Organophosphorus Flame Retardants: A Global Review of Indoor Contamination and Human Exposure in Europe and Epidemiological Evidence
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
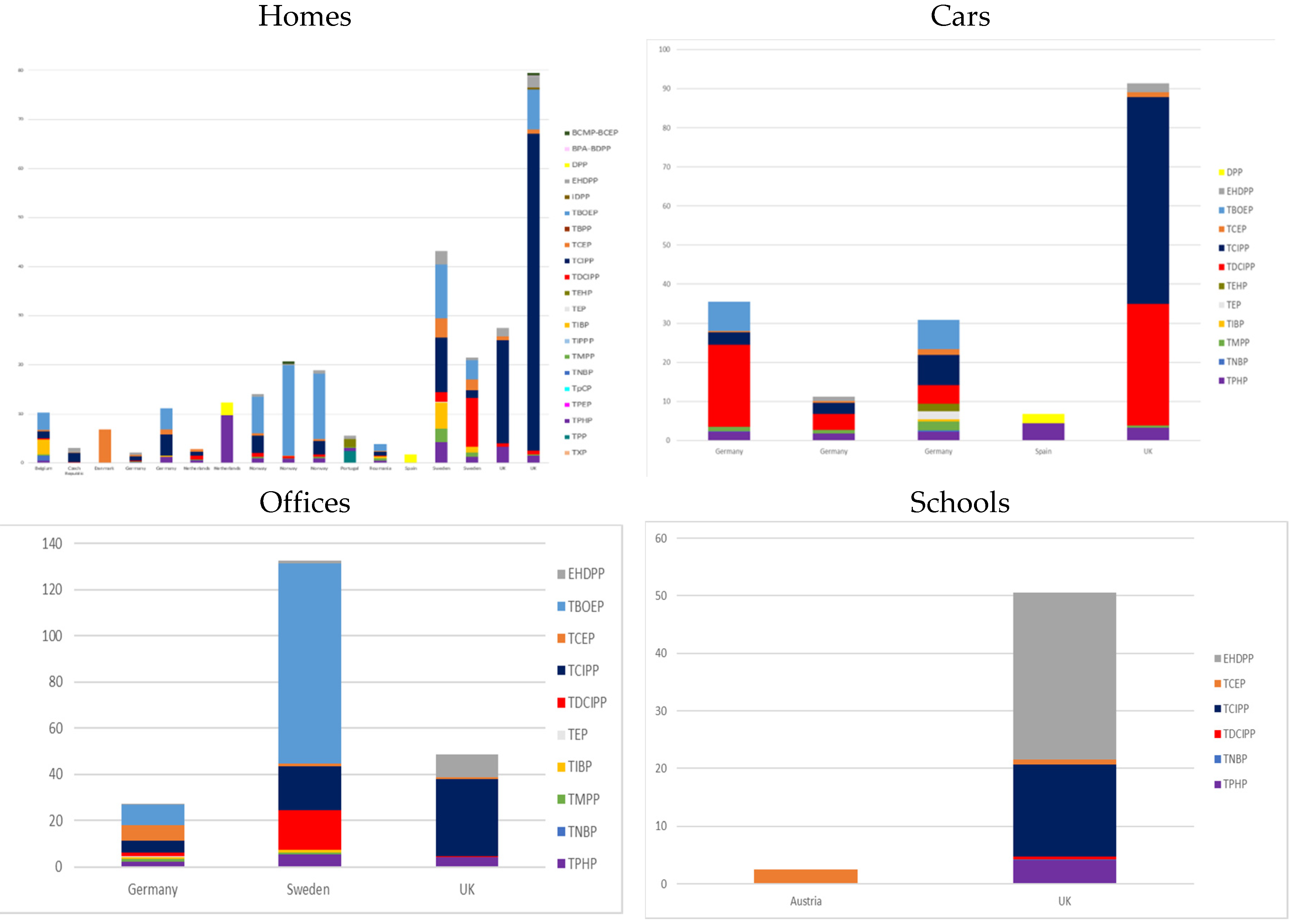
3.1. Indoor Contamination
3.1.1. Dust
3.1.2. Air
3.2. Human Exposure to OPFRs in Europe
3.2.1. Intake Estimation
3.2.2. Human Biological Measurements of Exposure
3.3. Epidemiological Associations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kemmlein, S.; Hahn, O.; Jann, O. Emissions of organophosphate and brominated flame retardants from selected consumer products and building materials. Atmos. Environ. 2003, 37, 5485–5493. [Google Scholar] [CrossRef]
- European Flame Retardant Association. Frequently Asked Questions on Flame Retardants. Available online: https://docplayer.net/11069706-Flame-retardants-frequently-asked-questions-the-european-flame-retardants-association.html (accessed on 10 July 2020).
- Building Evidence for Health. Available online: https://buildingevidence.forhealth.org/wp-content/uploads/sites/2/2017/05/ForHealth.org_FlameRetardants.pdf (accessed on 10 July 2020).
- Blum, A.; Behl, M.; Birnbaum, L.S.; Diamond, M.L.; Phillips, A.; Singla, V.; Sipes, N.S.; Stapleton, H.M.; Venier, M. Organophosphate Ester Flame Retardants: Are They a Regrettable Substitution for Polybrominated Diphenyl Ethers? Environ. Sci. Technol. Lett. 2019, 6, 638–649. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EU) 2017/227 of 9 February 2017 Amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as Regards Bis(Pentabromophenyl)Ether. Available online: http://data.europa.eu/eli/reg/2017/227/oj (accessed on 12 September 2020).
- European Union Comission Directive. Directive 2014/79/EU. Available online: https://eur-lex.europa.eu/legal-content/FR/TXT/HTML/?uri=CELEX:32014L0079 (accessed on 12 September 2020).
