Bayesian Estimation of Correlation between Measures of Blood Pressure Indices, Aerobic Capacity and Resting Heart Rate Variability Using Markov Chain Monte Carlo Simulation and 95% High Density Interval in Female School Teachers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Procedure and Instruments
2.3.1. Anthropometry
2.3.2. Blood Pressure
2.3.3. HRV Data Acquisition
2.3.4. Aerobic Capacity Test
2.4. HRV Data Management
2.4.1. Artifact Identification
2.4.2. Measurements of HRV Indices
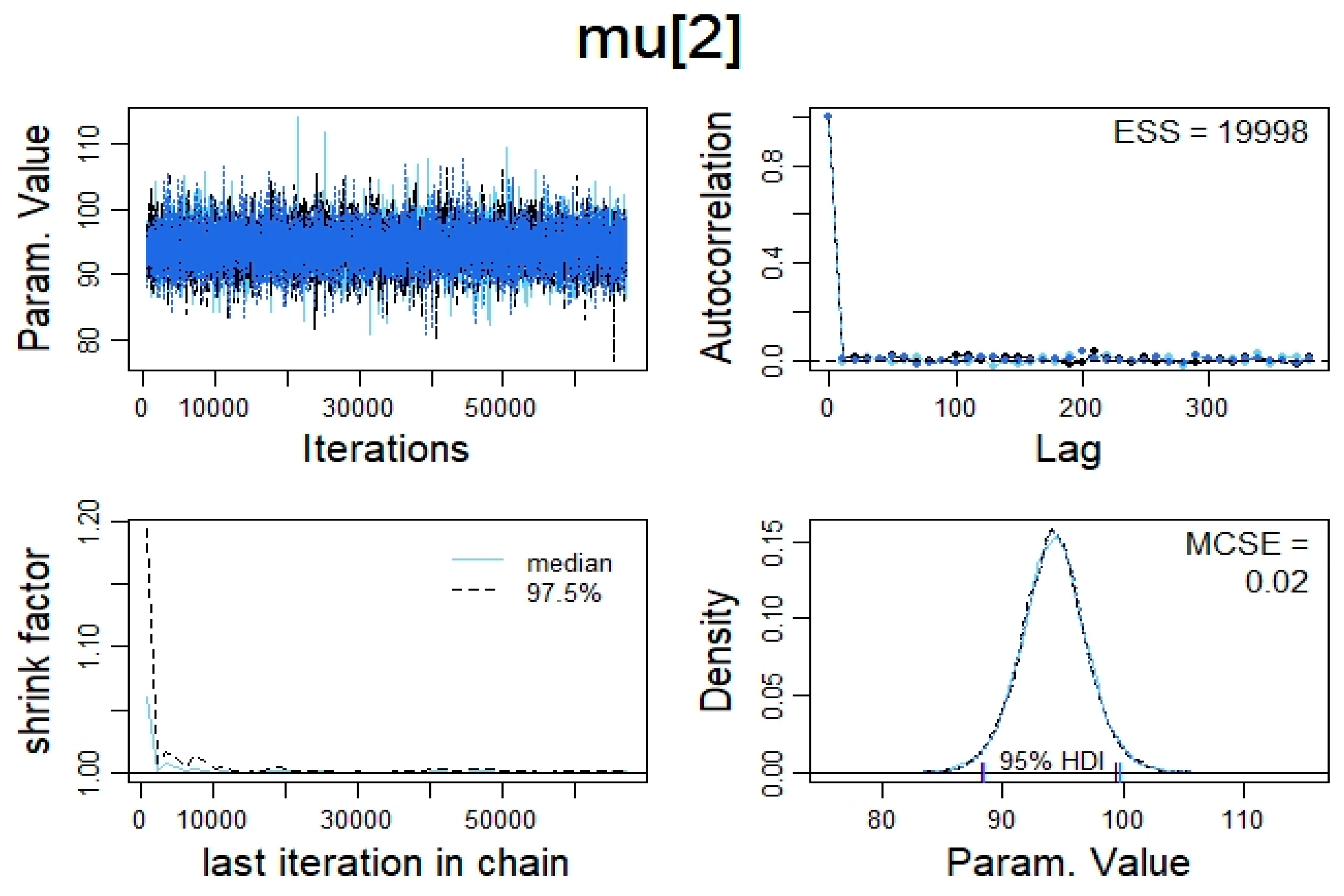
2.5. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. The Relationship within HRV Indices
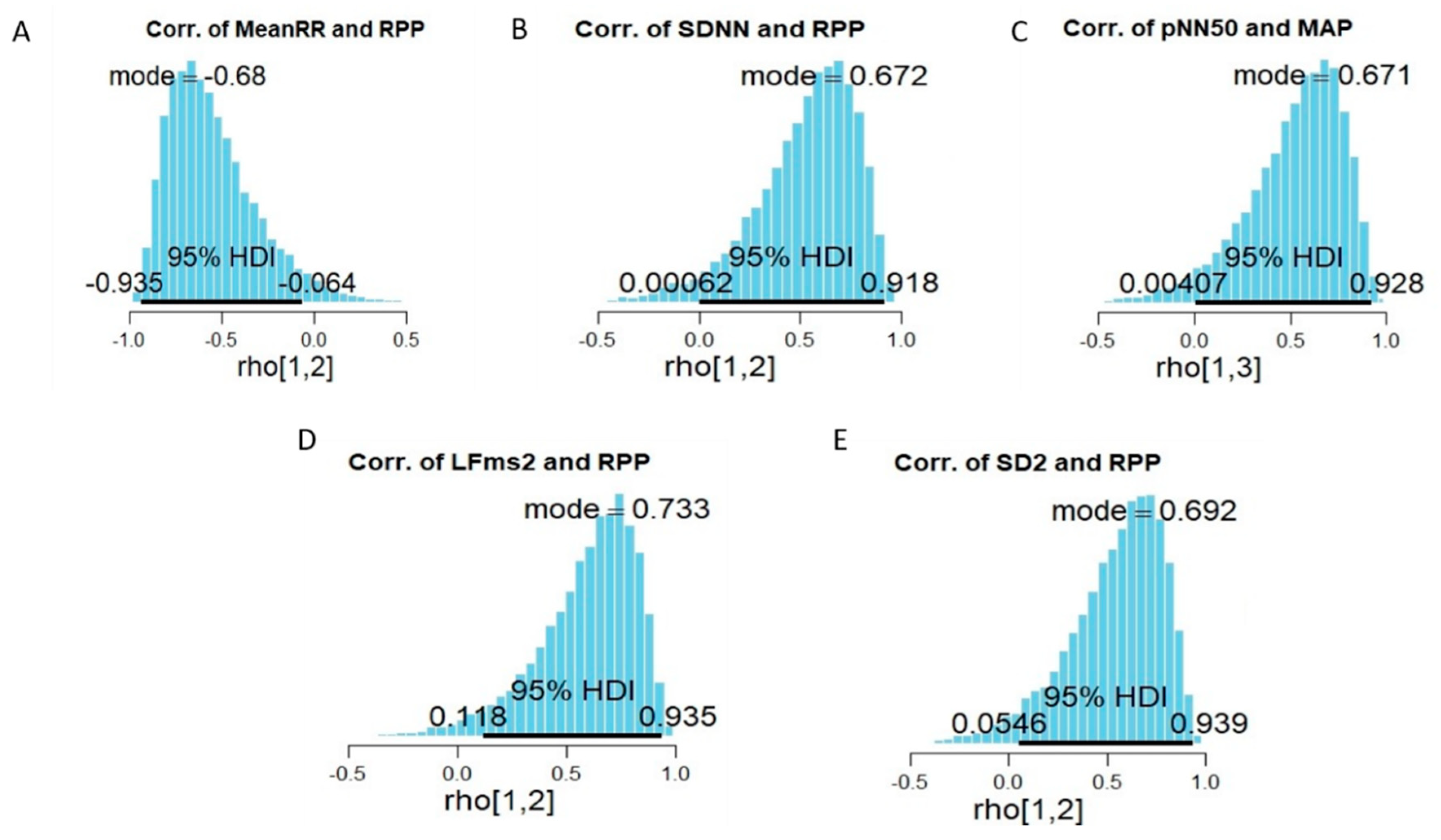
3.3. The Relationship between Measures of HRV Indices and Measurments of Blood Pressure Indices and Aerobic Capacity Performance
4. Discussion
4.1. Measures of HRV Indices Compared to Norms
4.2. The Association within HRV Indices
4.3. The Relationship between Measures of HRV Indices and Both Measures of Blood Pressure Indices and Aerobic Capacity Parameters
5. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Berntson, G.G.; Bigger, J.T., Jr.; Eckberg, D.L.; Grossman, P.; Kaufmann, P.G.; Malik, M.; Nagaraja, H.N.; Porges, S.W.; Saul, J.P.; Stone, P.H.; et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology 1997, 34, 623–648. [Google Scholar] [CrossRef] [PubMed]
- Sessa, F.; Anna, V.; Messina, G.; Cibelli, G.; Monda, V.; Marsala, G.; Ruberto, M.; Biondi, A.; Cascio, O.; Bertozzi, G.; et al. Heart rate variability as predictive factor for sudden cardiac death. Aging 2018, 10, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Task_Force. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research—Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Front. Psychol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Quintana, D.S.; Heathers, J.A.J. Considerations in the assessment of heart rate variability in biobehavioral research. Front. Psychol 2014, 5, 805. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An overview of heart rate variability metrics and norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Dong, J.G. The role of heart rate variability in sports physiology (Review). Exp. Ther. Med. 2016, 11, 1531–1536. [Google Scholar] [CrossRef]
