Correlation between Visual Functions and Retinal Morphology in Eyes with Early and Intermediate Age-Related Macular Degeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Visual Functions
2.3. Retinal Morphology
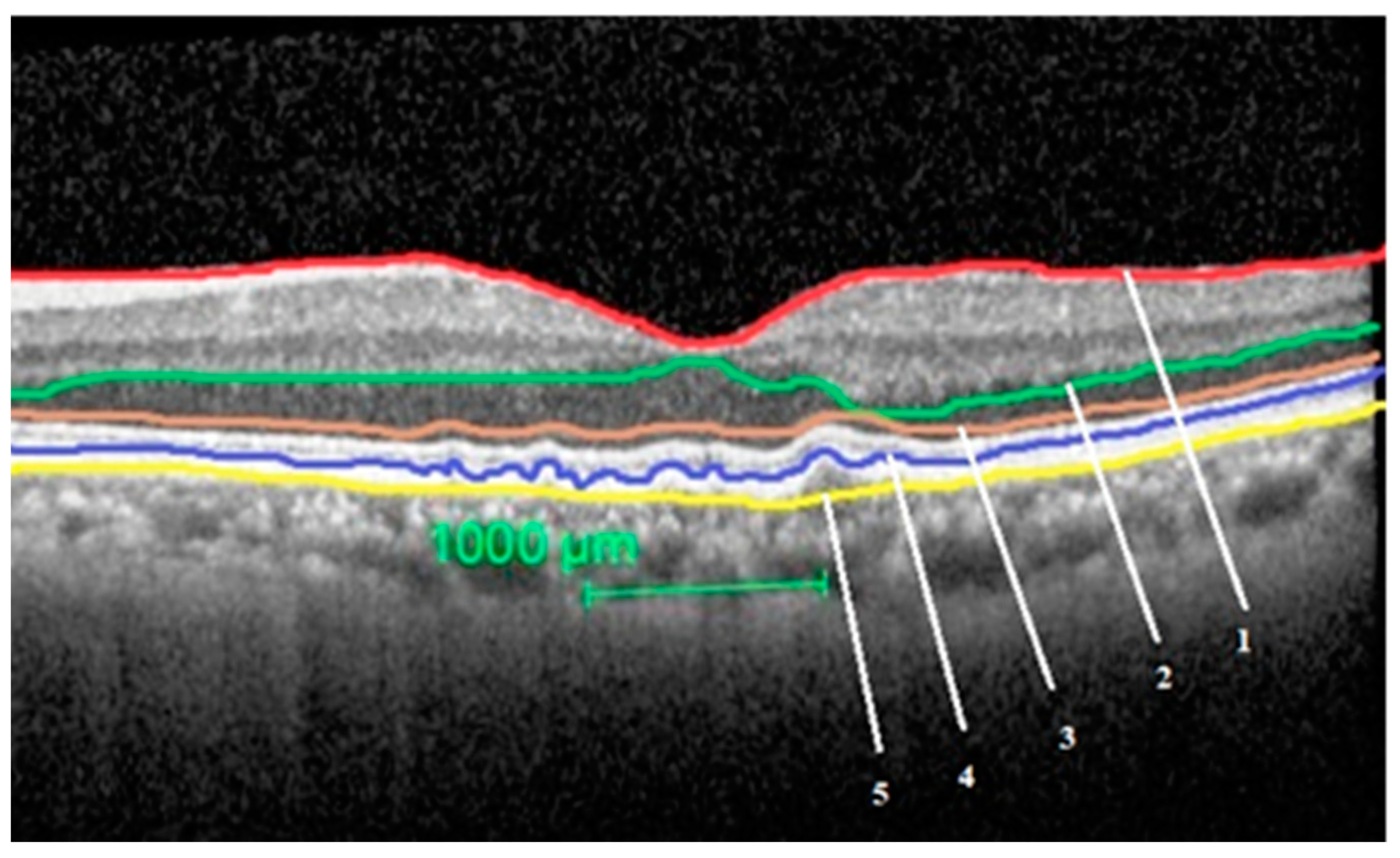
2.4. Image Analysis
- total retina (RT): distance between internal limiting membrane and Bruch membrane;
- retinal outer segment (ORT) or RPE-photoreceptor complex: distance between external limiting membrane and Bruch membrane;
- retinal pigment epithelium-Bruch membrane complex (RPET): distance between retinal pigment epithelium and Bruch membrane;
- outer nuclear layer (ONL): distance between outer layer of outer plexiform layer and external limiting membrane.
