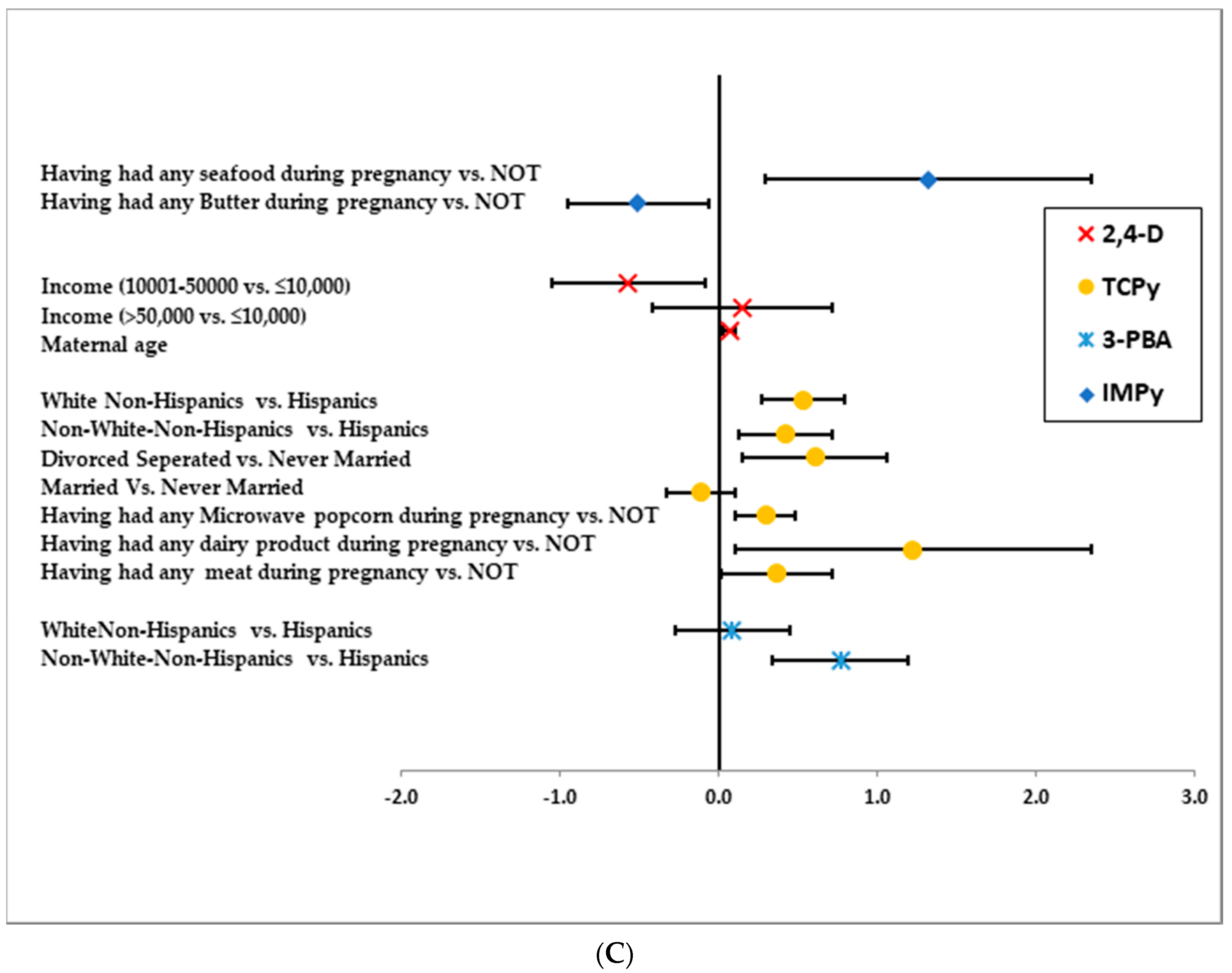
3.1. IMPy
3.2. 2,4-D
3.3. TCPy
3.4. 3-PBA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| N | Number |
| USDA | United States Department of Agriculture |
| NHANES | National Health and Nutrition Examination Survey |
| SD | Standard Deviation |
| SE | Standard Error |
| LOD | Lower than limit of Detection |
References
- Heudorf, U.; Angerer, J. Metabolites of pyrethroid insecticides in urine specimens: Current exposure in an urban population in Germany. Environ. Health Perspect. 2001, 109, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control. Glossary of Classes of Non-Persistent Pesticides. Available online: https://www.cdc.gov/nceh/clusters/Fallon/Glossary-Non%20Pers.pdf (accessed on 6 July 2019).
