Food Intake REstriction for Health OUtcome Support and Education (FIREHOUSE) Protocol: A Randomized Clinical Trial
Abstract
:1. Introduction
2. Methods
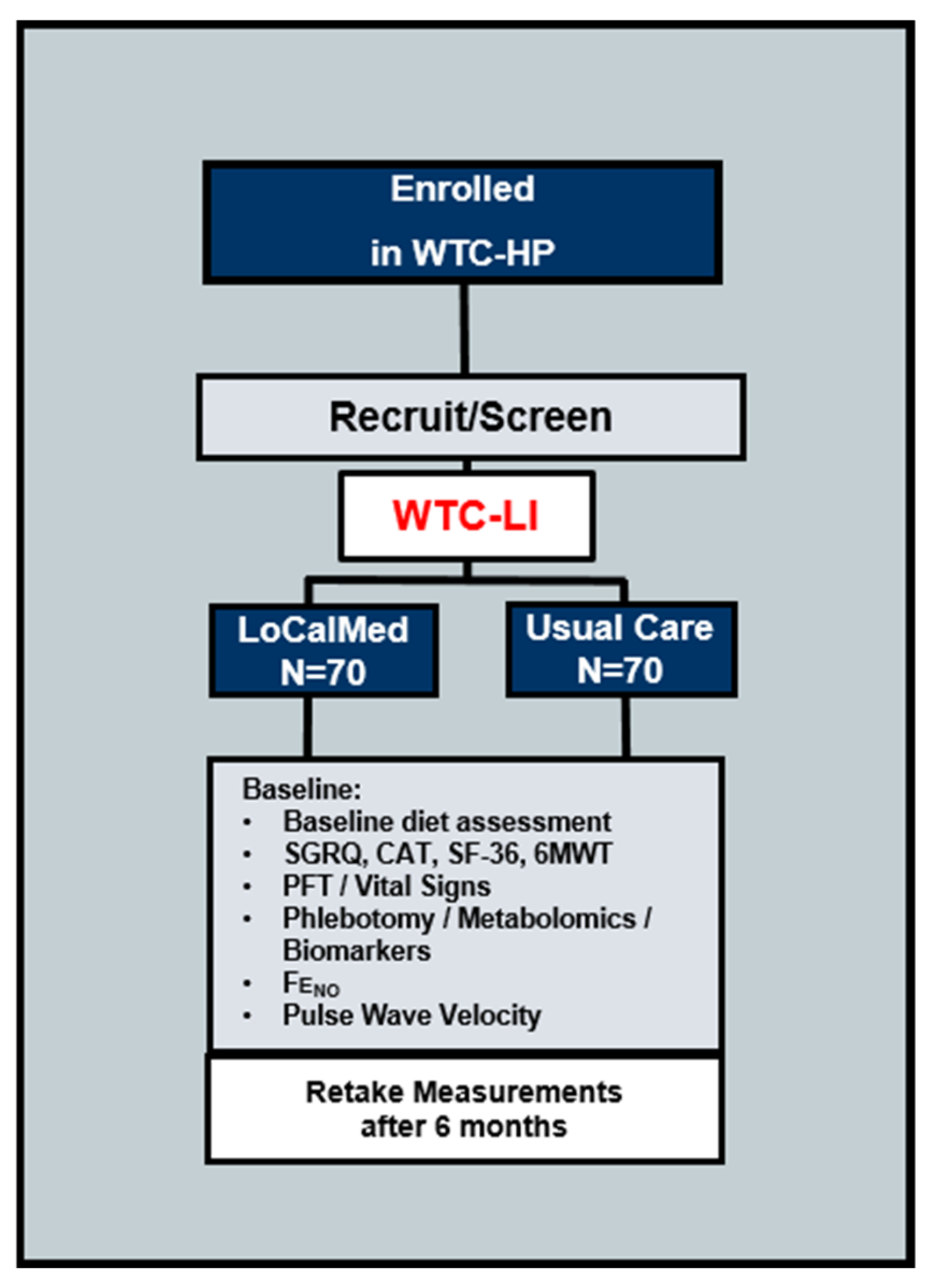
2.1. Design and Setting of the Study
2.1.1. Participant Characteristics
2.1.2. FIREHOUSE Intervention Usual Care Group
Mobile Self-Monitoring
Education and Behavioral Counseling
2.2. Statistical Analysis
Sample Size and Interim Analysis
2.3. COVID-19 Pandemic Precautions
3. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Adverse Event/Adverse Experience |
| BIA | Bioelectric Impedance Analysis |
| BMI | Body Mass Index (kg/m2) |
| CFR | Code of Federal Regulations |
| COPD | Chronic Obstructive Pulmonary Disease |
| COVID-19 | Coronavirus Disease-2019 |
| CSOC | Clinical Study Oversight Committee |
| CTSI-CRC | Clinical and Translational Science Institute-Clinical Research Center |
| DHHS | Department of Health and Human Services |
| DSMB | Data and Safety Monitoring Board |
| ECG | Electrocardiogram |
| FDNY | Fire Department of New York |
| FeNO | Fractional Exhaled Nitric Oxide |
| FEV1 | Forced Expiratory Volume in 1 second |
| FFR | Federal Financial Report |
| FFQ | Food Frequency Questionnaire |
| FIREHOUSE | Food Intake REstriction for Health OUtcome Support and Education |
| FVC | Forced Vital Capacity |
| FWA | Federal Wide Assurance |
| GCP | Good Clinical Practice |
| HIPAA | Health Insurance Portability and Accountability Act |
| HP | Health Program |
| ICF | Informed Consent Form |
| ICH | International Conference on Harmonization |
| IRB | Internal Review Board |
| LLN | Lower Limit of Normal |
| LoCalMed | Low Calorie Mediterranean Diet |
| MetSyn | Metabolic Syndrome |
| MOP | Manual of Procedures |
| MND | MyNetDiary |
| N | Number (typically refers to number of participants) |
| NIH | National Institutes of Health |
| NYU | New York University |
| OAD | Obstructive Airways Disease |
| OHRP | Office of Human Research Protections |
| OHSR | Office of Human Research Subjects |
| PI | Principal Investigator |
| PID | Participant ID Number |
| PPE | Personal Protective Equipment |
| PM | Particulate Matter |
| PWV | Pulse Wave Velocity |
| RCT | Randomized Clinical Trial |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| SF-36 | Short Form 36 |
| SGRQ | St. George’s Respiratory Questionnaire |
| SOP | Standard Operating Procedure |
| US | United States |
| WTC | World Trade Center |
| 9/11 | 11 September 2001 |
References
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and Management of the Metabolic Syndrome. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [Green Version]
