Dextromethorphan Attenuates Sensorineural Hearing Loss in an Animal Model and Population-Based Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Noise Exposure
2.2. DXM Application
2.3. Auditory Brainstem Response Recording
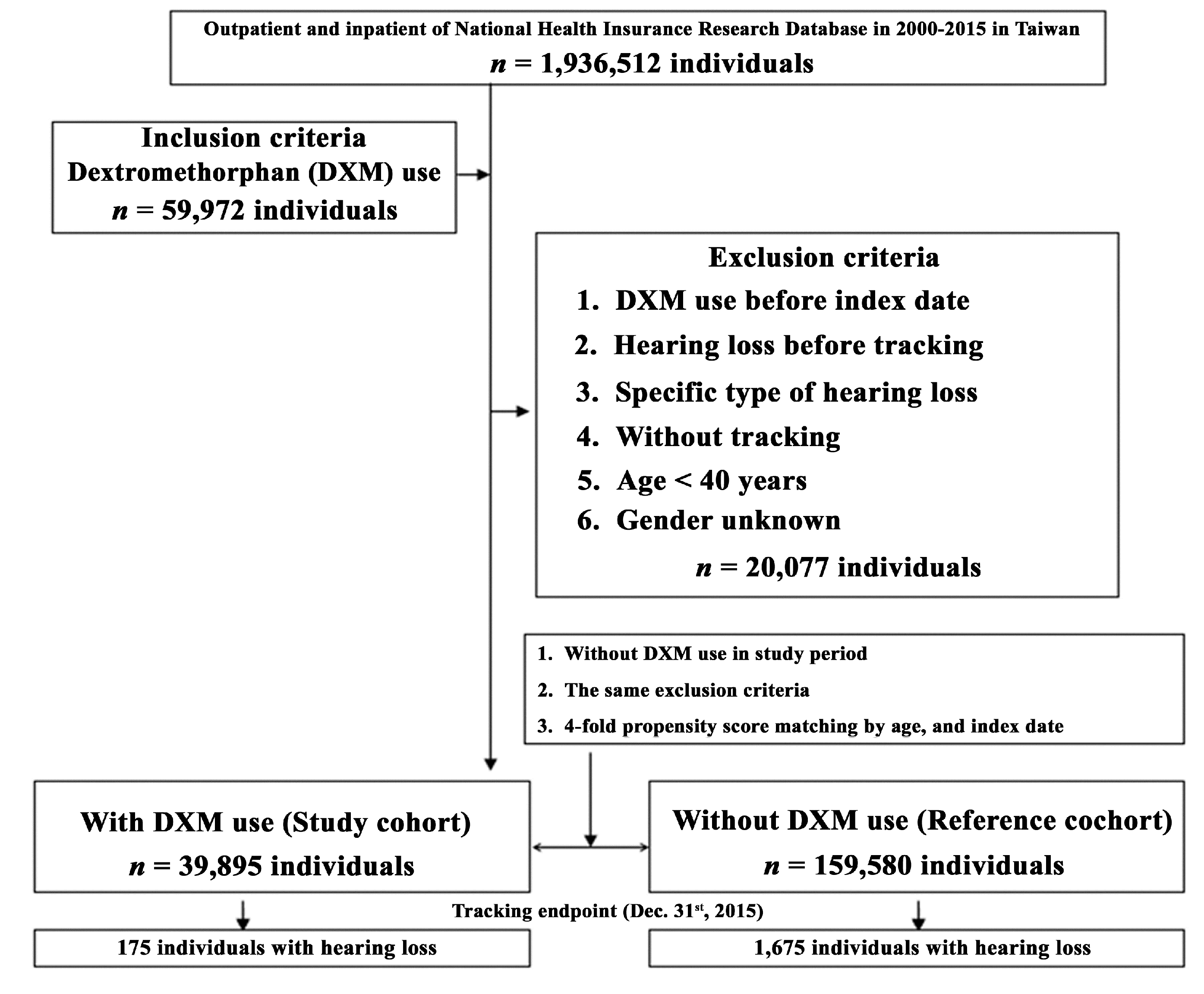
2.4. Population-Base Database
2.5. Duration of DXM Use and Sensitivity Analysis
2.6. Statistical Analysis
3. Results
3.1. Animal Study
3.2. Population-Based Human Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wilson, B.S.; Tucci, D.L.; Merson, M.H.; O’Donoghue, G.M. Global hearing health care: New findings and perspectives. Lancet 2017, 390, 2503–2515. [Google Scholar] [CrossRef]
- Disease, G.B.D.; Injury, I.; Prevalence, C. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Puel, J.L. Toward Cochlear Therapies. Physiol. Rev. 2018, 98, 2477–2522. [Google Scholar] [CrossRef] [PubMed]
- Gazia, F.; Abita, P.; Alberti, G.; Loteta, S.; Longo, P.; Caminiti, F.; Gargano, R. NICU Infants & SNHL: Experience of a western Sicily tertiary care centre. Acta Med. Mediterr. 2019, 35, 1001–1007. [Google Scholar]
- Valero, M.D.; Burton, J.A.; Hauser, S.N.; Hackett, T.A.; Ramachandran, R.; Liberman, M.C. Noise-induced cochlear synaptopathy in rhesus monkeys (Macaca mulatta). Hear. Res. 2017, 353, 213–223. [Google Scholar] [CrossRef]
- Kujawa, S.G.; Liberman, M.C. Adding insult to injury: Cochlear nerve degeneration after "temporary" noise-induced hearing loss. J. Neurosci. 2009, 29, 14077–14085. [Google Scholar] [CrossRef] [Green Version]
- Hickox, A.E.; Larsen, E.; Heinz, M.G.; Shinobu, L.; Whitton, J.P. Translational issues in cochlear synaptopathy. Hear. Res. 2017, 349, 164–171. [Google Scholar] [CrossRef]
- Moser, T.; Predoehl, F.; Starr, A. Review of hair cell synapse defects in sensorineural hearing impairment. Otol. Neurotol. 2013, 34, 995–1004. [Google Scholar] [CrossRef] [Green Version]
- Liberman, M.C.; Kujawa, S.G. Cochlear synaptopathy in acquired sensorineural hearing loss: Manifestations and mechanisms. Hear. Res. 2017, 349, 138–147. [Google Scholar] [CrossRef]
- Hong, J.; Chen, Y.; Zhang, Y.; Li, J.; Ren, L.; Yang, L.; Shi, L.; Li, A.; Zhang, T.; Li, H.; et al. N-Methyl-d-Aspartate Receptors Involvement in the Gentamicin-Induced Hearing Loss and Pathological Changes of Ribbon Synapse in the Mouse Cochlear Inner Hair Cells. Neural Plast. 2018, 2018, 3989201. [Google Scholar] [CrossRef] [Green Version]
- Jager, W.; Goiny, M.; Herrera-Marschitz, M.; Brundin, L.; Fransson, A.; Canlon, B. Noise-induced aspartate and glutamate efflux in the guinea pig cochlea and hearing loss. Exp. Brain Res. 2000, 134, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Agerman, K.; Ernfors, P.; Canlon, B. Complementary roles of neurotrophin 3 and a N-methyl-d-aspartate antagonist in the protection of noise and aminoglycoside-induced ototoxicity. Proc. Natl. Acad. Sci. USA 2000, 97, 7597–7602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, M. Glutamate receptor antagonist and neurotrophin can protect inner ear against damage. J. Otol. 2009, 4, 26–33. [Google Scholar]