2.5. Examiners
2.6. Statistical Analysis
3. Results
3.1. Visual Functions
3.2. Retinal Morphology
3.3. Correlation between Visual Functions with Retinal Morphology
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Coleman, H.R.; Chan, C.C.; Ferris, F.L., III; Chew, E.Y. Age-related macular degeneration. Lancet 2008, 372, 1835–1845. [Google Scholar] [CrossRef]
- Ferris, C.P., III; Frederick, L.; Wilkinson Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R. Clinical Classification of Age-related Macular Degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef]
- Girmens, J.F.; Sahel, J.A.; Marazova, K. Dry age-related macular degeneration: A currently unmet clinical need. Intractable Rare Dis. Res. 2012, 1, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Karampelas, M.; Sim, D.A.; Keane, P.A.; Papastefanou, V.P.; Sadda, S.R.; Tufail, A.; Dowler, J. Evaluation of retinal pigment epithelium–Bruch’s membrane complex thickness in dry age-related macular degeneration using optical coherence tomography. Br. J. Ophthalmol. 2013, 97, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Rogala, J.; Zanger, B.; Assaad, N.; Fletcher, E.L.; Kalloniatis, M.; Nivison-Smith, L. In Vivo Quantification of Retinal Changes Associated With Drusen in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1689–1700. [Google Scholar] [CrossRef] [PubMed]
- Sadigh, S.; Cideciyan, A.V.; Sumaroka, A.; Huang, W.C.; Luo, X.; Swider, M.; Steinberg, J.D.; Stambolian, D.; Jacobson, S.G. Abnormal thickening as well as thinning of the photoreceptor layer in intermediate age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1603–1612. [Google Scholar] [CrossRef]
- Muftuoglu, I.K.; Ramkumar, H.L.; Bartsch, D.U.; Meshi, A.; Gaber, R.; Freeman, W.R. Quantitative analysis of the inner retinal layer thicknesses in age-related macular degeneration using corrected optical coherence tomography segmentation. Retina 2018, 38, 1478–1484. [Google Scholar] [CrossRef]
- Dunaief, J.L.; Dentchev, T.; Ying, G.S.; Milam, A.H. The role of apoptosis in age-related macular degeneration. Arch. Ophthalmol. 2002, 120, 1435–1442. [Google Scholar] [CrossRef]
- Sarks, J.P.; Sarks, S.H.; Killingsworth, M.C. Evaluation of geographic atrophy of retinal pigment epithelium. Eye 1988, 2, 552–577. [Google Scholar] [CrossRef]
- Pappuru, R.R.; Ouyang, P.Y.; Nittala, M.G.; Hemmati, H.D.; Keane, P.A.; Walsh, A.C.; Sadda, S.R. Relationship between Outer Retinal Thickness Substructures and Visual Acuity in Eyes with Dry Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6743–6748. [Google Scholar] [CrossRef] [PubMed]
- Hazel, C.A.; Petre, K.L.; Armstrong, R.A.; Benson, M.T.; Frost, N.A. Visual function and subjective quality of life compared in subjects with acquired macular disease. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1309–1315. [Google Scholar]
- Wood, J.M.; Owens, D.A. Standard measures of visual acuity do not predict drivers’ recognition performance under day or night conditions. Optom. Vis. Sci. 2005, 82, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Keane, P.A.; Sadda, S.R. Predicting visual outcomes for macular disease using optical coherence tomography. Saudi J. Ophthalmol. 2011, 25, 145–158. [Google Scholar] [CrossRef]
- Nixon, D.R.; Flinn, N.A.P. Evaluation of contrast sensitivity and other visual function outcomes in neovascular age-related macular degeneration patients after treatment switch to aflibercept from ranibizumab. Clin. Ophthalmol. 2017, 11, 715–721. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cochran, W.G. Sampling Techniques; Wiley: New York, NY, USA, 1963. [Google Scholar]