- Bradley, M.A.; Luecken, L.J. Heart rate variability as an index of regulated emotional responding. Rev. Gen. Psychol. 2006, 10, 229–240. [Google Scholar] [CrossRef]
- Quintana, D.S.; Alvares, G.A.; Heathers, J.A. Guidelines for Reporting Articles on Psychiatry and Heart rate variability (GRAPH): Recommendations to advance research communication. Transl. Psychiatry 2016, 6, e803. [Google Scholar] [CrossRef]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef]
- Castaneda, D.; Esparza, A.; Ghamari, M.; Soltanpur, C.; Nazeran, H. A review on wearable photoplethysmography sensors and their potential future applications in health care. Int. J. Biosens. Bioelectron. 2018, 4, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, M.; Lewis, M.J.; Marson, R.E. Comparison of Polar 810s and an ambulatory ECG system for RR interval measurement during progressive exercise. Int. J. Sports Med. 2005, 26, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Nunan, D.; Donovan, G.; Jakovljevic, D.G.; Hodges, L.D.; Sandercock, G.R.; Brodie, D.A. Validity and reliability of short-term heart-rate variability from the Polar S810. Med. Sci. Sports Exerc. 2009, 41, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Hernando, D.; Garatachea, N.; Almeida, R.; Casajus, J.A.; Bailon, R. Validation of Heart Rate Monitor Polar RS800 for Heart Rate Variability Analysis During Exercise. J. Strength Cond. Res. 2016, 32, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Cassirame, J.; Vanhaesebrouck, R.; Chevrolat, S.; Mourot, L. Accuracy of the Garmin 920 XT HRM to perform HRV analysis. Australas. Phys. Eng. Sci. Med. 2017, 40, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Fatisson, J.; Oswald, V.; Lalonde, F. Influence diagram of physiological and environmental factors affecting heart rate variability: An extended literature overview. Heart Int. 2016, 11, e32–e40. [Google Scholar] [CrossRef]
- Triplett, N.T. Structure and function of body systems. In Essentials of Strength Training and Conditioning, 4th ed.; Haff, G.G., Triplett, N.T., Eds.; Human Kinetics: Champaign, IL, USA, 2016; pp. 1–18. [Google Scholar]