- Environmental Protection Agency. Pyrethrins and Pyrethroids. Available online: https://www.epa.gov/ingredients-used-pesticide-products/pyrethrins-and-pyrethroids (accessed on 13 August 2020).
- Whyatt, R.M.; Garfinkel, R.; Hoepner, L.A.; Holmes, D.; Borjas, M.; Williams, M.K.; Reyes, A.; Rauh, V.; Perera, F.P.; Camann, D.E. Within-and between-home variability in indoor-air insecticide levels during pregnancy among an inner-city cohort from New York City. Environ. Health Perspect. 2007, 115, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.G. Residential post-application pesticide exposure monitoring. In Occupational and Incidental Residential Exposure Assessment; Franklin, C.A., Worgan, J.P., Eds.; John Wiley & Sons: Sussex, UK, 2005; pp. 71–128. [Google Scholar]
- Lu, Z.; Gan, J.; Cui, X.; Delgado-Moreno, L.; Lin, K. Understanding the bioavailability of pyrethroids in the aquatic environment using chemical approaches. Environ. Int. 2019, 129, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Heudorf, U.; Angerer, J. Metabolites of Organophosphorous Insecticides in Urine Specimens from Inhabitants of a Residential Area. Environ. Res. 2001, 86, 80–87. [Google Scholar] [CrossRef]
- Gurunathan, S.; Robson, M.; Freeman, N.; Buckley, B.; Roy, A.; Meyer, R.; Bukowski, J.; Lioy, P.J. Accumulation of chlorpyrifos on residential surfaces and toys accessible to children. Environ. Health Perspect. 1998, 106, 9–16. [Google Scholar] [CrossRef]
- Davis, D.L.; Ahmed, A.K. Exposures from indoor spraying of chlorpyrifos pose greater health risks to children than currently estimated. Environ. Health Perspect. 1998, 106, 299–301. [Google Scholar] [CrossRef]
- Atwood, D.; Paisley-Jones, C. Pesticides Industry Sales and Usage; Environemental Protection Agency: Washington, DC, USA, 2017.
- Savage, E.P.; Keefe, T.J.; Wheeler, H.W.; Mounce, L.; Helwic, L.; Applehans, F.; Goes, E.; Goes, T.; Mihlan, G.; Rench, J. Household pesticide usage in the United States. Arch. Environ. Health Int. J. 1981, 36, 304–309. [Google Scholar] [CrossRef]
- U.S. EPA. Diazinon Revised Risk Assessment and Agreement with Registrants; United States Environmental Protection Agency: Washington, DC, USA, 2000.
- U.S. EPA. Chlorpyrifos Revised Risk Assessment and Agreement with Registrants; United States Environmental Protection Agency: Washington, DC, USA, 2001.
- Atwood, D.; Paisley-Jones, C. Pesticides Industry Sales and Usage: 2008–2012 Market Estimates; United States Environmental Protection Agency: Washington, DC, USA, 2017; pp. 14–16.
- USDA. Pesticides Data Program—Annual Summary, Calendar Year 2008; US Department of Agriculture Agricultural Marketing Service: Manassas, VA, USA, 2008.
- USDA. Pesticides Data Program—Annual Summary, Calendar Year 2009; US Department of Agriculture Agricultural Marketing Service: Manassas, VA, USA, 2009.
- USDA. Pesticides Data Program—Annual Summary, Calendar Year 2010; US Department of Agriculture Agricultural Marketing Service: Manassas, VA, USA, 2010.
- New York City Department of Environmental Protection. New York City 2008 Drinking Water Supply and Quality Report; New York City Department of Environmental Protection: New York, NY, USA, 2008; p. 11.
- New York City Department of Environmental Protection. New York City 2009 Drinking Water Supply and Quality Report; New York City Department of Environmental Protection: New York, NY, USA, 2009; p. 11.
- New York City Department of Environmental Protection. New York City 2010 Drinking Water Supply and Quality Report; New York City Department of Environmental Protection: New York, NY, USA, 2010; p. 11.
- New York City Department of Health and Mental Hygiene. Pesticide Use by New York City Agencies in 2010; Department of Health and Mental Hygiene: New York, NY, USA, 2011.