- European Union Comission Directive. Directive 2003/11/EC of the European Parliament and of the Council of 6 February 2003 Amending for the 24th time Council Directive 76/769/EEC Relating to Restrictions on the Marketing and Use of Certain Dangerous Substances and Preparations (Pentabromodiphenyl Ether, Octabromodiphenyl Ether). Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:042:0045:0046:EN:PDF (accessed on 3 June 2019).
- Stockholm Convention, UNEP/POPS/POPRC.4/18 Listing of Tetrabromodiphenyl Ether and Pentabromodiphenyl Ether. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiOuZmNqurrAhVFzKQKHZseBscQFjAAegQIBRAB&url=http%3A%2F%2Fchm.pops.int%2FPortals%2F0%2Fdownload.aspx%3Fd%3DUNEP-POPS-COP.4-SC-4-18.English.pdf&usg=AOvVaw0DuNRdjspaLgaK481RHSln (accessed on 14 September 2020).
- Covaci, A.; Harrad, S.; Abdallah, M.A.-E.; Ali, N.; Law, R.J.; Herzke, D.; de Wit, C.A. Novel brominated flame retardants: A review of their analysis, environmental fate and behaviour. Environ. Int. 2011, 37, 532–556. [Google Scholar] [CrossRef] [PubMed]
- Van der Veen, I.; de Boer, J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 2012, 88, 1119–1153. [Google Scholar] [CrossRef] [PubMed]
- Pinfa. Non-Halogenated Phosphorus, Inorganic and Nitrogen Flame Retardants 2017. Available online: https://www.pinfa.eu/wp-content/uploads/2018/05/pinfa_BC_edit-2017-web.pdf (accessed on 10 July 2020).
- Andresen, J.A.; Grundmann, A.; Bester, K. Organophosphorus flame retardants and plasticisers in surface waters. Sci. Total Environ. 2004, 332, 155–166. [Google Scholar] [CrossRef]
- Mendelsohn, E.; Hagopian, A.; Hoffman, K.; Butt, C.M.; Lorenzo, A.; Congleton, J.; Webster, T.F.; Stapleton, H.M. Nail polish as a source of exposure to triphenyl phosphate. Environ. Int. 2016, 86, 45–51. [Google Scholar] [CrossRef]
- Marklund, A.; Andersson, B.; Haglund, P. Traffic as a Source of Organophosphorus Flame Retardants and Plasticizers in Snow. Environ. Sci. Technol. 2005, 39, 3555–3562. [Google Scholar] [CrossRef]
- Schindler, B.K.; Weiss, T.; Schütze, A.; Koslitz, S.; Broding, H.C.; Bünger, J.; Brüning, T. Occupational exposure of air crews to tricresyl phosphate isomers and organophosphate flame retardants after fume events. Archiv. Toxicol. 2013, 87, 645–648. [Google Scholar] [CrossRef]
- Kojima, H.; Takeuchi, S.; Itoh, T.; Iida, M.; Kobayashi, S.; Yoshida, T. In vitro endocrine disruption potential of organophosphate flame retardants via human nuclear receptors. Toxicology 2013, 314, 76–83. [Google Scholar] [CrossRef]
- Behl, M.; Hsieh, J.-H.; Shafer, T.J.; Mundy, W.R.; Rice, J.R.; Boyd, W.A.; Freedman, J.H.; Hunter, E.S.; Jarema, K.A.; Padilla, S.; et al. Use of alternative assays to identify and prioritize organophosphorus flame retardants for potential developmental and neurotoxicity. Neurotoxicol. Teratol. 2015, 52, 181–193. [Google Scholar] [CrossRef]
- Langer, S.; Fredricsson, M.; Weschler, C.J.; Bekö, G.; Strandberg, B.; Remberger, M.; Toftum, J.; Clausen, G. Organophosphate esters in dust samples collected from Danish homes and daycare centers. Chemosphere 2016, 154, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Bergh, C.; Torgrip, R.; Emenius, G.; Östman, C. Organophosphate and phthalate esters in air and settled dust—A multi-location indoor study: Organophosphate and phthalate esters in air and settled dust. Indoor Air 2011, 21, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Luongo, G.; Östman, C. Organophosphate and phthalate esters in settled dust from apartment buildings in Stockholm. Indoor Air 2016, 26, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Fromme, H.; Lahrz, T.; Kraft, M.; Fembacher, L.; Mach, C.; Dietrich, S.; Burkardt, R.; Völkel, W.; Göen, T. Organophosphate flame retardants and plasticizers in the air and dust in German daycare centers and human biomonitoring in visiting children (LUPE 3). Environ. Int. 2014, 71, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Tay, J.-H.; Covaci, A.; Padilla-Sánchez, J.A.; Papadopoulou, E.; Haug, L.S.; Neels, H.; Sellström, U.; de Wit, C.A. Assessment of dietary exposure to organohalogen contaminants, legacy and emerging flame retardants in a Norwegian cohort. Environ. Int. 2017, 102, 236–243. [Google Scholar] [CrossRef]
- Ballesteros-Gómez, A.; Aragón, Á.; Van den Eede, N.; de Boer, J.; Covaci, A. Impurities of Resorcinol Bis(Diphenyl Phosphate) in Plastics and Dust Collected on Electric/Electronic Material. Environ. Sci. Technol. 2016, 50, 1934–1940. [Google Scholar] [CrossRef]
- Björnsdotter, M.K.; Romera-García, E.; Borrull, J.; de Boer, J.; Rubio, S.; Ballesteros-Gómez, A. Presence of diphenyl phosphate and aryl-phosphate flame retardants in indoor dust from different microenvironments in Spain and the Netherlands and estimation of human exposure. Environ. Int. 2018, 112, 59–67. [Google Scholar] [CrossRef]
- Brommer, S.; Harrad, S.; Van den Eede, N.; Covaci, A. Concentrations of organophosphate esters and brominated flame retardants in German indoor dust samples. J. Environ. Monit. 2012, 14, 2482. [Google Scholar] [CrossRef]