- Cheung, C.M.G.; Tai, E.S.; Kawasaki, R.; Tay, W.T.; Lee, J.L.; Hamzah, H.; Wong, T.Y. Prevalence of and Risk Factors for Age-Related Macular Degeneration in a Multiethnic Asian Cohort. Arch. Ophthalmol. 2012, 130, 480–486. [Google Scholar] [PubMed]
- Buari, N.H.; Yusof, N.H.; Mohd-Satali, A.; Chen, A.H. Repeatability of the universiti teknologi mara reading charts. Bangladesh J. Med. Sci. 2015, 14, 226–240. [Google Scholar] [CrossRef]
- Zhichao, W.U.; Guymer, R.H.; Finger, R.P. Low luminance deficit and night vision symptoms in intermediate age-related macular degeneration. Br. J. Ophthalmol. 2016, 100, 395–398. [Google Scholar]
- Patel, P.J.; Chen, F.K.; Rubin, G.S.; Tufail, A. Intersession Repeatability of Contrast Sensitivity Scores in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2621–2625. [Google Scholar] [CrossRef]
- Vujosevic, S.; Pucci, P.; Casciano, M.; Longhin, E.; Convento, E.; Bini, S.; Midena, E. Long-term longitudinal modifications in mesopic Microperimetry, in early and intermediate age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 301–309. [Google Scholar] [CrossRef]
- Keane, P.A.; Patel, P.J.; Ouyang, Y.; Chen, F.K.; Ikeji, F.; Walsh, A.C.; Tufail, A.; Sadda, S.R. Effects of retinal morphology on contrast sensitivity and reading ability in neovascular age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5431–5437. [Google Scholar] [CrossRef] [PubMed]
- Haegerstrom-Portnoy, G.; Schneck, M.E.; Lott, L.A.; Brabyn, J.A. The relation between visual acuity and other spatial vision measures. Optom. Vis. Sci. 2000, 77, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Pelli, D.G.; Robson, J.G.; Wilkins, A.J. The design of a new letter chart for measuring contrast sensitivity. Clin. Vis. Sci. 1988, 2, 187–199. [Google Scholar]
- Patel, P.J.; Chen, F.K.; Cruz, L.D.; Rubin, G.S.; Tufail, A. Test–Retest Variability of Reading Performance Metrics Using MNREAD in Patients with Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3854–3859. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Sato, T.; Kishi, S. Outer nuclear layer thickness at fovea determines visual outcome in resolved central serous retinopathy. Am. J. Ophthalmol. 2009, 148, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Menghini, M.; Lujan, B.J.; Zayit-Soudry, S.; Syed, R.; Porco, T.C.; Bayabo, K.; Carroll, J.; Roorda, A.; Duncan, J.L. Correlation of outer nuclear layer thickness with cone density values in patients with retinitis pigmentosa and healthy subjects. Investig. Ophthalmol. Vis. Sci. 2015, 56, 372–381. [Google Scholar] [CrossRef]
- Hogan, M.; Alvarado, J.; Weddell, J. Histology of the Human Eye; WB Saunders: Philadelphia, PA, USA, 1971. [Google Scholar]
- Tao, W.; Wen, R.; Goddard, M.B.; Sherman, S.D.; O’Rourke, P.J.; Stabila, P.F.; Bell, W.J.; Dean, B.J.; Kauper, K.A.; Budz, V.A.; et al. Encapsulated cell-based delivery of CNTF reduces photoreceptor degeneration in animal model of retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3292–3298. [Google Scholar]
- McGill, T.J.; Prusky, G.; Douglas, R.M.; Yasumura, D.; Matthes, M.T.; Lowe, R.J.; Duncan, J.L.; Yang, H.; Ahern, K.; Daniello, K.M.; et al. Discordant anatomical, electrophysiological, and visual behavioral profiles of retinal degeneration in rat models of retinal degenerative disease. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6232–6244. [Google Scholar] [CrossRef]