- DeMers, D.; Wachs, D. Physiology, Mean Arterial Pressure; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Ansari, M.; Javadi, H.; Pourbehi, M.; Mogharrabi, M.; Rayzan, M.; Semnani, S.; Jallalat, S.; Amini, A.; Abbaszadeh, M.; Barekat, M.; et al. The association of rate pressure product (RPP) and myocardial perfusion imaging (MPI) findings: A preliminary study. Perfusion 2012, 27, 207–213. [Google Scholar] [CrossRef]
- Chapleau, M.W. Baroreceptor Reflexes. In Primer on the Autonomic Nervous System; Low, P.A., Polinsky, R.J., Robertson, D., Robertson, D., Paton, J.F.R., Low, P.A., Eds.; Elsevier Science & Technology: San Diego, CA, USA, 2011; pp. 161–165. [Google Scholar]
- Figueroa, M.A.; Demeersman, R.E.; Manning, J. The autonomic and rate pressure product responses of tai chi practitioners. N. Am. J. Med. Sci. 2012, 4, 270–275. [Google Scholar] [CrossRef]
- Kazmi, S.Z.H.; Zhang, H.; Aziz, W.; Monfredi, O.; Abbas, S.A.; Shah, S.A.; Kazmi, S.S.H.; Butt, W.H. Inverse Correlation between Heart Rate Variability and Heart Rate Demonstrated by Linear and Nonlinear Analysis. PLoS ONE 2016, 11, e0157557. [Google Scholar] [CrossRef]
- Papp, M.E.; Lindfors, P.; Storck, N.; Wändell, P.E. Increased heart rate variability but no effect on blood pressure from 8 weeks of hatha yoga—A pilot study. BMC Res. Notes 2013, 6, 59. [Google Scholar] [CrossRef]
- Swank, A.; Sharp, C. Adaptations to aerobic endurance training programs. In Essentials of Strength Training and Conditioning, 4th ed.; Haff, G.G., Triplett, N.T., Eds.; Human Kinetics: Champaign, IL, USA, 2016; pp. 115–134. [Google Scholar]
- Herda, T.J.; Cramer, J.T. Bioenergetics of Exercise and Training. In Essentials of Strength Training and Conditioning, 4th ed.; Haff, G.G., Triplett, N.T., Eds.; Human Kinetics: Champaign, IL, USA, 2016; pp. 43–64. [Google Scholar]
- De Meersman, R.E. Heart rate variability and aerobic fitness. Am. Heart J. 1993, 125, 726–731. [Google Scholar] [CrossRef]