- Macintosh, D.L.; Kabiru, C.W.; Ryan, P.B. Longitudinal investigation of dietary exposure to selected pesticides. Environ. Health Perspect. 2001, 109, 145–150. [Google Scholar] [CrossRef]
- Riederer, A.M.; Bartell, S.M.; Barr, D.B.; Ryan, P.B. Diet and nondiet predictors of urinary 3-phenoxybenzoic acid in NHANES 1999-2002. Environ. Health Perspect. 2008, 116, 1015–1022. [Google Scholar] [CrossRef]
- Hayes, W.J.; Laws, E.R. Handbook of Pesticide Toxicology; FAO: Rome, Italy, 1991. [Google Scholar]
- Human Biomonitoring Commission. Innere Belastung der Allgemeinbevölkerung in Deutschland mit Organophosphaten und Referenzwerte für die Organophosphat-Metabolite DMP, DMTP und DEP im Urin. Bundesgesundheitsbl. Gesundh. Gesundh. 2003, 46, 1107–1111. [Google Scholar] [CrossRef]
- Human Biomonitoring Commission. Innere Belastung der Allgemeinbevölkerung in Deutschland mit Pyrethroiden und Referenzwerte für Pyrethroid-Metabolite im Urin. Bundesgesundheitsbl. Gesundh. Gesundh. 2005, 48, 1187–1193. [Google Scholar]
- Egeghy, P.P.; Cohen Hubal, E.A.; Tulve, N.S.; Melnyk, L.J.; Morgan, M.K.; Fortmann, R.C.; Sheldon, L.S. Review of pesticide urinary biomarker measurements from selected US EPA children’s observational exposure studies. Int. J. Environ. Res. Public Health 2011, 8, 1727–1754. [Google Scholar] [CrossRef] [PubMed]
- Timchalk, C. Organophosphate pharmacokinetics. In Handbook of Pesticide Toxicology; Elsevier: Amsterdam, The Netherlands, 2001; Volume 2, pp. 936–939. [Google Scholar]
- Kamrin, M.A. Pesticide Profiles: Toxicity, Environmental Impact, and Fate; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Baker, S.E.; Olsson, A.O.; Barr, D.B. Isotope dilution high-performance liquid chromatography-tandem mass spectrometry method for quantifying urinary metabolites of synthetic pyrethroid insecticides. Arch. Environ. Contam. Toxicol. 2004, 46, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Demoute, J.-P. A brief review of the environmental fate and metabolism of pyrethroids. Pestic. Sci. 1989, 27, 375–385. [Google Scholar] [CrossRef]
- Furlong, M.A.; Barr, D.B.; Wolff, M.S.; Engel, S.M. Prenatal exposure to pyrethroid pesticides and childhood behavior and executive functioning. Neurotoxicology 2017, 62, 231–238. [Google Scholar] [CrossRef]
- Jervais, G.L.B.; Buhl, K.; Stone, D. 2,4-D Technical Fact Sheet. Available online: http://npic.orst.edu/factsheets/archive/2,4-DTech.html (accessed on 29 July 2020).
- Cornell Cooperative Extension. 2,4-D. Available online: http://pmep.cce.cornell.edu/profiles/extoxnet/24d-captan/24d-ext.html#18 (accessed on 29 July 2020).