- Kwon, S.; Crowley, G.; Caraher, E.J.; Haider, S.H.; Lam, R.; Veerappan, A.; Yang, L.; Liu, M.; Zeig-Owens, R.; Schwartz, T.M.; et al. Validation of Predictive Metabolic Syndrome Biomarkers of World Trade Center Lung Injury. Chest 2019, 156, 486–496. [Google Scholar] [CrossRef]
- Chen, J.-C.; Schwartz, J. Metabolic Syndrome and Inflammatory Responses to Long-Term Particulate Air Pollutants. Environ. Health Perspect. 2008, 116, 612–617. [Google Scholar] [CrossRef] [Green Version]
- Naveed, B.; Weiden, M.D.; Kwon, S.; Gracely, E.J.; Comfort, A.L.; Ferrier, N.; Kasturiarachchi, K.J.; Cohen, H.; Aldrich, T.K.; Rom, W.N.; et al. Metabolic Syndrome Biomarkers Predict Lung Function Impairment. Am. J. Respir. Crit. Care Med. 2012, 185, 392–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varraso, R.; Fung, T.T.; Barr, R.G.; Hu, F.B.; Willett, W.; Camargo, C.A. Prospective study of dietary patterns and chronic obstructive pulmonary disease among US women. Am. J. Clin. Nutr. 2007, 86, 488–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varraso, R.; Fung, T.T.; Hu, F.B.; Willett, W.; Camargo, C.A. Prospective study of dietary patterns and chronic obstructive pulmonary disease among US men. Thorax 2007, 62, 786–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webber, M.P.; Yip, J.; Zeig-Owens, R.; Moir, W.; Ungprasert, P.; Crowson, C.S.; Hall, C.B.; Jaber, N.; Weiden, M.D.; Matteson, E.L.; et al. Post-9/11 sarcoidosis in WTC-exposed firefighters and emergency medical service workers. Respir. Med. 2017, 132, 232–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, N.P.; Park, S.; Anh, N.H.; Nghi, T.D.; Yoon, S.J.; Park, J.H.; Lim, J.; Kwon, S.W. High-Throughput Omics and Statistical Learning Integration for the Discovery and Validation of Novel Diagnostic Signatures in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 296. [Google Scholar] [CrossRef] [Green Version]
- Weiden, M.D.; Ferrier, N.; Nolan, A.; Rom, W.N.; Comfort, A.; Gustave, J.; Zeig-Owens, R.; Zheng, S.; Goldring, R.M.; Berger, K.I.; et al. Obstructive airways disease with air trapping among firefighters exposed to World Trade Center dust. Chest 2009, 137, 566–574. [Google Scholar] [CrossRef] [Green Version]