- Melanson, E.L.; Freedson, P.S. The effect of endurance training on resting heart rate variability in sedentary adult males. Eur. J. Appl. Physiol. 2001, 85, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Catai, A.M.; Chacon-Mikahil, M.P.; Martinelli, F.S.; Forti, V.A.; Silva, E.; Golfetti, R.; Martins, L.E.; Szrajer, J.S.; Wanderley, J.S.; Lima-Filho, E.C.; et al. Effects of aerobic exercise training on heart rate variability during wakefulness and sleep and cardiorespiratory responses of young and middle-aged healthy men. Braz. J. Med. Biol. Res. 2002, 35, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Kouidi, E.; Haritonidis, K.; Koutlianos, N.; Deligiannis, A. Effects of athletic training on heart rate variability triangular index. Clin. Physiol. Funct. Imaging 2002, 22, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Marocolo, M.; Nadal, J.; Benchimol Barbosa, P.R. The effect of an aerobic training program on the electrical remodeling of heart high-frequency components of the signal-averaged electrocardiogram is a predictor of the maximal aerobic power. Braz. J. Med. Biol. Res. 2007, 40, 199–208. [Google Scholar] [CrossRef]
- Schmitt, L.; Fouillot, J.P.; Millet, G.P.; Robach, P.; Nicolet, G.; Brugniaux, J.; Richalet, J.P. Altitude, heart rate variability and aerobic capacities. Int. J. Sports Med. 2008, 29, 300–306. [Google Scholar] [CrossRef]
- Grant, C.C.; Clark, J.R.; Janse van Rensburg, D.C.; Viljoen, M. Relationship between exercise capacity and heart rate variability: Supine and in response to an orthostatic stressor. Auton Neurosci. 2009, 151, 186–188. [Google Scholar] [CrossRef]
- Leite, M.R.; Ramos, E.M.; Kalva-Filho, C.A.; Rodrigues, F.M.; Freire, A.P.; Tacao, G.Y.; de Toledo, A.C.; Cecílio, M.J.; Vanderlei, L.C.; Ramos, D. Correlation between heart rate variability indexes and aerobic physiological variables in patients with COPD. Respirology 2015, 20, 273–278. [Google Scholar] [CrossRef]
- Flatt, A.A.; Esco, M.R. Evaluating individual training adaptation with smartphone-derived heart rate variability in a collegiate female soccer team. J. Strength Cond. Res. 2016, 30, 378–385. [Google Scholar] [CrossRef]