- Brommer, S.; Harrad, S. Sources and human exposure implications of concentrations of organophosphate flame retardants in dust from UK cars, classrooms, living rooms, and offices. Environ. Int. 2015, 83, 202–207. [Google Scholar] [CrossRef]
- Cequier, E.; Ionas, A.C.; Covaci, A.; Marcé, R.M.; Becher, G.; Thomsen, C. Occurrence of a Broad Range of Legacy and Emerging Flame Retardants in Indoor Environments in Norway. Environ. Sci. Technol. 2014, 48, 6827–6835. [Google Scholar] [CrossRef]
- Christia, C.; Poma, G.; Besis, A.; Samara, C.; Covaci, A. Legacy and emerging organophosphοrus flame retardants in car dust from Greece: Implications for human exposure. Chemosphere 2018, 196, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Coelho, S.D.; Sousa, A.C.A.; Isobe, T.; Kim, J.-W.; Kunisue, T.; Nogueira, A.J.A.; Tanabe, S. Brominated, chlorinated and phosphate organic contaminants in house dust from Portugal. Sci. Total Environ. 2016, 569–570, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Dirtu, A.C.; Ali, N.; Van den Eede, N.; Neels, H.; Covaci, A. Country specific comparison for profile of chlorinated, brominated and phosphate organic contaminants in indoor dust. Case study for Eastern Romania, 2010. Environ. Int. 2012, 49, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Harrad, S.; Brommer, S.; Mueller, J.F. Concentrations of organophosphate flame retardants in dust from cars, homes, and offices: An international comparison. Emerg. Contam. 2016, 2, 66–72. [Google Scholar] [CrossRef]
- Hutter, H.-P.; Haluza, D.; Piegler, K.; Hohenblum, P.; Fröhlich, M.; Scharf, S.; Uhl, M.; Damberger, B.; Tappler, P.; Kundi, M.; et al. Semivolatile compounds in schools and their influence on cognitive performance of children. Int. J. Occup. Med. Environ. Health 2013, 26. [Google Scholar] [CrossRef] [PubMed]
- Kademoglou, K.; Xu, F.; Padilla-Sanchez, J.A.; Haug, L.S.; Covaci, A.; Collins, C.D. Legacy and alternative flame retardants in Norwegian and UK indoor environment: Implications of human exposure via dust ingestion. Environ. Int. 2017, 102, 48–56. [Google Scholar] [CrossRef]
- Sugeng, E.J.; Leonards, P.E.G.; van de Bor, M. Brominated and organophosphorus flame retardants in body wipes and house dust, and an estimation of house dust hand-loadings in Dutch toddlers. Environ. Res. 2017, 158, 789–797. [Google Scholar] [CrossRef]
- Van den Eede, N.; Neels, H.; Jorens, P.G.; Covaci, A. Analysis of organophosphate flame retardant diester metabolites in human urine by liquid chromatography electrospray ionisation tandem mass spectrometry. J. Chromatogr. A 2013, 1303, 48–53. [Google Scholar] [CrossRef]
- Vykoukalová, M.; Venier, M.; Vojta, Š.; Melymuk, L.; Bečanová, J.; Romanak, K.; Prokeš, R.; Okeme, J.O.; Saini, A.; Diamond, M.L.; et al. Organophosphate esters flame retardants in the indoor environment. Environ. Int. 2017, 106, 97–104. [Google Scholar] [CrossRef]
- Xu, F.; Giovanoulis, G.; van Waes, S.; Padilla-Sanchez, J.A.; Papadopoulou, E.; Magnér, J.; Haug, L.S.; Neels, H.; Covaci, A. Comprehensive Study of Human External Exposure to Organophosphate Flame Retardants via Air, Dust, and Hand Wipes: The Importance of Sampling and Assessment Strategy. Environ. Sci. Technol. 2016, 50, 7752–7760. [Google Scholar] [CrossRef]
- Zhou, L.; Hiltscher, M.; Püttmann, W. Occurrence and human exposure assessment of organophosphate flame retardants in indoor dust from various microenvironments of the Rhine/Main region, Germany. Indoor Air 2017, 27, 1113–1127. [Google Scholar] [CrossRef]
- Wei, G.-L.; Li, D.-Q.; Zhuo, M.-N.; Liao, Y.-S.; Xie, Z.-Y.; Guo, T.-L.; Li, J.-J.; Zhang, S.-Y.; Liang, Z.-Q. Organophosphorus flame retardants and plasticizers: Sources, occurrence, toxicity and human exposure. Environ. Pollut. 2015, 196, 29–46. [Google Scholar] [CrossRef]
- Bergh, C.; Magnus Åberg, K.; Svartengren, M.; Emenius, G.; Östman, C. Organophosphate and phthalate esters in indoor air: A comparison between multi-storey buildings with high and low prevalence of sick building symptoms. J. Environ. Monit. 2011, 13, 2001. [Google Scholar] [CrossRef]
- Wong, F.; de Wit, C.A.; Newton, S.R. Concentrations and variability of organophosphate esters, halogenated flame retardants, and polybrominated diphenyl ethers in indoor and outdoor air in Stockholm, Sweden. Environ. Pollut. 2018, 240, 514–522. [Google Scholar] [CrossRef]
- Zhou, L.; Hiltscher, M.; Gruber, D.; Püttmann, W. Organophosphate flame retardants (OPFRs) in indoor and outdoor air in the Rhine/Main area, Germany: Comparison of concentrations and distribution profiles in different microenvironments. Environ. Sci. Pollut. Res. 2017, 24, 10992–11005. [Google Scholar] [CrossRef]
- Persson, J.; Wang, T.; Hagberg, J. Organophosphate flame retardants and plasticizers in indoor dust, air and window wipes in newly built low-energy preschools. Sci. Total Environ. 2018, 628–629, 159–168. [Google Scholar] [CrossRef]