- Weiden, M.D.; Naveed, B.; Kwon, S.; Cho, S.J.; Comfort, A.L.; Prezant, D.J.; Rom, W.N.; Nolan, A. Cardiovascular biomarkers predict susceptibility to lung injury in World Trade Center dust-exposed firefighters. Eur. Respir. J. 2012, 41, 1023–1030. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, F.-C.; Wu, C.-Z.; Su, S.-C.; Sun, M.-T.; Hsieh, C.-H.; Hung, Y.-J.; He, C.-T.; Pei, D. Baseline forced expiratory volume in the first second as an independent predictor of development of the metabolic syndrome. Metabolism 2010, 59, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Lipovec, N.C.; Beijers, R.J.; Borst, B.V.D.; Doehner, W.; Lainscak, M.; Schols, A.M. The Prevalence of Metabolic Syndrome in Chronic Obstructive Pulmonary Disease: A Systematic Review. COPD J. Chronic Obstr. Pulm. Dis. 2016, 13, 399–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Wang, H.; Zuo, Y.; Farmer, S.R. Functional Interaction between Peroxisome Proliferator-Activated Receptor γ and β-Catenin. Mol. Cell. Boil. 2006, 26, 5827–5837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, E.S.; Giles, W.H.; Dietz, W.H. Prevalence of the metabolic syndrome among US adults: Findings from the third National Health and Nutrition Examination Survey. JAMA 2002, 287, 356–359. [Google Scholar] [CrossRef]
- Gan, W.Q.; Man, S.F.P.; Senthilselvan, A.; Sin, D.D. Association between chronic obstructive pulmonary disease and systemic inflammation: A systematic review and a meta-analysis. Thorax 2004, 59, 574–580. [Google Scholar] [CrossRef] [Green Version]
- Fiordelisi, A.; Piscitelli, P.; Trimarco, B.; Coscioni, E.; Iaccarino, G.; Sorriento, D. The mechanisms of air pollution and particulate matter in cardiovascular diseases. Heart Fail. Rev. 2017, 22, 337–347. [Google Scholar] [CrossRef]
- Braun, S. The Link between the Metabolic Syndrome and Cancer. Int. J. Boil. Sci. 2011, 7, 1003–1015. [Google Scholar] [CrossRef]
- Leone, N.; Courbon, D.; Thomas, F.; Bean, K.; Jégo, B.; Leynaert, B.; Guize, L.; Zureik, M. Lung Function Impairment and Metabolic Syndrome. Am. J. Respir. Crit. Care Med. 2009, 179, 509–516. [Google Scholar] [CrossRef] [Green Version]
- McClean, K.M.; Kee, F.; Young, I.S.; Elborn, J. Obesity and the lung: 1 {middle dot} Epidemiology. Thorax 2008, 63, 649–654. [Google Scholar] [CrossRef] [Green Version]
- Shai, I.; Schwarzfuchs, D.; Henkin, Y.; Shahar, D.R.; Witkow, S.; Greenberg, I.; Golan, R.; Fraser, D.; Bolotin, A.; Vardi, H.; et al. Weight Loss with a Low-Carbohydrate, Mediterranean, or Low-Fat Diet. N. Engl. J. Med. 2008, 359, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Kwon, S.; Crowley, G.; Mikhail, M.; Nolan, A.; Clementi, E.A.; Zeig-Owens, R.; Schwartz, T.; Liu, M.; Prezant, D.J.; Nolan, A. Metabolic Syndrome Biomarkers of World Trade Center Airway Hyperreactivity: A 16-Year Prospective Cohort Study. Int. J. Environ. Res. Public Health 2019, 16, 1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowley, G.; Kwon, S.; Haider, S.H.; Caraher, E.J.; Lam, R.; St-Jules, D.E.; Liu, M.; Prezant, D.J.; Nolan, A. Metabolomics of World Trade Center-Lung Injury: A machine learning approach. BMJ Open Respir. Res. 2018, 5, e000274. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J. Role of polyunsaturated fatty acids in lung disease. Am. J. Clin. Nutr. 2000, 71, 393S–396S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seegmiller, A.C. Abnormal Unsaturated Fatty Acid Metabolism in Cystic Fibrosis: Biochemical Mechanisms and Clinical Implications. Int. J. Mol. Sci. 2014, 15, 16083–16099. [Google Scholar] [CrossRef] [Green Version]
- Angelillo, V.A.; Bedi, S.; Durfee, D.; Dahl, J.; Patterson, A.J.; O’Donohue, W.J. Effects of Low and High Carbohydrate Feedings in Ambulatory Patients with Chronic Obstructive Pulmonary Disease and Chronic Hypercapnia. Ann. Intern. Med. 1985, 103, 883. [Google Scholar] [CrossRef]
- McDonald, V.M.; Gibson, P.G.; Scott, H.A.; Baines, P.J.; Hensley, M.J.; Pretto, J.J.; Wood, L.G. Should we treat obesity in COPD? The effects of diet and resistance exercise training. Respirology 2016. [Google Scholar] [CrossRef]
- Aaron, S.D.; Fergusson, D.; Dent, R.; Chen, Y.; Vandemheen, K.L.; Dales, R.E. Effect of weight reduction on respiratory function and airway reactivity in obese women. Chest 2004, 125, 2046–2052. [Google Scholar] [CrossRef] [Green Version]
- Hakala, K.; Stenius-Aarniala, B.; Sovijarvi, A. Effects of weight loss on peak flow variability, airways obstruction, and lung volumes in obese patients with asthma. Chest 2000, 118, 1315–1321. [Google Scholar] [CrossRef] [Green Version]
- Sevick, M.A.; Woolf, K.; Mattoo, A.; Katz, S.D.; Li, H.; St-Jules, D.E.; Jagannathan, R.; Hu, L.; Pompeii, M.L.; Ganguzza, L.; et al. The Healthy Hearts and Kidneys (HHK) study: Design of a 2 × 2 RCT of technology-supported self-monitoring and social cognitive theory-based counseling to engage overweight people with diabetes and chronic kidney disease in multiple lifestyle changes. Contemp. Clin. Trials 2017, 64, 265–273. [Google Scholar] [CrossRef]
- Torbjornsen, A.; Jenum, A.K.; Cvancarova, M.; Årsand, E.; Holmen, H.; Wahl, A.K.; Ribu, L.; Quinlan, L.; Pal, K.; Fischer, F. A Low-Intensity Mobile Health Intervention With and Without Health Counseling for Persons With Type 2 Diabetes, Part 1: Baseline and Short-Term Results From a Randomized Controlled Trial in the Norwegian Part of RENEWING HEALTH. JMIR mHealth uHealth 2014, 2, e52. [Google Scholar] [CrossRef]
- Defining Adult Overweight and Obesity 2020. Available online: https://www.cdc.gov/obesity/adult/defining.html (accessed on 4 September 2020).