- Flatt, A.A.; Esco, M.R.; Nakamura, F.Y. Individual Heart Rate Variability Responses to Preseason Training in High Level Female Soccer Players. J. Strength Cond. Res. 2017, 31, 531–538. [Google Scholar] [CrossRef]
- Materko, W. Stratification of the level of aerobic fitness based on heart rate variability parameters in adult males at rest. Motricidade 2018, 14, 51–57. [Google Scholar] [CrossRef]
- Materko, W.; Bartels, R.; Peçanha, T.; Perrout de Lima, J.R.; Carvalho, A.R.S.; Nadal, J. Maximum oxygen uptake prediction model based on heart rate variability parameters for young healthy adult males at rest. Open Access Biostat. Bioinform. 2018, 2, 1–7. [Google Scholar] [CrossRef]
- Phoemsapthawee, J.; Prasertsri, P.; Leelayuwat, N. Heart rate variability responses to a combined exercise training program: Correlation with adiposity and cardiorespiratory fitness changes in obese young men. J. Exerc. Rehabil. 2019, 15, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Vitale, J.A.; Bonato, M.; La Torre, A.; Banfi, G. Heart rate variability in sport performance: Do time of day and chronotype play a role? J. Clin. Med. 2019, 8, 723. [Google Scholar] [CrossRef] [PubMed]
- Nunan, D.; Sandercock, G.R.; Brodie, D.A. A quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing Clin. Electrophysiol. 2010, 33, 1407–1417. [Google Scholar] [CrossRef]
- Society, B.A.I.H. Validated BP Monitors for For Specialist Use. Available online: https://bihsoc.org/bp-monitors/for-specialist-use/ (accessed on 26 February 2020).
- Kallioinen, N.; Hill, A.; Horswill, M.S.; Ward, H.E.; Watson, M.O. Sources of inaccuracy in the measurement of adult patients’ resting blood pressure in clinical settings: A systematic review. J. Hypertens. 2017, 35, 421–441. [Google Scholar] [CrossRef]
- Berntson, G.G.; Quigley, K.S.; Jang, J.F.; Boysen, S.T. An approach to artifact identification: Application to heart period data. Psychophysiology 1990, 27, 586–598. [Google Scholar] [CrossRef]
- Vyaire, m. Vyntus CPX Powered by SentrySuite. Available online: https://www.vyaire.com/Documents/international/brochures/respiratory-care/cardiopulmonary/RC_Vyntus-CPX_BR_EN.pdf (accessed on 10 December 2018).