- Bing, D.; Lee, S.C.; Campanelli, D.; Xiong, H.; Matsumoto, M.; Panford-Walsh, R.; Wolpert, S.; Praetorius, M.; Zimmermann, U.; Chu, H.; et al. Cochlear NMDA receptors as a therapeutic target of noise-induced tinnitus. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015, 35, 1905–1923. [Google Scholar] [CrossRef]
- Furman, A.C.; Kujawa, S.G.; Liberman, M.C. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J. Neurophysiol. 2013, 110, 577–586. [Google Scholar] [CrossRef]
- Nguyen, L.; Thomas, K.L.; Lucke-Wold, B.P.; Cavendish, J.Z.; Crowe, M.S.; Matsumoto, R.R. Dextromethorphan: An update on its utility for neurological and neuropsychiatric disorders. Pharmacol. Ther. 2016, 159, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, K.; Tsuji, S.; Wada, T.; Ito, Z.; Hara, A.; Kusakari, J. Effect of ketamine, dextromethorphan, and MK-801 on cochlear dysfunction induced by transient ischemia. Ann. Otol. Rhinol. Laryngol. 2002, 111, 44–49. [Google Scholar] [CrossRef]
- Werling, L.L.; Lauterbach, E.C.; Calef, U. Dextromethorphan as a potential neuroprotective agent with unique mechanisms of action. Neurologist 2007, 13, 272–293. [Google Scholar] [CrossRef]
- Rizzo, S.; Bentivegna, D.; Thomas, E.; La Mattina, E.; Mucia, M.; Salvago, P.; Sireci, F.; Martines, F. Sudden Sensorineural Hearing Loss, an Invisible Male: State of the Art. In Hearing Loss: Etiology, Management and Societal Implications; Hughes, J.D., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2017; pp. 75–86. [Google Scholar]
- Chen, J.; Shi, R. Current advances in neurotrauma research: Diagnosis, neuroprotection, and neurorepair. Neural Regen. Res. 2014, 9, 1093–1095. [Google Scholar] [CrossRef]
- Liu, C.T.; Huang, Y.S.; Chen, H.C.; Ma, K.H.; Wang, C.H.; Chiu, C.H.; Shih, J.H.; Kang, H.H.; Shiue, C.Y.; Li, I.H. Evaluation of brain SERT with 4-[(18)F]-ADAM/micro-PET and hearing protective effects of dextromethorphan in hearing loss rat model. Toxicol. Appl. Pharmacol. 2019, 378, 114604. [Google Scholar] [CrossRef]
- Shih, C.P.; Chen, H.C.; Lin, Y.C.; Chen, H.K.; Wang, H.; Kuo, C.Y.; Lin, Y.Y.; Wang, C.H. Middle-ear dexamethasone delivery via ultrasound microbubbles attenuates noise-induced hearing loss. Laryngoscope 2019, 129, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.P.; Lee, Y.H.; Chung, C.Y.; Hsu, J.C.; Yu, I.L.; Chang, N.T.; Chan, C.L. Comparisons of medical utilizations and categorical diagnoses of emergency visits between the elderly with catastrophic illness certificates and those without. BMC Health Serv. Res. 2013, 13, 152. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, J.; Corfas, G.; Liberman, M.C. Round-window delivery of neurotrophin 3 regenerates cochlear synapses after acoustic overexposure. Sci. Rep. 2016, 6, 24907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liberman, M.C. Noise-induced and age-related hearing loss: New perspectives and potential therapies. F1000Research 2017, 6, 927. [Google Scholar] [CrossRef]