- Alves, A.; Kucharska, A.; Erratico, C.; Xu, F.; Den Hond, E.; Koppen, G.; Vanermen, G.; Covaci, A.; Voorspoels, S. Human biomonitoring of emerging pollutants through non-invasive matrices: State of the art and future potential. Anal. Bioanal. Chem. 2014, 406, 4063–4088. [Google Scholar] [CrossRef]
- De Boer, J.; Ballesteros-Gómez, A.; Leslie, H.A.; Brandsma, S.H.; Leonards, P.E.G. Flame retardants: Dust—And not food—Might be the risk. Chemosphere 2016, 150, 461–464. [Google Scholar] [CrossRef]
- Van den Eede, N.; Dirtu, A.C.; Neels, H.; Covaci, A. Analytical developments and preliminary assessment of human exposure to organophosphate flame retardants from indoor dust. Environ. Int. 2011, 37, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Abou-Elwafa Abdallah, M.; Pawar, G.; Harrad, S. Human dermal absorption of chlorinated organophosphate flame retardants; implications for human exposure. Toxicol. Appl. Pharmacol. 2016, 291, 28–37. [Google Scholar] [CrossRef]
- Stapleton, H.M.; Klosterhaus, S.; Eagle, S.; Fuh, J.; Meeker, J.D.; Blum, A.; Webster, T.F. Detection of Organophosphate Flame Retardants in Furniture Foam and US House Dust. Environ. Sci. Technol. 2009, 43, 7490–7495. [Google Scholar] [CrossRef]
- Beser, M.I.; Pardo, O.; Beltrán, J.; Yusà, V. Determination of 21 perfluoroalkyl substances and organophosphorus compounds in breast milk by liquid chromatography coupled to orbitrap high-resolution mass spectrometry. Anal. Chim. Acta 2019, 1049, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Cequier, E. A high-throughput method for determination of metabolites of organophosphate flame retardants in urine by ultra performance liquid chromatography–high resolution mass spectrometry. Anal. Chim. Acta 2014, 845, 98–104. [Google Scholar] [CrossRef]
- Cequier, E.; Sakhi, A.K.; Marcé, R.M.; Becher, G.; Thomsen, C. Human exposure pathways to organophosphate triesters—A biomonitoring study of mother–child pairs. Environ. Int. 2015, 75, 159–165. [Google Scholar] [CrossRef]
- Kucharska, A.; Cequier, E.; Thomsen, C.; Becher, G.; Covaci, A.; Voorspoels, S. Assessment of human hair as an indicator of exposure to organophosphate flame retardants. Case study on a Norwegian mother–child cohort. Environ. Int. 2015, 83, 50–57. [Google Scholar] [CrossRef]
- Larsson, K.; de Wit, C.A.; Sellström, U.; Sahlström, L.; Lindh, C.H.; Berglund, M. Brominated Flame Retardants and Organophosphate Esters in Preschool Dust and Children’s Hand Wipes. Environ. Sci. Technol. 2018, 52, 4878–4888. [Google Scholar] [CrossRef]
- Reemtsma, T.; Lingott, J.; Roegler, S. Determination of 14 monoalkyl phosphates, dialkyl phosphates and dialkyl thiophosphates by LC-MS/MS in human urinary samples. Sci. Total Environ. 2011, 409, 1990–1993. [Google Scholar] [CrossRef]
- Schindler, B.K.; Förster, K.; Angerer, J. Determination of human urinary organophosphate flame retardant metabolites by solid-phase extraction and gas chromatography–tandem mass spectrometry. J. Chromatogr. B 2009, 877, 375–381. [Google Scholar] [CrossRef]
- Sundkvist, A.M.; Olofsson, U.; Haglund, P. Organophosphorus flame retardants and plasticizers in marine and fresh water biota and in human milk. J. Environ. Monit. 2010, 12, 943. [Google Scholar] [CrossRef]
- Völkel, W.; Fuchs, V.; Wöckner, M.; Fromme, H. Toxicokinetic of tris(2-butoxyethyl) phosphate (TBOEP) in humans following single oral administration. Arch. Toxicol. 2018, 92, 651–660. [Google Scholar] [CrossRef]
- Doherty, B.T.; Hoffman, K.; Keil, A.P.; Engel, S.M.; Stapleton, H.M.; Goldman, B.D.; Olshan, A.F.; Daniels, J.L. Prenatal exposure to organophosphate esters and cognitive development in young children in the Pregnancy, Infection, and Nutrition Study. Environ. Res. 2019, 169, 33–40. [Google Scholar] [CrossRef]
- Ait Bamai, Y.; Araki, A.; Nomura, T.; Kawai, T.; Tsuboi, T.; Kobayashi, S.; Miyashita, C.; Takeda, M.; Shimizu, H.; Kishi, R. Association of filaggrin gene mutations and childhood eczema and wheeze with phthalates and phosphorus flame retardants in house dust: The Hokkaido study on Environment and Children’s Health. Environ. Int. 2018, 121, 102–110. [Google Scholar] [CrossRef]
- Araki, A.; Bastiaensen, M.; Ait Bamai, Y.; Van den Eede, N.; Kawai, T.; Tsuboi, T.; Ketema, R.M.; Covaci, A.; Kishi, R. Associations between allergic symptoms and phosphate flame retardants in dust and their urinary metabolites among school children. Environ. Int. 2018, 119, 438–446. [Google Scholar] [CrossRef]
- Carignan, C.C.; Mínguez-Alarcón, L.; Williams, P.L.; Meeker, J.D.; Stapleton, H.M.; Butt, C.M.; Toth, T.L.; Ford, J.B.; Hauser, R. Paternal urinary concentrations of organophosphate flame retardant metabolites, fertility measures, and pregnancy outcomes among couples undergoing in vitro fertilization. Environ. Int. 2018, 111, 232–238. [Google Scholar] [CrossRef]
- Deziel, N.C.; Yi, H.; Stapleton, H.M.; Huang, H.; Zhao, N.; Zhang, Y. A case-control study of exposure to organophosphate flame retardants and risk of thyroid cancer in women. BMC Cancer 2018, 18. [Google Scholar] [CrossRef]