- Garrow, J.S.; Webster, J. Quetelet’s index (W/H2) as a measure of fatness. Int. J. Obes. 1985, 9, 147–153. [Google Scholar]
- Lawlor, D.A.; Benfield, L.; Logue, J.; Tilling, K.; Howe, L.D.; Fraser, A.; Cherry, L.; Watt, P.; Ness, A.R.; Smith, G.D.; et al. Association between general and central adiposity in childhood, and change in these, with cardiovascular risk factors in adolescence: Prospective cohort study. BMJ 2010, 341, c6224. [Google Scholar] [CrossRef] [Green Version]
- Flegal, K.M.; Graubard, B.I. Estimates of excess deaths associated with body mass index and other anthropometric variables. Am. J. Clin. Nutr. 2009, 89, 1213–1219. [Google Scholar] [CrossRef]
- Willett, K.; Jiang, R.; Lenart, E.; Spiegelman, N.; Willett, W. Comparison of Bioelectrical Impedance and BMI in Predicting Obesity-Related Medical Conditions*. Obesity 2006, 14, 480–490. [Google Scholar] [CrossRef] [Green Version]
- Thomas, D.M.; Martin, C.K.; Heymsfield, S.; Redman, L.M.; Schoeller, D.A.; Levine, J.A. A simple model predicting individual weight change in humans. J. Boil. Dyn. 2011, 5, 579–599. [Google Scholar] [CrossRef] [Green Version]
- Rejeski, W.J.; Brubaker, P.H.; Goff, D.C.; Bearon, L.B.; McClelland, J.W.; Perri, M.G.; Ambrosius, W.T. Translating weight loss and physical activity programs into the community to preserve mobility in older, obese adults in poor cardiovascular health. Arch. Intern. Med. 2011, 171, 880–886. [Google Scholar] [CrossRef]
- Johns, D.J.; Hartmann-Boyce, J.; Jebb, S.A.; Aveyard, P. Behavioural Weight Management Review Group Diet or exercise interventions vs combined behavioral weight management programs: A systematic review and meta-analysis of direct comparisons. J. Acad. Nutr. Diet. 2014, 114, 1557–1568. [Google Scholar] [CrossRef] [Green Version]
- Johns Hopkins University. Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. 2020. Available online: https://coronavirus.jhu.edu/map.html (accessed on 4 September 2020).
- DOH, N. Available online: https://www1.nyc.gov/site/doh/covid/covid-19-data.page (accessed on 4 September 2020).
- Gao, Y.; Qiu, H.-B.; Zhou, S.; Wang, Z.-N.; Zhang, J.-C.; Zhang, Z.-L.; Qian, Z.-X.; Wang, H.-B.; Yu, S.-H.; Luo, Y.-F.; et al. Accumulated Clinical Experiences from Successful Treatment of 1377 Severe and Critically Ill COVID-19 Cases. Curr. Med Sci. 2020. [Google Scholar] [CrossRef]
- Liang, W.-H.; Guan, W.-J.; Li, C.-C.; Li, Y.-M.; Liang, H.-R.; Zhao, Y.; Liu, X.-Q.; Sang, L.; Chen, R.-C.; Tang, C.-L.; et al. Clinical characteristics and outcomes of hospitalised patients with COVID-19 treated in Hubei (epicentre) and outside Hubei (non-epicentre): A nationwide analysis of China. Eur. Respir. J. 2020. [Google Scholar] [CrossRef] [Green Version]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, X.; Yang, P.; Zhang, S. COVID-19 Complicated by Acute Pulmonary Embolism. Radiol. Cardiothorac. Imaging 2020, 2, e200067. [Google Scholar] [CrossRef] [Green Version]
- Onder, G.; Rezza, G.; Brusaferro, S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA 2020. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, L.; Liu, L.; Zhao, X.; Zhang, Z.; Xue, L.; Yan, X.; Huang, S.; Li, Y.; Cheng, J.; et al. Overweight and obesity are risks factors of severe illness in patients with COVID-19. Obesity 2020. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Arentz, M.; Yim, E.; Klaff, L.; Lokhandwala, S.; Riedo, F.X.; Chong, M.; Lee, M. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA 2020, 323, 1612. [Google Scholar] [CrossRef] [Green Version]