- Pujol, R.; Puel, J.L.; Eybalin, M. Implication of non-NMDA and NMDA receptors in cochlear ischemia. Neuroreport 1992, 3, 299–302. [Google Scholar] [CrossRef]
- Lipton, S.A.; Rosenberg, P.A. Excitatory amino acids as a final common pathway for neurologic disorders. N. Engl. J. Med. 1994, 330, 613–622. [Google Scholar] [CrossRef]
- Basile, A.S.; Huang, J.M.; Xie, C.; Webster, D.; Berlin, C.; Skolnick, P. N-methyl-d-aspartate antagonists limit aminoglycoside antibiotic-induced hearing loss. Nat. Med. 1996, 2, 1338–1343. [Google Scholar] [CrossRef]
- Ruel, J.; Chabbert, C.; Nouvian, R.; Bendris, R.; Eybalin, M.; Leger, C.L.; Bourien, J.; Mersel, M.; Puel, J.L. Salicylate enables cochlear arachidonic-acid-sensitive NMDA receptor responses. J. Neurosci. 2008, 28, 7313–7323. [Google Scholar] [CrossRef]
- Kobel, M.; Le Prell, C.G.; Liu, J.; Hawks, J.W.; Bao, J. Noise-induced cochlear synaptopathy: Past findings and future studies. Hear. Res. 2017, 349, 148–154. [Google Scholar] [CrossRef]
- Mepani, A.M.; Kirk, S.A.; Hancock, K.E.; Bennett, K.; de Gruttola, V.; Liberman, M.C.; Maison, S.F. Middle Ear Muscle Reflex and Word Recognition in “Normal-Hearing” Adults: Evidence for Cochlear Synaptopathy? Ear Hear. 2020, 41, 25–38. [Google Scholar] [CrossRef]
- Cunningham, L.L.; Tucci, D.L. Restoring synaptic connections in the inner ear after noise damage. N. Engl. J. Med. 2015, 372, 181–182. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.; Gomez-Casati, M.E.; Gigliello, A.R.; Liberman, M.C.; Corfas, G. Neurotrophin-3 regulates ribbon synapse density in the cochlea and induces synapse regeneration after acoustic trauma. eLife 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Sly, D.J.; Campbell, L.; Uschakov, A.; Saief, S.T.; Lam, M.; O’Leary, S.J. Applying Neurotrophins to the Round Window Rescues Auditory Function and Reduces Inner Hair Cell Synaptopathy After Noise-induced Hearing Loss. Otol. Neurotol. 2016, 37, 1223–1230. [Google Scholar] [CrossRef] [PubMed]




| DXM Use | With | Without | p | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Total | 39,895 | 20.00 | 159,580 | 80.00 | |
| Hearing loss | 175 | 0.44 | 1675 | 1.05 | <0.001 |
| Variables | |||||
| Gender | 0.999 | ||||
| Male | 20,121 | 50.43 | 80,484 | 50.43 | |
| Female | 19,774 | 49.57 | 79,096 | 49.57 | |
| Age (years) | 55.28 ± 26.45 | 54.70 ± 25.51 | <0.001 | ||
| Catastrophic illness | 2671 | 6.70 | 12,104 | 7.58 | <0.001 |
| DM | 3884 | 9.74 | 16,011 | 10.03 | 0.076 |
| HTN | 9112 | 22.84 | 35,142 | 22.02 | <0.001 |