- Hoffman, K.; Stapleton, H.M.; Lorenzo, A.; Butt, C.M.; Adair, L.; Herring, A.H.; Daniels, J.L. Prenatal exposure to organophosphates and associations with birthweight and gestational length. Environ. Int. 2018, 116, 248–254. [Google Scholar] [CrossRef]
- Castorina, R.; Butt, C.; Stapleton, H.M.; Avery, D.; Harley, K.G.; Holland, N.; Eskenazi, B.; Bradman, A. Flame retardants and their metabolites in the homes and urine of pregnant women residing in California (the CHAMACOS cohort). Chemosphere 2017, 179, 159–166. [Google Scholar] [CrossRef]
- Hoffman, K.; Lorenzo, A.; Butt, C.M.; Hammel, S.C.; Henderson, B.B.; Roman, S.A.; Scheri, R.P.; Stapleton, H.M.; Sosa, J.A. Exposure to flame retardant chemicals and occurrence and severity of papillary thyroid cancer: A case-control study. Environ. Int. 2017, 107, 235–242. [Google Scholar] [CrossRef]
- Lipscomb, S.T.; McClelland, M.M.; MacDonald, M.; Cardenas, A.; Anderson, K.A.; Kile, M.L. Cross-sectional study of social behaviors in preschool children and exposure to flame retardants. Environ. Health 2017, 16. [Google Scholar] [CrossRef]
- Preston, E.V.; McClean, M.D.; Claus Henn, B.; Stapleton, H.M.; Braverman, L.E.; Pearce, E.N.; Makey, C.M.; Webster, T.F. Associations between urinary diphenyl phosphate and thyroid function. Environ. Int. 2017, 101, 158–164. [Google Scholar] [CrossRef]
- Soubry, A.; Hoyo, C.; Butt, C.M.; Fieuws, S.; Price, T.M.; Murphy, S.K.; Stapleton, H.M. Human exposure to flame-retardants is associated with aberrant DNA methylation at imprinted genes in sperm. Environ. Epigenet 2017, 3, dvx003. [Google Scholar] [CrossRef]
- Canbaz, D.; van Velzen, M.J.M.; Hallner, E.; Zwinderman, A.H.; Wickman, M.; Leonards, P.E.G.; van Ree, R.; van Rijt, L.S. Exposure to organophosphate and polybrominated diphenyl ether flame retardants via indoor dust and childhood asthma. Indoor Air 2016, 26, 403–413. [Google Scholar] [CrossRef]
- Zhao, F.; Wan, Y.; Zhao, H.; Hu, W.; Mu, D.; Webster, T.F.; Hu, J. Levels of Blood Organophosphorus Flame Retardants and Association with Changes in Human Sphingolipid Homeostasis. Environ. Sci. Technol. 2016, 50, 8896–8903. [Google Scholar] [CrossRef]
- Araki, A.; Saito, I.; Kanazawa, A.; Morimoto, K.; Nakayama, K.; Shibata, E.; Tanaka, M.; Takigawa, T.; Yoshimura, T.; Chikara, H.; et al. Phosphorus flame retardants in indoor dust and their relation to asthma and allergies of inhabitants. Indoor Air 2014, 24, 3–15. [Google Scholar] [CrossRef]
- Meeker, J.D.; Cooper, E.M.; Stapleton, H.M.; Hauser, R. Urinary Metabolites of Organophosphate Flame Retardants: Temporal Variability and Correlations with House Dust Concentrations. Environ. Health Perspect. 2013, 121, 580–585. [Google Scholar] [CrossRef]
- Kanazawa, A.; Saito, I.; Araki, A.; Takeda, M.; Ma, M.; Saijo, Y.; Kishi, R. Association between indoor exposure to semi-volatile organic compounds and building-related symptoms among the occupants of residential dwellings. Indoor Air 2010, 20, 72–84. [Google Scholar] [CrossRef]
- Meeker, J.D.; Johnson, P.I.; Camann, D.; Hauser, R. Polybrominated diphenyl ether (PBDE) concentrations in house dust are related to hormone levels in men. Sci. Total Environ. 2009, 407, 3425–3429. [Google Scholar] [CrossRef]







| Author | Date | Country | Population | Exposure Assessment | Compounds of Interest | Health Outcome | Covariates | Human Health Findings |
|---|---|---|---|---|---|---|---|---|
| Doherty et al. [58] | 2019 | USA | 149 children of 36 months | Urine sample collected from mothers between 24- and 29-week gestation | DPHP, BDCIPP, IP-PPP, BCIPHIPP | Children’s cognitive function (Composite, Fine Motor, Visual Reception, Receptive Language, Expressive Language) was assessed using the Mullen Scales of Early Learning (MSEL) at age between 2 and 3 years | Maternal age, education, income, race/ethnicity, BMI, and child’s sex | Concentrations of IP-PPP (ng/mL) were associated with MSEL Cognitive Composite Score (β= −2.61; 95% CI: −5.69, 0.46), Fine Motor Scale (β= −3.08; 95% CI: −5.26, −0.91) and the Expressive Language Scale (β= −1.21; 95% CI: −2.91, 0.49) |
| 227 children of 36 months | Urine sample collected from mothers between 24- and 29-week gestation | DPHP, BDCIPP, IP-PPP, and BCIPHIPP | Children’s language (Vocabulary, Grammatical Complexity) was assessed using the MacArthur-Bates Communicative Development Inventories (MB-CDI) at age between 2 and 3 years | Maternal age, education, income, race/ethnicity, BMI, and child’s sex | Prenatal IP-PPP concentrations were inversely associated with age-standardized scores on the MB-CDI Vocabulary assessment (β= −1.19; 95% CI: −2.53, 0.16) | |||
| Ait Bamai et al. [59] | 2018 | Japan | 296 children | House dust samples collected at age 7 of children | TMP, TEP, TPP, TBP, TCIP | Eczema and wheeze were assessed in children aged 7 years using the International Study of Asthma and Allergies in Childhood questionnaire | Sex, household income, maternal smoking, and parental history of atopy. | Among children without any filaggrin mutations, TDCIPP was associated with wheeze (OR: 1.22, 95% CI: 1.00–1.48) |