- Kislinger, T.; Fu, C.; Huber, B.; Qu, W.; Taguchi, A.; Du Yan, S.; Hofmann, M.; Yan, S.-F.; Pischetsrieder, M.; Stern, D.; et al. N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J. Boil. Chem. 1999, 274, 31740–31749. [Google Scholar] [CrossRef] [Green Version]
- Semba, R.D.; Sun, K.; Schwartz, A.V.; Varadhan, R.; Harris, T.B.; Satterfield, S.; Garcia, M.; Ferrucci, L.; Newman, A.B. Health ABC Study Serum carboxymethyl-lysine, an advanced glycation end product, is associated with arterial stiffness in older adults. J. Hypertens. 2015, 33, 797–803. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.-L.; Cheng, H.-M.; Sung, S.-H.; Chuang, S.-Y.; Li, C.-H.; Spurgeon, H.A.; Ting, C.-T.; Najjar, S.S.; Lakatta, E.G.; Yin, F.C.; et al. Wave reflection and arterial stiffness in the prediction of 15-year all-cause and cardiovascular mortalities: A community-based study. Hypertens. 2010, 55, 799–805. [Google Scholar] [CrossRef] [Green Version]
- Heier, M.; Margeirsdottir, H.D.; Gaarder, M.; Stensæth, K.H.; Brunborg, C.; Torjesen, P.A.; Seljeflot, I.; Hanssen, K.F.; Dahl-Jørgensen, K. Soluble RAGE and atherosclerosis in youth with type 1 diabetes: A 5-year follow-up study. Cardiovasc. Diabetol. 2015, 14, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Jaisson, S.; Souchon, P.-F.; Desmons, A.; Salmon, A.-S.; Delemer, B.; Gillery, P. Early Formation of Serum Advanced Glycation End-Products in Children with Type 1 Diabetes Mellitus: Relationship with Glycemic Control. J. Pediatr. 2016, 172, 56–62. [Google Scholar] [CrossRef]
- Petersen, K.S.; Blanch, N.; Keogh, J.; Clifton, P. Weight Loss, Dietary Intake and Pulse Wave Velocity. Pulse 2015, 3, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Petersen, K.S.; Blanch, N.; Keogh, J.B.; Clifton, P.M. Effect of Weight Loss on Pulse Wave Velocity. Arter. Thromb. Vasc. Boil. 2015, 35, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Pase, M.; Grima, N.A.; Sarris, J. Do long-chain n-3 fatty acids reduce arterial stiffness? A meta-analysis of randomised controlled trials. Br. J. Nutr. 2011, 106, 974–980. [Google Scholar] [CrossRef] [Green Version]
- Pase, M.; Grima, N.A.; Sarris, J. The effects of dietary and nutrient interventions on arterial stiffness: A systematic review. Am. J. Clin. Nutr. 2010, 93, 446–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Roisin, R.; Drakulovic, M.; Rodriguez, D.A.; Roca, J.; Barberà, J.A.; Wagner, P.D. Ventilation-perfusion imbalance and chronic obstructive pulmonary disease staging severity. J. Appl. Physiol. 2009, 106, 1902–1908. [Google Scholar] [CrossRef] [PubMed]
- Liebow, A.A. Pulmonary emphysema with special reference to vascular changes. Am. Rev. Respir. Dis. 1959, 80, 67–93. [Google Scholar] [PubMed]
- Tsukiji, J.; Cho, S.J.; Echevarria, G.C.; Kwon, S.; Joseph, P.; Schenck, E.J.; Naveed, B.; Prezant, D.J.; Rom, W.N.; Schmidt, A.M.; et al. Lysophosphatidic acid and apolipoprotein A1 predict increased risk of developing World Trade Center-lung injury: A nested case-control study. Biomarkers 2014, 19, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Maziak, W.; Loukides, S.; Culpitt, S.; Sullivan, P.; Kharitonov, S.A.; Barnes, P.J. Exhaled Nitric Oxide in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 1998, 157, 998–1002. [Google Scholar] [CrossRef] [Green Version]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Male sex, over age 21 years at enrollment | Pre-existing conditions including (and not necessarily limited to) active cancer, severe heart disease, significant cognitive impairment, eating disorders, significant psychiatric illness, end-stage COPD, severe pulmonary HTN, or organ transplant |