- Gibson, A.L.; Wagner, D.R.; Heyward, V.H. Advanced Fitness Assessment and Exercise Prescription; Human Kinetics: Champaign, IL, USA, 2019. [Google Scholar]
- Nevill, A.M.; Ramsbottom, R.; Williams, C. Scaling physiological measurements for individuals of different body size. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 65, 110–117. [Google Scholar] [CrossRef]
- Kaufmann, T.; Sutterlin, S.; Schulz, S.M.; Vogele, C. ARTiiFACT: A tool for heart rate artifact processing and heart rate variability analysis. Behav. Res. Methods 2011, 43, 1161–1170. [Google Scholar] [CrossRef]
- Hooten, M.B.; Hefley, T.J. Bringing Bayesian Models to Life; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2019. [Google Scholar]
- Kruschke, J.K. Doing Bayesian Data Analysis. A Tutorial with R, JAGS, and Stan, 2nd ed.; Elsevier: Oxford, UK, 2015. [Google Scholar]
- Kruschke, J.K.; Liddell, T.M. Bayesian data analysis for newcomers. Psychon. Bull. Rev. 2018, 25, 155–177. [Google Scholar] [CrossRef]
- Kruschke, J.K.; Liddell, T.M. The Bayesian New Statistics: Hypothesis testing, estimation, meta-analysis, and power analysis from a Bayesian perspective. Psychon. Bull. Rev. 2018, 25, 178–206. [Google Scholar] [CrossRef]
- DeCarlo, L.T. On the meaning and use of kurtosis. Psychol. Methods 1997, 2, 292–307. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: New York, NY, USA, 1988. [Google Scholar]
- Kruschke, J.K. Posterior predictive checks can and should be Bayesian: Comment on Gelman and Shalizi, ‘Philosophy and the practice of Bayesian statistics’. Br. J. Math. Stat. Psychol. 2013, 66, 45–56. [Google Scholar] [CrossRef]
- Jarrin, D.C.; McGrath, J.J.; Giovanniello, S.; Poirier, P.; Lambert, M. Measurement fidelity of heart rate variability signal processing: The devil is in the details. Int. J. Psychophysiol. 2012, 86, 88–97. [Google Scholar] [CrossRef]
- Rajendra Acharya, U.; Paul Joseph, K.; Kannathal, N.; Lim, C.M.; Suri, J.S. Heart rate variability: A review. Med. Biol. Eng. Comput. 2006, 44, 1031–1051. [Google Scholar] [CrossRef]
- Stein, P.K. Assessing heart rate variability from real-world Holter reports. Card. Electrophysiol. Rev. 2002, 6, 239–244. [Google Scholar] [CrossRef]
- Sendelides, T.; Metaxas, T.; Koutlianos, N.; Kouidi, E.; Deligiannis, A. Heart rate variability changes in soccer players. OÉsterr. J. Sportmed. 2003, 33, 10–13. [Google Scholar]
- Sherldine, T. The Usefulness of the Rate Pressure Product (RPP) for cardiac rehabilitation exercise prescription. Intern. Med. Res. Open J. 2017, 2, 1–3. [Google Scholar]
- Nasario-Junior, O.; Benchimol-Barbosa, P.R.; Pedrosa, R.C.; Nadal, J. Beat-to-beat ventricular repolarization duration variability assessed by cardiac acceleration and deceleration phases in athletes. EC Cardiol. 2015, 1, 33–42. [Google Scholar]
| Time Domain | ||
| SDNN | Standard deviation of all Normal–Normal intervals in a time series | SDNN indicate total variability [3,7,8] |
| RMSSD | The root mean square of successive differences | RMSSD and pNN50 (%) reflects vagal tone/PNS activities [4,7,10] |
| pNN50 (%)) | Percent of successive intervals with a difference greater that 50 ms compared to previous interval | |
| Frequency Domain | ||
| HF | High-frequency band (i.e., 0.15 to 0.4 Hz) | HF reflects vagal tone/PNS activities [4,8,10] |
| LF | Low-frequency (LF) band (i.e., 0.04 and 0.15 Hz) | LF reflects baroreceptor activity at rest (vagal influenced) and SNS activities during stress [3,4,8,10] |
| VLF | Very-low-frequency (VLF) band (i.e., 0.0033 to 0.04 Hz) | VLF and ULF reflect long-term thermo- and hormonal regulation mechanisms [3,10] |