- Slotkin, T.A. Cholinergic systems in brain development and disruption by neurotoxicants: Nicotine, environmental tobacco smoke, organophosphates. Toxicol. Appl. Pharmacol. 2004, 198, 132–151. [Google Scholar] [CrossRef]
- Sanborn, M.; Kerr, K.J.; Sanin, L.H.; Cole, D.C.; Bassil, K.L.; Vakil, C. Non-cancer health effects of pesticides: Systematic review and implications for family doctors. Can. Fam. Physician 2007, 53, 1712–1720. [Google Scholar]
- Neta, G.; Goldman, L.R.; Barr, D.; Sjodin, A.; Apelberg, B.J.; Witter, F.R.; Halden, R.U. Distribution and determinants of pesticide mixtures in cord serum using principal component analysis. Environ. Sci. Technol. 2010, 44, 5641–5648. [Google Scholar] [CrossRef]
- Engel, S.M.; Berkowitz, G.S.; Barr, D.B.; Teitelbaum, S.L.; Siskind, J.; Meisel, S.J.; Wetmur, J.G.; Wolff, M.S. Prenatal organophosphate metabolite and organochlorine levels and performance on the Brazelton Neonatal Behavioral Assessment Scale in a multiethnic pregnancy cohort. Am. J. Epidemiol. 2007, 165, 1397–1404. [Google Scholar] [CrossRef]
- Eskenazi, B.; Marks, A.R.; Bradman, A.; Harley, K.; Barr, D.B.; Johnson, C.; Morga, N.; Jewell, N.P. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ. Health Perspect. 2007, 115, 792–798. [Google Scholar] [CrossRef]
- Rauh, V.; Arunajadai, S.; Horton, M.; Perera, F.; Hoepner, L.; Barr, D.B.; Whyatt, R. Seven-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environ. Health Perspect. 2011, 119, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Young, J.G.; Eskenazi, B.; Gladstone, E.A.; Bradman, A.; Pedersen, L.; Johnson, C.; Barr, D.B.; Furlong, C.E.; Holland, N.T. Association between in utero organophosphate pesticide exposure and abnormal reflexes in neonates. Neurotoxicology 2005, 26, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, G.S.; Obel, J.; Deych, E.; Lapinski, R.; Godbold, J.; Liu, Z.; Landrigan, P.J.; Wolff, M.S. Exposure to indoor pesticides during pregnancy in a multiethnic, urban cohort. Environ. Health Perspect. 2003, 111, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Barr, D.B.; Pearson, M.; Bartell, S.; Bravo, R. A longitudinal approach to assessing urban and suburban children’s exposure to pyrethroid pesticides. Environ. Health Perspect. 2006, 114, 1419–1423. [Google Scholar] [CrossRef] [PubMed]
- Saieva, C.; Aprea, C.; Tumino, R.; Masala, G.; Salvini, S.; Frasca, G.; Giurdanella, M.C.; Zanna, I.; Decarli, A.; Sciarra, G.; et al. Twenty-four-hour urinary excretion of ten pesticide metabolites in healthy adults in two different areas of Italy (Florence and Ragusa). Sci. Total. Environ. 2004, 332, 71–80. [Google Scholar] [CrossRef]
- Berman, T.; Goldsmith, R.; Goen, T.; Spungen, J.; Novack, L.; Levine, H.; Amitai, Y.; Shohat, T.; Grotto, I. Urinary concentrations of organophosphate pesticide metabolites in adults in Israel: Demographic and dietary predictors. Environ. Int. 2013, 60, 183–189. [Google Scholar] [CrossRef]
- Ye, M.; Beach, J.; Martin, J.W.; Senthilselvan, A. Associations between dietary factors and urinary concentrations of organophosphate and pyrethroid metabolites in a Canadian general population. Int. J. Hyg. Environ. Health 2015, 218, 616–626. [Google Scholar] [CrossRef]
- Fortes, C.; Mastroeni, S.; Pilla, M.A.; Antonelli, G.; Lunghini, L.; Aprea, C. The relation between dietary habits and urinary levels of 3-phenoxybenzoic acid, a pyrethroid metabolite. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 52, 91–96. [Google Scholar] [CrossRef]
- Hyland, C.; Bradman, A.; Gerona, R.; Patton, S.; Zakharevich, I.; Gunier, R.B.; Klein, K. Organic diet intervention significantly reduces urinary pesticide levels in U.S. children and adults. Environ. Res. 2019, 171, 568–575. [Google Scholar] [CrossRef]
- Oates, L.; Cohen, M.; Braun, L.; Schembri, A.; Taskova, R. Reduction in urinary organophosphate pesticide metabolites in adults after a week-long organic diet. Environ. Res. 2014, 132, 105–111. [Google Scholar] [CrossRef]