- Curcio, C.; Medeiros, N.E.; Millican, C.L. Photoreceptor loss in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1236–1249. [Google Scholar]
- Bird, A.C.; Phillips, R.L.; Hageman, G.S. Geographic atrophy a histopathological assessment. JAMA Ophthalmol. 2014, 132, 338–345. [Google Scholar] [CrossRef]
- Schuman, S.G.; Koreishi, A.F.; Farsiu, S.; Jung, S.H.; Izatt, J.A.; Toth, C.A. Photoreceptor Layer Thinning over Drusen in Eyes with Age related Macular Degeneration Imaged In Vivo with Spectral Domain Optical Coherence Tomography. Ophthalmology 2009, 116, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Acton, J.H.; Smith, R.T.; Hood, D.C.; Greenstein, V.C. Relationship between retinal layer thickness and the visual field in early age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7618–7624. [Google Scholar] [CrossRef] [PubMed]
- Hageman, G.S.; Luthert, P.J.; Chong, N.H.; Johnson, L.V.; Anderson, D.H.; Mullins, R.F. An integrated hypothesis that considers drusen asbiomarkers of immune-mediated processes at the RPE-Bruch’smembrane interface in aging and age-related macular degeneration. Prog. Retin. Eye Res. 2001, 20, 705–732. [Google Scholar] [CrossRef]
- De Jong, P.T. Age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1474–1485. [Google Scholar] [CrossRef] [PubMed]
- Ramrattan, R.S.; Van der Schaft, T.L.; Mooy, C.M.; de Bruijn, W.C.; Mulder, P.G.; de Jong, P.T. Morphometric analysis of Bruch’s choroid in aging. Investig. Ophthalmol. Vis. Sci. 1994, 35, 2857–2864. [Google Scholar]
- Starita, C.; Hussain, A.A.; Pagliarini, S.; Marshall, J. Hydrodynamics of ageing Bruch’s membrane: Implications for macular disease. Exp. Eye Res. 1996, 62, 565–572. [Google Scholar] [CrossRef]
- Gilmore, A.P. Anoikis. Cell Death Differ. 2005, 12, 1473–1477. [Google Scholar] [CrossRef]
- Sevilla, M.B.; McGwin, G., Jr.; Lad, M.E.; Clark, M.; Yuan, E.L.; Farsiu, S.; Curcio, C.A.; Owsley, C.; Toth, C.A. Relating retinal morphology and function in aging and early to intermediate age-related macular degeneration subjects. Am. J. Ophthalmol. 2016, 165, 65–77. [Google Scholar] [CrossRef]

| Parameters | Early ARMD (n = 7) | Intermediate ARMD (n = 18) | Total (n = 25) |
|---|---|---|---|
| Age (mean ± S.D.) | 61.00 ± 4.21 years | 67.78 ± 5.62 years | 65.96 ± 5.22 years |
| Gender | Number (percentage) | Number (percentage) | Number (percentage) |
| Male | 2 (28.57%) | 12 (66.66%) | 14 (56%) |
| Female | 5 (71.42%) | 6 (33.33%) | 11 (44%) |
| Ethnicity | |||
| Malay | 3 (42.85%) | 5 (27.77%) | 8 (32%) |
| Chinese | 3 (42.85%) | 11 (61.11%) | 14 (56%) |
| Indian | 1 (14.28%) | 2 (11.11%) | 3 (12%) |
| Distance visual acuity (mean ± S.D.) | 0.05 ± 0.04 logMAR | 0.36 ± 0.11 logMAR | 0.28 ± 0.17 logMAR |
| Visual Parameters | Early ARMD Mean/Median ± S.D. | Intermediate ARMD Mean/Median ± S.D. | Combined Early and Intermediate ARMD Mean/Median ± S.D. | Range |
|---|---|---|---|---|
| Mean distance visual acuity (logMAR) | 0.05 ± 0.04 | 0.36 ± 0.11 | 0.28 ± 0.17 | 0 to 0.56 |
| Mean near visual acuity (logMAR) | 0.03 ± 0.05 | 0.32 ± 0.16 | 0.25 ± 0.20 | 0 to 0.6 |
| Mean contrast sensitivity (log contrast unit) | 1.59 ± 0.12 | 1.20 ± 0.24 | 1.30 ± 0.28 | 0.9 to 1.8 |
| Mean reading speed * (wpm) | 178 | 117 | 141 ± 45 | 75 to 206 |
| OCT Parameters Mean ± S.D. (n = 7) | Early ARMD | Intermediate ARMD | Combined Early and Intermediate ARMD | Range |
|---|---|---|---|---|
| Average total retinal thickness (micron) | 262.57 ± 17.33 | 257.36 ± 32.29 | 258.77 ± 28.78 | 205 to 297 |