| TCEP, TEHP, TBEP, TDCPP, TPhP, TCP | ||||||||
| Araki et al. [60] | 2018 | Japan | 128 elementary school-aged children | Multisurface dust | TMP, TEP, TPP, TNBP, | International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire | Sex, grade, annual income, and dampness index | Association between TDCIPP in house dust and eczema (OR:3.75; 95% CI: 1.39, 10.2) |
| TCIPP, TCEP, TEHP, TBEP, | ||||||||
| TDCPP, TPHP, TMPP | ||||||||
| 113 to 128 elementary school-aged children | Urine samples collected from children | 5-HO-EHDPHP, EHPHP, | International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire | Sex, grade, annual income, dampness index, and creatinine | Association between ΣuTCIPP and rhinoconjunctivitis (4th quartile vs. 1st quartile) (OR= 5.01; 95% CI: 1.53, 6.5; p = 0.008], TBEP-OH (>LOD vs. <LOD) and eczema (OR= 2.86; 95% CI: 1.04, 7.85; p = 0.041), BDCIPP (3rd tertile vs. 1st tertile) and at least one of the symptoms (wheeze, rhino-conjunctivitis, eczema) (OR= 3.91; 95% CI: 1.24, 12.3; p = 0.019] | |||
| BBOEP, 3-HO-TBEP, BBOEHEP, BCIPP, BCIPHIPP, DPHP, 4-HO-DPHP, | ||||||||
| 3-HO-TPHP, 4-HO-TPHP, BDCIPP, DNBP, uTCEP | ||||||||
| Carignan et al. [61] | 2018 | USA | 201 couples from the Environment and Reproductive Health (EARTH) | One or two spot urine samples per in vitro fertilization cycle | BCIP, BDCIPP, DPHP, IP-PPP, tb-PPP | Proportion of fertilized oocytes, number of best quality embryos, proportion of cycles resulting in implantation, clinical pregnancy and live birth | Year of IVF treatment cycle, primary infertility diagnosis, and maternal urinary PFR metabolites as well as paternal and maternal age, body mass index, and race/ethnicity. | Paternal urinary concentrations of BDCIPP were associated with fertilization (95% CI: 0.01, 0.12; p-trend = 0.06) |
| USA | 211 women from the Environment and Reproductive Health (EARTH) | One or two urine samples per IVF cycle | BCIP, BDCIPP, DPHP, IP-PPP, tb-PPP | Proportion of fertilized oocytes, number of best quality embryos, proportion of cycles resulting in implantation, clinical pregnancy and live birth | Maternal age, body mass index, race/ethnicity, year of IVF treatment cycle, and primary Society for Assisted Reproductive Technology (SART) infertility diagnosis at study entry | Association between the levels of two individual metabolites (DPHP and tb-PPP) and of total metabolites, and reduced probability of successful fertilization, implantation, clinical pregnancy, and live birth | ||
| Deziel et al. [62] | 2018 | USA | 200 women (100 papillary thyroid cancer cases and 100 controls) | Single spot urine samples | BCIPP, BCIHPP, BDCIPP | Age, BMI, education level, family history of thyroid cancer, previous benign thyroid disease, and alcohol consumption | No association between BCIPHIPP, BCIPP, DPHP, BDCIPP, IP-PPP, tb-PPP and papillary thyroid cancer (PTC) | |
| IP-PPP, DPHP and tb-PPP | ||||||||
| Hoffman et al. [63] | 2018 | USA | 248 pairs women–child | Urine samples collected between 24–30 weeks gestation | BDCIPP, DPHP, IP-PPP, | Gestational age in days (combination of last menstrual period and earliest-ultrasounds data) and birthweight | Maternal age, race, education, parity, prepregnancy BMI and season of urine sample collection | Among female infants, IP-PPP was associated with birth (β = −1.00 week; 95% CI: −1.85, −0.15 weeks; p= 0.02). Among male infants, DPHP was associated with gestational duration (β = 0.75 weeks; 95% CI: 0.01, 1.50 weeks; p = 0.05) |
| BCIPHIPP, BCIPP, tb-PPP | ||||||||
| Preterm birth (defined as <37 weeks gestation) | Maternal age, race, education, parity, prepregnancy BMI and season of urine sample collection | Among females infants, preterm birth was associated with IP-PPP (OR: 4.58; 95% CI: 1.23, 17.06) and BDCIPP (OR: 3.99; CI: 1.08, 14.78). Among male infants maternal urinary IP-PPP concentrations were associated with preterm birth (OR: 0.21; 95% CI: 0.06, 0.68). | ||||||
| Castorina et al. [64] | 2017 | USA | 248 to 249 pairs women–child | Urine samples collected during the 2nd prenatal study visit | BDCIPP, DPHP, IP-PPP, tb-PPP | Children’s cognitive abilities was assessed by a single bilingual psychometrician at age 7 using the Wechsler Intelligence Scale for Children, 4th edition (WISC-IV) (Full-Scale IQ, Working memory, Perceptual reasoning, Verbal comprehension, Processing speed) | Maternal education, PPVT scores, CES-D scores, country of birth and prenatal urinary DAP metabolite levels, HOME z-score, language of WISC testing, child sex and age at assessment, and household poverty | Association between DPHP and Full-Scale IQ (β: −2.9; 95% CI: −6.3, 0.5), DPHP and Working memory (WISC-IV scale) (β: −3.9; 95% CI: −7.3, −0.5), ΣPFR metabolites and Working memory (WISC-IV scale) (β: −4.6, 95% CI: −8.9, −0.3). |