- Lu, C.; Toepel, K.; Irish, R.; Fenske Richard, A.; Barr Dana, B.; Bravo, R. Organic Diets Significantly Lower Children’s Dietary Exposure to Organophosphorus Pesticides. Environ. Health Perspect. 2006, 114, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Bradman, A.; Quirós-Alcalá, L.; Castorina, R.; Schall Raul, A.; Camacho, J.; Holland Nina, T.; Barr Dana, B.; Eskenazi, B. Effect of Organic Diet Intervention on Pesticide Exposures in Young Children Living in Low-Income Urban and Agricultural Communities. Environ. Health Perspect. 2015, 123, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Helsel, D.R.; Hirsch, R.M. Methods for Data Below the Reporting Limit. In Statistical Methods in Water Resources; Helsel, D.R., Hirsch, R.M., Eds.; Elsevier: Amsterdam, The Netherlands, 1992; Volume 49. [Google Scholar]
- Sinha, P.; Lambert, M.B.; Trumbull, V.L. Evaluation of statistical methods for left-censored environmental data with nonuniform detection limits. Environ. Toxicol. Chem. 2006, 25, 2533–2540. [Google Scholar] [CrossRef] [PubMed]
- Glorennec, P.; Serrano, T.; Fravallo, M.; Warembourg, C.; Monfort, C.; Cordier, S.; Viel, J.-F.; Le Gléau, F.; Le Bot, B.; Chevrier, C. Determinants of children’s exposure to pyrethroid insecticides in western France. Environ. Int. 2017, 104, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Seiwert, M.; Angerer, J.; Kolossa-Gehring, M.; Hoppe, H.W.; Ball, M.; Schulz, C.; Thumulla, J.; Seifert, B. GerES IV pilot study: Assessment of the exposure of German children to organophosphorus and pyrethroid pesticides. Int. J. Hyg. Environ. Health 2006, 209, 221–233. [Google Scholar] [CrossRef]
- Whyatt, R.M.; Barr, D.B.; Camann, D.E.; Kinney, P.L.; Barr, J.R.; Andrews, H.F.; Hoepner, L.A.; Garfinkel, R.; Hazi, Y.; Reyes, A.; et al. Contemporary-use pesticides in personal air samples during pregnancy and blood samples at delivery among urban minority mothers and newborns. Environ. Health Perspect. 2003, 111, 749–756. [Google Scholar] [CrossRef] [PubMed]
- McKelvey, W.; Jacobson, J.B.; Kass, D.; Barr, D.B.; Davis, M.; Calafat, A.M.; Aldous, K.M. Population-based biomonitoring of exposure to organophosphate and pyrethroid pesticides in New York City. Environ. Health Perspect. 2013, 121, 1349–1356. [Google Scholar] [CrossRef]
- Horton, M.K.; Bousleiman, S.; Jones, R.; Sjodin, A.; Liu, X.; Whyatt, R.; Factor-Litvak, P. Predictors of serum concentrations of polybrominated flame retardants among healthy pregnant women in an urban environment: A cross-sectional study. Environ. Health Glob. Access Sci. Source 2013, 12, 23. [Google Scholar] [CrossRef]
- Cowell, W.J.; Stapleton, H.M.; Holmes, D.; Calero, L.; Tobon, C.; Perzanowski, M.; Herbstman, J.B. Prevalence of historical and replacement brominated flame retardant chemicals in New York City homes. Emerg. Contam. 2017, 3, 32–39. [Google Scholar] [CrossRef]
- Perera, F.P.; Rauh, V.; Tsai, W.-Y.; Kinney, P.; Camann, D.; Barr, D.; Bernert, T.; Garfinkel, R.; Tu, Y.-H.; Diaz, D.; et al. Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ. Health Perspect. 2003, 111, 201–205. [Google Scholar] [CrossRef]
- Davis, M.D.; Wade, E.L.; Restrepo, P.R.; Roman-Esteva, W.; Bravo, R.; Kuklenyik, P.; Calafat, A.M. Semi-automated solid phase extraction method for the mass spectrometric quantification of 12 specific metabolites of organophosphorus pesticides, synthetic pyrethroids, and select herbicides in human urine. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 929, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control. Biomonitoring Summary, Methyl Parathion. Available online: https://www.cdc.gov/biomonitoring/Methyl_Eethyl_Parathion_BiomonitoringSummary.html#:~:text=General%20Information,with%20limited%20applications%20in%20agriculture (accessed on 14 June 2020).
- Steffes, M. Laboratory Procedure Manual; University of Minnesota: Minneapolis, MN, USA, 2011. [Google Scholar]
- SAS Institute Inc. SAS 9.4 for Windows; SAS Institute Inc.: Cary, NC, USA, 2012. [Google Scholar]
- Barr, D.B.; Wilder, L.C.; Caudill, S.P.; Gonzalez, A.J.; Needham, L.L.; Pirkle, J.L. Urinary creatinine concentrations in the U.S. population: Implications for urinary biologic monitoring measurements. Environ. Health Perspect. 2005, 113, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Hornung, R.W.; Reed, L.D. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 1990, 5, 46–51. [Google Scholar] [CrossRef]
- Centers for Disease Control & Prevention. Fourth National Report on Human Exposure to Environmental Chemicals; Centers for Disease Control & Prevention: Atlanta, GA, USA, 2009.