| FDNY rescue and recovery worker | Concomitant use of interfering medication(s) or devices within one month prior to enrollment |
| Documented WTC exposure | Severe GI illness that would prevent diet adherence |
| Enrolled in the FDNY WTC health program | Severe kidney disease requiring dialysis |
| Willing and able to consent for themselves to study enrollment | Severe liver disease requiring frequent medical intervention |
| Willing and able to participate in study procedures, to modify their diet and activity level | Participation in other diet modification studies |
| Able to perform ADLs independently | High-dose steroid (>20 mg prednisone or equivalent) or other hormonal treatments or chemotherapy within one month |
| Light duty or retired FDNY firefighters | Life expectancy < 6-months |
| FEV1 less than LLN of predicted at any time post 9/11 | Recent significant weight loss > five percent TBW within one month |
| Spirometry within the last 36- months, and at post-9/11 visit | Significant alcohol use |
| BMI > 27 kg/m2 and < 50 kg/m2 | |
| Able to demonstrate minimal proficiency using a smart phone | |
| Have means to accommodate transportation to/from in-person visits |
| Enrollment | Pre-Randomization Baseline | Post-Randomization | Close-Out | ||
|---|---|---|---|---|---|
| TIMEPOINT (Visit) | 0 | 1 | T/N | 2 | F/U |
| ENROLLMENT | |||||
| Eligibility screen | x | ||||
| Informed consent | x | ||||
| INTERVENTIONS | |||||
| LoCalMed | x | x | |||
| Usual Care | x | ||||
| ASSESSMENTS | |||||
| Physical exam | x | x | |||
| Phlebotomy | x | x | |||
| EKG/PWV | |||||
| FeNO | x | x | |||
| Spirometry | x | x | |||
| Genome | x | x | |||
| Microbiome | |||||
| Questionnaires | x | x | x | ||
| INSTRUCTIVE COMPONENTS | |||||
| Technology training | x | x | |||
| Nutrition consultation | x | x | |||
| Week | Education Materials (Videos) | Social Cognitive Theory (Coaching) |
|---|---|---|
| 1 | Introduction to the FIREHOUSE study. | Goals for life. |
| 2 | Self-monitoring of diet and physical activity—making sense of the numbers. | Where am I? Establishing the relevance of behavior change. |
| 3 | Being a Calorie Detective. Portion control and empty calories. | Setting goals. |
| 4 | Introducing physical activity into your life. Finding time for fitness. Exercise safety. | Self-Reward. Turning goals into habits. |
| 6 | Being a Fat Detective. Healthy and unhealthy fats, the contribution of fat to total calorie intake. | Social support. Developing and working your social support network. |
| 8 | Building duration and intensity of aerobic exercise. | Problem solving: Barriers and setbacks. Introduction to the problem-solving model. |
| 10 | Changing seasons, special occasions, life events, and eating at restaurants. | Problem solving: Behavioral triggers and stimulus control. |
| 12 | The role of sleep and stress in weight gain and loss. | Problem solving: Stress management. |
| 14 | Adding color and fiber to your diet. | Problem solving: Emotional eating. |
| 16 | The role of breakfast and meal frequency in weight loss success. | Problem solving: Eliminating negative self-talk. |
| 18 | Snacking and sugar-sweetened beverages. Empty calories. | Problem solving: Food cravings, addictions, and habitual over-eating. |
| 20 | Building muscles with strength training. | Problem solving: Anticipating high-risk situations. |