| Urine samples collected during the 2nd prenatal study visit | BDCIPP, DPHP, IP-PPP, tb-PPP | Children’s behavior was assessed by maternal and teacher report at age 7 using the Behavior Assessment System for Children 2 (BASC-2) (ADHD Index, Inattention DSM-IV, Hyperactive/Impulsive DSM-IV, total subscale DSM-IV) and the Conners’ ADHD/DSM-IV Scales (CADS) (Hyperactivity scale, Attention problems scale) | Sex, age at assessment, maternal country of birth, HOME score at 7-years, prenatal DAPs, and maternal depression and education | Association between IP-PPP and Hyperactivity scale (BASC-2—Maternal Report (T-score) (β: 2.4; 95% CI: 0.1, 4.7), BDCIPP and Attention problems scale (BASC-2—Teacher Report (T-score) (β: 1.1; 95% CI: −0.1,2.3; p- < 0.1) | ||||
| Hoffman et al. [65] | 2017 | USA | 70 cases and 70 controls | Dust samples from homes | TCEP, TCIPP, TDCPP and TPHP | Diagnostic of papillary thyroid cancer (PTC) | Indicator of tumor aggressiveness for FR exposure above the median | Higher levels of TCEP associated with increased odds of PTC (OR: 2.42; 95% CI: (1.10, 5.33) |
| Lipscomb et al. [66] | 2017 | USA | 72 children aged 3–5 years | Passive wristband samplers worn continuously for 7 days | TPP, TCIPP, TCEP, TDCPP | Children’s social behaviors were assessed using the Social Skills Improvement System-Rating Scales (SSIS-RS) by their teacher in the preschools they were attending (seven subscales representing positive behaviors: Communication, Cooperation, Assertion, Responsibility, Empathy, Engagement, and Self-Control; four subscales representing behavior problem domains: Externalizing, Bullying, Hyperactivity/Inattention, and Internalizing) | Gender, age, family context, and child’s exposure to adverse experiences | lnΣOPFR levels were associated with responsibility (β = −0.25, p < 0.001) and externalizing problems (β = 0.31, p < 0.05) |
| Preston et al. [67] | 2017 | USA | 51 adults | 133 urine samples collected at months 1,6 and 12 | DPHP | Free thyroxine (fT4), total thyroxine (TT4), total triiodothyronine (TT3), and thyroid stimulating hormone (TSH) in serum samples | Sampling round, time of sample collection, specific gravity-corrected iodine and BDE-47 and sex | DPHP was associated with a 0.43 μg/dL (95% CI: 0.47, 1.36) increase in mean TT4 levels |
| 25 women | 61 urine samples collected at months 1,6 and 12 | DPHP | Free thyroxine (fT4), total thyroxine (TT4), total triiodothyronine (TT3), and thyroid stimulating hormone (TSH) in serum samples | Sampling round, time of sample collection, specific gravity-corrected iodine and BDE-47 | DPHP was associated with a 0.91 μg/dL (95% CI: 0.47, 1.36) increase in mean TT4 levels | |||
| 26 men | 61 urine samples collected at months 1,6 and 12 | DPHP | Free thyroxine (fT4), total thyroxine (TT4), total triiodothyronine (TT3), and thyroid stimulating hormone (TSH) in serum samples | Sampling round, time of sample collection, specific gravity-corrected iodine and BDE-47 | No significant association between DPHP and TT4, fT4, TT3, TSH | |||
| Soubry et al. [68] | 2017 | USA | 67 men | Urines samples | BCIP, BDCIPP, DPHP, IP-PPP, tb-PPP | DNA extracted from sperm samples | Age, obesity-status and multiple testing, exposure to monoisopropylphenyl | Association between BDCIPP, DPHP, IP-PPP and hyper- or hypomethylation of different genes specific to the metabolites |
| diphenyl phosphate | ||||||||
| Canbaz et al. [69] | 2016 | Sweden | 110 children who developed asthma at 4 or at 8 years, matched with 110 controls from a large perspective study | Dust collected from the mother’s mattress two months after childbirth | TCEP, TCIPP, TDCPP, TBEP, TPhP, EHDPHP, mmp-TMPP | Asthma at 4 or 8 years was defined based on at least two of the following three criteria: (i) >1 episode of wheeze in the last 12 months; (ii) a doctor’s diagnosis of asthma; (iii) asthma medicine prescribed occasionally or regularly over the last 12 months | No association between PEFRs concentrations and development of childhood asthma | |
| Zhao et al. [70] | 2016 | China | 154 men and 101 women | One blood sample | TCIPP, TBEP, TPHP, TEP, TNBP, EHDPP | Blood samples | Negative association between EHDPP, TPHP, and TNBP levels and sphingosine 1-phosphate concentration | Association between levels of the six PEFRs and increased sphingomyelin concentration (p < 0.001 for all OPFRs). The S1P level in the highest quartile of EHDPP was 36% lower (95% CI: −39%, −33%; p < 0.001) than that in the lowest quartile, 16% lower (95% CI: −19%, −14%; p < 0.001) than that in the highest TPHP quartile, and 36% lower (95% CI: −38%, −33%; p < 0.001) than that in the highest TNBP quartile |
| Araki et al. [71] | 2014 | Japan | 516 inhabitants (adults and children) in 156 different homes | Floor dust | TMP, TEP, TPP, TNBP, TCIPP, TCEP, TEHP, TBEP, TDCPP, T PHP, TMPP | All inhabitants of each home were asked to complete a self-administered questionnaire participants who reported having received medical treatment for bronchial asthma, atopic dermatitis, allergic rhinitis, and/or allergic conjunctivitis at any time during the preceding 2 years were classified as positive | Gender, age, tobacco smoke, ETS exposure, | Association between TNBP in multi-surface dust and asthma (OR: 5.34; 95% CI: 1.45, 19.7), TNBP in multi-surface dust and allergic rhinitis (OR: 2.55; 95% CI: 1.29, 45.01) |