- Glass, D.C.; Gray, C.N. Estimating mean exposures from censored data: Exposure to benzene in the Australian petroleum industry. Ann. Occup. Hyg. 2001, 45, 275–282. [Google Scholar] [CrossRef]
- Turnbull, B.W. The Empirical Distribution Function with Arbitrarily Grouped, Censored and Truncated Data. J. R. Stat. Soc. Ser. B (Methodol.) 1976, 38, 290–295. [Google Scholar] [CrossRef]
- Li, A.J.; Kannan, K. Urinary concentrations and profiles of organophosphate and pyrethroid pesticide metabolites and phenoxyacid herbicides in populations in eight countries. Environ. Int. 2018, 121, 1148–1154. [Google Scholar] [CrossRef]
- Shahar, D.; Shai, I.; Vardi, H.; Shahar, A.; Fraser, D. Diet and eating habits in high and low socioeconomic groups. Nutrition 2005, 21, 559–566. [Google Scholar] [CrossRef]
- Smith, A.M.; Baghurst, K.I. Public health implications of dietary differences between social status and occupational category groups. J. Epidemiol. Community Health 1992, 46, 409–416. [Google Scholar] [CrossRef]
- Shimakawa, T.; Sorlie, P.; Carpenter, M.A.; Dennis, B.; Tell, G.S.; Watson, R.; Williams, O.D. Dietary intake patterns and sociodemographic factors in the atherosclerosis risk in communities study. ARIC Study Investigators. Prev. Med. 1994, 23, 769–780. [Google Scholar] [CrossRef]
- Yoo, M.; Lim, Y.-H.; Kim, T.; Lee, D.; Hong, Y.-C. Association between urinary 3-phenoxybenzoic acid and body mass index in Korean adults: 1(st) Korean National Environmental Health Survey. Ann. Occup Environ. Med. 2016, 28, 2. [Google Scholar] [CrossRef]
- Colapinto, C.K.; Arbuckle, T.E.; Dubois, L.; Fraser, W. Tea consumption in pregnancy as a predictor of pesticide exposure and adverse birth outcomes: The MIREC Study. Environ. Res. 2015, 142, 77–83. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, J.; Cheung, W.; Leung, D. Determination of pesticide residue transfer rates (percent) from dried tea leaves to brewed tea. J. Agric. Food Chem. 2014, 62, 966–983. [Google Scholar] [CrossRef] [PubMed]
- Canadian Food Inspection Agency. Food Safety Action Plan. Report: 2010–2011-Targeted Surveys Chemistry. Pesticide in Coffee, Fruit Juice and Tea; Canadian Food Inspection Agency: Ottawa, ON, Canada, 2011.
- Shokunbi, O.S.; Oladejo, D.O.; Ngozi, E.O.; Amah, G.H. Effects of Processing on the Levels of Pesticides in Some Commonly Consumed Meats from Sagamu, South-Western Nigeria. Curr. J. Appl. Sci. Technol. 2018, 1–13. [Google Scholar] [CrossRef]
- Coulibaly, K.; Smith, J.S. Effect of pH and cooking temperature on the stability of organophosphate pesticides in beef muscle. J. Agric. Food Chem. 1994, 42, 2035–2039. [Google Scholar] [CrossRef]
- Li, A.J.; Martinez-Moral, M.-P.; Kannan, K. Temporal variability in urinary pesticide concentrations in repeated-spot and first-morning-void samples and its association with oxidative stress in healthy individuals. Environ. Int. 2019, 130, 104904. [Google Scholar] [CrossRef]



| S/H Cohort (n = 121) | TDID Cohort (n = 153) | p-Value | |
|---|---|---|---|
| n% | n% | ||
| Education | |||
| Not Completed High School | 25 (20.7) | 22 (14.4) | 0.49 |
| 4yr college | 32 (26.4) | 40 (26.1) | |
| 4 + yr college | 29 (24.0) | 37 (24.2) | |
| Completed High School | 35 (28.9) | 54 (35.3) | |