| Depression | 1097 | 2.75 | 4048 | 2.54 | 0.016 |
| Insomnia | 1845 | 4.62 | 6822 | 4.27 | 0.002 |
| Stroke | 2111 | 5.29 | 8705 | 5.45 | 0.197 |
| CKD | 4344 | 10.89 | 17,035 | 10.67 | 0.217 |
| Hyperlipidaemia | 1184 | 2.97 | 4499 | 2.82 | 0.111 |
| Epilepsy | 429 | 1.08 | 1375 | 0.86 | <0.001 |
| AID | 3245 | 8.13 | 13,401 | 8.40 | 0.088 |
| IHD | 2675 | 6.71 | 12,604 | 7.90 | <0.001 |
| COPD | 2973 | 7.45 | 12,801 | 8.02 | <0.001 |
| Pneumonia | 5671 | 14.21 | 20,754 | 13.01 | <0.001 |
| Head injury | 6881 | 17.25 | 26,279 | 16.47 | <0.001 |
| Asthma | 4213 | 10.56 | 17,987 | 11.27 | <0.001 |
| Alcohol abuse/dependence | 381 | 0.96 | 1275 | 0.80 | 0.002 |
| Tobacco abuse/dependence | 291 | 0.73 | 1248 | 0.78 | 0.282 |
| CLD | 4041 | 10.13 | 16,124 | 10.10 | <0.001 |
| Parkinson’s disease | 900 | 2.26 | 3251 | 2.04 | 0.006 |
| Urbanization level | <0.001 | ||||
| 1 (The highest) | 12,121 | 30.38 | 44,026 | 27.10 | |
| 2 | 13,986 | 35.06 | 54,521 | 33.57 | |
| 3 | 5111 | 12.81 | 20,782 | 12.79 | |
| 4 (The lowest) | 8677 | 21.75 | 43,101 | 26.54 | |
| Level of care | <0.001 | ||||
| Hospital center | 14,562 | 36.50 | 46,128 | 28.91 | |
| Regional hospital | 15,756 | 39.49 | 65,117 | 40.81 | |
| Local hospital | 9577 | 24.01 | 48,335 | 30.29 | |
| DXM Dose | Population | Events | PYs | Rate (per 105 PYs) | Adjusted HR | 95% CI | 95% CI | p |
|---|---|---|---|---|---|---|---|---|
| Without | 159,580 | 1675 | 2,012,465.11 | 83.23 | Reference | |||
| With | 39,895 | 175 | 513,401.02 | 34.09 | 0.725 | 0.624 | 0.803 | <0.001 |
| 1–30 days | 8423 | 43 | 114,267.35 | 37.63 | 0.797 | 0.684 | 0.888 | <0.001 |
| 31–90 days | 19,450 | 88 | 248,640.12 | 35.39 | 0.756 | 0.642 | 0.834 | <0.001 |
| ≥91 days | 12,022 | 44 | 150,493.55 | 29.24 | 0.622 | 0.531 | 0.697 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-C.; Wang, C.-H.; Chien, W.-C.; Chung, C.-H.; Shih, C.-P.; Lin, Y.-C.; Li, I.-H.; Lin, Y.-Y.; Kuo, C.-Y. Dextromethorphan Attenuates Sensorineural Hearing Loss in an Animal Model and Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2020, 17, 6336. https://doi.org/10.3390/ijerph17176336
Chen H-C, Wang C-H, Chien W-C, Chung C-H, Shih C-P, Lin Y-C, Li I-H, Lin Y-Y, Kuo C-Y. Dextromethorphan Attenuates Sensorineural Hearing Loss in an Animal Model and Population-Based Cohort Study. International Journal of Environmental Research and Public Health. 2020; 17(17):6336. https://doi.org/10.3390/ijerph17176336
Chicago/Turabian StyleChen, Hsin-Chien, Chih-Hung Wang, Wu-Chien Chien, Chi-Hsiang Chung, Cheng-Ping Shih, Yi-Chun Lin, I-Hsun Li, Yuan-Yung Lin, and Chao-Yin Kuo. 2020. "Dextromethorphan Attenuates Sensorineural Hearing Loss in an Animal Model and Population-Based Cohort Study" International Journal of Environmental Research and Public Health 17, no. 17: 6336. https://doi.org/10.3390/ijerph17176336