| Average total retinal volume (mm3) | 0.21 ± 0.01 | 0.20 ± 0.25 | 0.202 ± 0.25 | 0.14 to 0.24 |
| Average outer retinal layers thickness (micron) | 89.28 ± 11.27 | 91.68 ± 7.60 | 91.04 ± 8.56 | 76 to 112 |
| Average outer retinal layers volume (mm3) | 0.06 ± 0.03 | 0.07 ± 0.06 | 0.068 ± 0.005 | 0.06 to 0.08 |
| Average retinal pigment epithelium–Bruch membrane thickness (micron) | 22.00 ± 3.61 | 26.57 ± 4.66 | 25.35 ± 4.80 | 17 to 34 |
| Average retinal pigment epithelium-Bruch membrane volume (mm3) | 0.01 ± 0.001 | 0.02 ± 0.006 | 0.02 ± 0.006 | 0.01 to 0.03 |
| Average outer nuclear layers thickness (micron) | 88.85 ± 3.80 | 70.89 ± 20.28 | 75.73 ± 19.13 | 34 to 102 |
| Average outer nuclear layers volume (mm3) | 0.07 ± 0.00 | 0.06 ± 0.015 | 0.06 ± 0.014 | 0.03 to 0.08 |
| Parameters | Average Outer Nuclear Layers Thickness | Average Outer Nuclear Layers Volume | Average Retinal Pigment Epithelium-Bruch Membrane Thickness | Average Retinal Pigment Epithelium-Bruch Membrane Volume | Average Total Retinal Thickness | Average Total Retinal Volume | Average Outer Retinal Thickness | Average Outer Retinal Volume |
|---|---|---|---|---|---|---|---|---|
| Distance visual acuity | r = −0.57 p = 0.003 | r = −0.58 p = 0.002 | r = 0.55 p = 0.004 | r = 0.31 p = 0.122 | r = −0.19 p = 0.363 | r = −0.27 p = 0.184 | r = −0.21 p = 0.297 | r = −0.05 p = 0.792 |
| Near visual acuity | r = −0.45 p = 0.020 | r = −0.51 p = 0.008 | r = 0.53 p = 0.006 | r = 0.13 p = 0.010 | r = −0.02 p = 0.939 | r = −0.10 p = 0.618 | r = −0.19 p = 0.355 | r = −0.12 p = 0.565 |
| Contrast sensitivity | r = 0.52 p = 0.006 | r = 0.53 p = 0.005 | r = −0.61 p = 0.000 | r = −0.43 p = 0.027 | r = 0.25 p = 0.223 | r = 0.31 p = 0.129 | r = −0.30 p = 0.137 | r = −0.01 p = 0.964 |
| Reading speed * | r = 0.60 p = 0.006 | r = 0.69 p = 0.001 | r = −0.72 p = 0.000 | r = −0.14 p = 0.571 | r = 0.03 p = 0.900 | r = 0.24 p = 0.347 | r = −0.18 p = 0.476 | r = 0.24 p = 0.344 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghoshal, R.; Sharanjeet-Kaur, S.; Mohamad Fadzil, N.; Mutalib, H.A.; Ghosh, S.; Ngah, N.F.; Abd Aziz, R.A. Correlation between Visual Functions and Retinal Morphology in Eyes with Early and Intermediate Age-Related Macular Degeneration. Int. J. Environ. Res. Public Health 2020, 17, 6379. https://doi.org/10.3390/ijerph17176379
Ghoshal R, Sharanjeet-Kaur S, Mohamad Fadzil N, Mutalib HA, Ghosh S, Ngah NF, Abd Aziz RA. Correlation between Visual Functions and Retinal Morphology in Eyes with Early and Intermediate Age-Related Macular Degeneration. International Journal of Environmental Research and Public Health. 2020; 17(17):6379. https://doi.org/10.3390/ijerph17176379
Chicago/Turabian StyleGhoshal, Rituparna, Sharanjeet Sharanjeet-Kaur, Norliza Mohamad Fadzil, Haliza Abdul Mutalib, Somnath Ghosh, Nor Fariza Ngah, and Roslin Azni Abd Aziz. 2020. "Correlation between Visual Functions and Retinal Morphology in Eyes with Early and Intermediate Age-Related Macular Degeneration" International Journal of Environmental Research and Public Health 17, no. 17: 6379. https://doi.org/10.3390/ijerph17176379
APA StyleGhoshal, R., Sharanjeet-Kaur, S., Mohamad Fadzil, N., Mutalib, H. A., Ghosh, S., Ngah, N. F., & Abd Aziz, R. A. (2020). Correlation between Visual Functions and Retinal Morphology in Eyes with Early and Intermediate Age-Related Macular Degeneration. International Journal of Environmental Research and Public Health, 17(17), 6379. https://doi.org/10.3390/ijerph17176379