| Marital Status | |||
| Never Married | 41 (33.9) | 47 (30.7) | 0.09 |
| Married/Lived with same partner > 7yrs | 67 (55.4) | 99 (64.7) | |
| Divorced/Widowed/Separated | 13 (10.7) | 7 (4.6) | |
| Household income | |||
| Less than or = 10,000 | 21 (16.4) | 63 (49.7) | 0.0001 |
| 10,001–50,000 | 76 (62.8) | 37(24.2) | |
| >50,000 | 22 (18.2) | 40 (26.1) | |
| Missing | 2 | 13 | |
| Smoking Status (pregnancy) | |||
| Smoker | 6 (5.0) | 3(2.0) | 0.16 |
| Non-smoker | 115 (95.0) | 150 (98.0) | |
| Employment status | |||
| Employed | 68 (56.2) | 85 (55.6) | 0.91 |
| Unemployed | 53 (43.8) | 68 (44.4) | |
| Race/ethnicity | |||
| White-nonHispanic | NA | 28 (18.3) | NA * |
| African American-Non-Hispanic | 52 (43.0) | 10(6.5) | |
| Asian-nonHispanic | NA | 8 (5.2) | |
| Hispanic | 69 (57.0) | 107 (69.9) | |
| Mean (SD) | Mean (SD) | ||
| Maternal pre-pregnancy BMI | 27.2 (6.1) | 25.0 (6.4) | 0.002 |
| Maternal Age | 31.2 (4.4) | 28.9 (5.5) | 0.0006 |
| Biomarker Name | Mean (SD) (µg/g Creatinine) a | Geometric Mean (95%CI) (µg/g Creatinine) | Median (µg/g Creatinine) a | LOD (µg/L) | # of Values > LOD | # of Values < LOD (%) |
|---|---|---|---|---|---|---|
| TDID cohort | ||||||
| IMPY | 0.3 (0.4) | NC b | 0.2 | 0.1 | 62 | 91 (59.5%) |
| 2,4-D | 0.6(1.5) | NC b | 0.3 | 0.15 | 89 | 64 (41.8%) |
| TCPy | 1.6 (1.2) | 1.1 (1.0,1.3) | 1.2 | 0.1 | 135 | 18 (11.8%) |
| 3-PBA | 0.9 (3.1) | 0.4 (0.3,0.4) | 0.3 | 0.1 | 113 | 40 (26.1%) |
| S/H cohort | ||||||
| IMPY | 0.2 (0.4) | 0.1 (0.1,0.2) | 0.1 | 0.1 | 78 | 43 (35.5%) |
| 2,4-D | 0.2 (0.3) | 0.2 (0.2,0.2) | 0.2 | 0.15 | 90 | 31 (25.6%) |
| TCPy | 1.1 (0.9) | 0.8 (0.7,1.0) | 0.9 | 0.1 | 108 | 13 (10.7%) |
| 3-PBA | 0.7 (1.8) | 0.3 (0.2,0.3) | 0.3 | 0.1 | 89 | 32 (26.4%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balalian, A.A.; Liu, X.; Siegel, E.L.; Herbstman, J.B.; Rauh, V.; Wapner, R.; Factor-Litvak, P.; Whyatt, R. Predictors of Urinary Pyrethroid and Organophosphate Compound Concentrations among Healthy Pregnant Women in New York. Int. J. Environ. Res. Public Health 2020, 17, 6164. https://doi.org/10.3390/ijerph17176164
Balalian AA, Liu X, Siegel EL, Herbstman JB, Rauh V, Wapner R, Factor-Litvak P, Whyatt R. Predictors of Urinary Pyrethroid and Organophosphate Compound Concentrations among Healthy Pregnant Women in New York. International Journal of Environmental Research and Public Health. 2020; 17(17):6164. https://doi.org/10.3390/ijerph17176164
Chicago/Turabian StyleBalalian, Arin A., Xinhua Liu, Eva Laura Siegel, Julie Beth Herbstman, Virginia Rauh, Ronald Wapner, Pam Factor-Litvak, and Robin Whyatt. 2020. "Predictors of Urinary Pyrethroid and Organophosphate Compound Concentrations among Healthy Pregnant Women in New York" International Journal of Environmental Research and Public Health 17, no. 17: 6164. https://doi.org/10.3390/ijerph17176164
APA StyleBalalian, A. A., Liu, X., Siegel, E. L., Herbstman, J. B., Rauh, V., Wapner, R., Factor-Litvak, P., & Whyatt, R. (2020). Predictors of Urinary Pyrethroid and Organophosphate Compound Concentrations among Healthy Pregnant Women in New York. International Journal of Environmental Research and Public Health, 17(17), 6164. https://doi.org/10.3390/ijerph17176164