| 22 | Weight loss plateaus. | Problem solving: Lapse and relapse. |
| 24 | Putting it all together; review of lifestyle recommendations. | Problem solving: Coping with lapses and setting new goals. |
| Outcome Measure | Description | |
|---|---|---|
| Primary Endpoint | Body mass index (BMI) in kg/m2 | Body mass divided by square of individual’s heights, with attempt to quantify and standardize amount of tissue mass across persons |
| Secondary Endpoints | FEV1 | Usual spirometric technique with reproducibility and acceptability based on ATS/ERS guidelines. Allows best correlation with symptoms and pulmonary function |
| Bioelectrical impedance analysis | InBody270 BIA scale. Measure lean body mass and total body fat percentage | |
| St. George’s Respiratory Questionnaire | Standardized/validated airways disease-specific survey to assess symptoms, activity hindrance, and overall impact | |
| Short Form 36 | Standardized/validated general health survey to assess mental, emotional, and functional health status | |
| Pulse wave velocity | SphygmoCor (Atcor Medical) by carotid–femoral pulse discretion to measure vascular stiffness | |
| FeNO | NIOX VERO portable, to assess airway inflammation | |
| Vital Signs | BP, HR, RR, body temp, neck/waist/hip circumferences | |
| Electrocardiogram | Standard 12-lead ECG to assess axis changes | |
| Phlebotomy | Routine cell counts, metabolic panels, lipid panel. Possible further analysis of metabolomic fingerprints | |
| Exploratory Endpoints | Microbiome | GenoTek Oragene-Gut personal stool collection kit |
| Genome | DNA GenoTek Oragene-Discover saliva collection kit |
| Interim Analysis | Completion of Subjects/Group | Critical Value Z ± | p Value |
|---|---|---|---|
| 1 | 30 | 3.951 | 0.000 |
| 2 | 50 | 2.686 | 0.007 |
| 3 | 70 | 2.129 | 0.033 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, S.; Riggs, J.; Crowley, G.; Lam, R.; Young, I.R.; Nayar, C.; Sunseri, M.; Mikhail, M.; Ostrofsky, D.; Veerappan, A.; et al. Food Intake REstriction for Health OUtcome Support and Education (FIREHOUSE) Protocol: A Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2020, 17, 6569. https://doi.org/10.3390/ijerph17186569
Kwon S, Riggs J, Crowley G, Lam R, Young IR, Nayar C, Sunseri M, Mikhail M, Ostrofsky D, Veerappan A, et al. Food Intake REstriction for Health OUtcome Support and Education (FIREHOUSE) Protocol: A Randomized Clinical Trial. International Journal of Environmental Research and Public Health. 2020; 17(18):6569. https://doi.org/10.3390/ijerph17186569
Chicago/Turabian StyleKwon, Sophia, Jessica Riggs, George Crowley, Rachel Lam, Isabel R. Young, Christine Nayar, Maria Sunseri, Mena Mikhail, Dean Ostrofsky, Arul Veerappan, and et al. 2020. "Food Intake REstriction for Health OUtcome Support and Education (FIREHOUSE) Protocol: A Randomized Clinical Trial" International Journal of Environmental Research and Public Health 17, no. 18: 6569. https://doi.org/10.3390/ijerph17186569
APA StyleKwon, S., Riggs, J., Crowley, G., Lam, R., Young, I. R., Nayar, C., Sunseri, M., Mikhail, M., Ostrofsky, D., Veerappan, A., Zeig-Owens, R., Schwartz, T., Colbeth, H., Liu, M., Pompeii, M. L., St-Jules, D., Prezant, D. J., Sevick, M. A., & Nolan, A. (2020). Food Intake REstriction for Health OUtcome Support and Education (FIREHOUSE) Protocol: A Randomized Clinical Trial. International Journal of Environmental Research and Public Health, 17(18), 6569. https://doi.org/10.3390/ijerph17186569