| recent renovations, wall-to-wall carpeting, dampness | ||||||||
| index, hair/fur-bearing pets in the dwelling, | ||||||||
| mechanical ventilation equipment usage, and total | ||||||||
| fungi | ||||||||
| Meeker et al. [72] | 2013 | USA | 33 men | Urine samples | BDCIPP, DPHP | Blood and semen samples | Age, BMI, and time of sample collection, abstinence period | Association between BDCIPP levels and decreases in sperm quality parameters, and concentrations of total T3 (% change: 6.6; 95% CI: 1.6,12,8, p = 0.02) and TSH in serum (% change: 40.3; 95% IC: 11.4, 77.1, p = 0.006). DPHP was |
| associated with a 57% (95% CI: −77.8, −18.8, p = 0.01) decrease in sperm concentration and a 20% (95% CI: –41.1, 0.5) decrease in sperm motility | ||||||||
| Hutter et al. [32] | 2013 | Austria | 436 children | Air | TCEP, TDCPP | The cognitive evaluation was accomplished by a neurodevelopment test | Social status, gender and region (urban/rural) | Significant correlations of TCEP in PM10 and PM2.5 and school dust samples with cognitive performance. Cognitive performance decreased with increasing concentrations of TCEP |
| Bergh et al. [40] | 2011 | Sweden | Adults (men and women) | Air | TEP, TiPrP, TPrP, TiBP, TBP, TCEP, TCIPP, TPeP, THP, TDCPP, TPP, DPEHP, TEHP, TToP, d27-TBP cis | No association between OPFRs levels and reported Sick Building Syndrome symptoms | ||
| Kanazawa et al. [73] | 2010 | Japan | 134 adults (70 women and 64 men) | Floor dust | TBP, TBEP, TDCPP | Age (ordinal variable in increments of 10 years), gender, history of allergy, time spent | Association between TBP and mucosal symptoms of Sick Building Syndrome (OR: 15, 95% CI: 2.7–80), TBOEP (OR: 0.3, 95% CI: 0.1–0.7), TDCIPP (OR: 2.2, 95% CI: 1.0–4.6) | |
| at home (h/day; ≤12, >12), and condensation and moldy odor | ||||||||
| Meeker et al. [74] | 2009 | USA | 38 men | House dust | TDCPP, TPP | Serum and semen samples: hormones (Free T4, Total T3, TSH, FSH, LH, Inhibin B, Testosterone, SHBG, FAI, estradiol, Prolactin, Sperm concentration, sperm mobility, sperm morphology) | Age, BMI | Association between TDCIPP and Free T4 (β: −2.8; 95% CI: −4.6, −1.0; p: 0.004), TDCPP and prolactin (β:17.3; 95% CI: 4.1–32.2; p: 0.008), TPP and prolactin (β: 9.7; 95% CI: 2.3,18.9; p: 0.02) |
| 50 men | Age, BMI and abstinence period | Association between TPP and sperm concentration (β: −18.8; 95% CI: −30.1, −4.5; p: 0.01) |
| Compound | Indoor Contamination | Human Exposure | Epidemiological Evidence of Adverse Effect | ||||
|---|---|---|---|---|---|---|---|
| Reproductive | Thyroid | Respiratory/Immune | Neuro-Development | Dermal | |||
| BCMP-BCEP |  | ||||||
| BDP |  |  | |||||
| DPHP |  |  | |||||
| EHDPP |  |  |  |  | |||
| iDDPHP |  | ||||||
| iDPP |  | ||||||
| ITP |  |  |  |  | |||
| PBDPP |  | ||||||
| TBOEP |  |  |  |  | |||
| TBPP |  | ||||||
| TCEP |  |  |  |  |  |  | |
| TCIPP |  |  |  |  |  |  |  |
| TDBPP |  | ||||||
| TDCIPP |  |  |  |  |  |  |  |
| TEHP |  |  |  |  | |||
| TEP |  |  |  |  | |||
| THP |  |  | |||||
| TIBP |  | ||||||
| TIPP |  |  | |||||
| TiPPP |  | ||||||
| TmCP |  |  | |||||
| TMP |  |  |  |  | |||
| TMPP |  |  |  |  | |||
| TNBP |  |  |  |  | |||
| ToCP |  |  | |||||
| TpCP |  |  | |||||
| TPEP |  | ||||||
| TPHP |  |  |  |  |  |  |  |
| TPP |  |  |  |  |  |  | |
| TXP |  | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chupeau, Z.; Bonvallot, N.; Mercier, F.; Le Bot, B.; Chevrier, C.; Glorennec, P. Organophosphorus Flame Retardants: A Global Review of Indoor Contamination and Human Exposure in Europe and Epidemiological Evidence. Int. J. Environ. Res. Public Health 2020, 17, 6713. https://doi.org/10.3390/ijerph17186713
Chupeau Z, Bonvallot N, Mercier F, Le Bot B, Chevrier C, Glorennec P. Organophosphorus Flame Retardants: A Global Review of Indoor Contamination and Human Exposure in Europe and Epidemiological Evidence. International Journal of Environmental Research and Public Health. 2020; 17(18):6713. https://doi.org/10.3390/ijerph17186713
Chicago/Turabian StyleChupeau, Zohra, Nathalie Bonvallot, Fabien Mercier, Barbara Le Bot, Cecile Chevrier, and Philippe Glorennec. 2020. "Organophosphorus Flame Retardants: A Global Review of Indoor Contamination and Human Exposure in Europe and Epidemiological Evidence" International Journal of Environmental Research and Public Health 17, no. 18: 6713. https://doi.org/10.3390/ijerph17186713
APA StyleChupeau, Z., Bonvallot, N., Mercier, F., Le Bot, B., Chevrier, C., & Glorennec, P. (2020). Organophosphorus Flame Retardants: A Global Review of Indoor Contamination and Human Exposure in Europe and Epidemiological Evidence. International Journal of Environmental Research and Public Health, 17(18), 6713. https://doi.org/10.3390/ijerph17186713