| ULF | Ultra-low-frequency (ULF) band (i.e., <0.0033 Hz) | |
| Poincaré Plot | ||
| SD1 | Standard descriptor 1 | SD1 reflects fast IBI variability which is a reflection of PNS activities to the heart [4,7] |
| SD2 | Standard descriptor 2 | SD2 reflects the long-term IBI variability, which represents both SNS and PSN activities [4,7] |
| Study (Date) | Focus | Findings |
|---|---|---|
| De Meersman [26] (year 1993) | Compared different age groups on both VO2max and HRV (assessed by the percent change in mean HR). |
|
| Melanson and Freedson [27] (year 2001) | Effect of a 12-week endurance training on resting heart rate variability in sedentary adult males. |
|
| Catai et al. [28] (year 2002) | Effects of aerobic exercise training on heart rate variability during wakefulness and sleep and cardiorespiratory responses of young and middle-aged healthy men. |
|
| Kouidi et al. [29] (year 2002) | Effect of athletic training on time domain HRV indices. |
|
| Marocolo et al. [30] (year 2007) | Effect of aerobic training program on the electrical remodeling of heart high-frequency components. |
|
| Schmitt et al. [31] (year 2008) | Altitude, heart rate variability and aerobic capacities. |
|
| Grant et al. [32] (year 2009) | Relationship between exercise capacity and heart rate variability. |
|
| Leite et al. [33] (year 2015) | Correlation between heart rate variability indexes and aerobic physiological variables. |
|
| Flatt and Esco [34] (year 2016) | Evaluating individual raining adaptation with smartphone-derived heart rate variability in a collegiate female soccer team. |
|
| Flatt et al. [35] (year 2017) | Individual heart rate variability responses to preseason training in high level female soccer players. |
|
| Materko [36] (year 2018) | Stratification of the level of aerobic fitness based on heart rate variability parameters in adult males at rest. |
|
| Materko et al. [37] (year 2018) | Maximum oxygen uptake prediction model based on heart rate variability parameters. |
|
| Phoemsapthawee et al. [38] (year 2019) | Clarifying the casual link between body composition, aerobic fitness and the alterations in cardiac autonomic modulation after a 12-week exercise training. |
|
| Based on the Last 6 min | Based on 5 min | |||
|---|---|---|---|---|
| Participant Number | Total Data Point | Artifact | Percentage | Total Data Point Analyzed |
| P. 1 | 392 | 17 | 4.3 | 333 |
| P. 2 | 445 | 0 | 0.0 | 369 |
| P. 3 | 434 | 0 | 0.0 | 363 |
| P. 4 | 396 | 1 | 0.3 | 329 |
| P. 5 | 457 | 4 | 0.9 | 383 |
| P. 6 | 391 | 2 | 0.5 | 324 |
| P. 7 | 420 | 0 | 0.0 | 349 |
| P. 8 | 369 | 2 | 0.5 | 309 |
| Variable | Mean ± (95% HDI) | SD ± (95% HDI) | Shapiro–Wilk’s Test (Sig.) | Skewness | Kurtosis |
|---|---|---|---|---|---|
| Mean HR (bpm) | 69.3 (65.5–73.1) | 4.93 (3.11–8.78) | 0.870 | 0.17 | −1.14 |
| Min HR (bpm) | 64.5 (61.3–67.5) | 3.91 (2.46–7.06) | 0.811 | −0.11 | −1.08 |
| Max HR (bpm) | 76 (71.2–81) | 6.13 (4.01–11.3) | 0.979 | 0.00 | 0.92 |
| MeanRR (ms) | 873 (824–924) | 58.7 (40.1–111) | 0.896 | 0.06 | −1.09 |
| SDNN (ms) | 28.9 (20.4–37.1) | 9.93 (6.59–18.5) | 0.911 | 0.24 | −0.22 |
| RMSSD (ms) | 23.8 (17.4–30.8) | 8.17 (5.23–14.8) | 0.185 | 0.09 | −2.18 |
| pNN50 (%) | 5.34 (1.11–9.51) | 5.22 (3.39–9.48) | 0.132 | 0.49 | −1.64 |
| HF (ms2) | 179 (92.2–273) | 110 (73.2–205) | 0.677 | 0.56 | −0.73 |
| LF (ms2) | 696 (244–1150) | 552 (354–1010) | 0.255 | 1.36 | 2.34 |
| HF (n.u) | 28.2 (13.1–42.9) | 18.6 (11.6–33.1) | 0.158 | 1.22 | 1.65 |
| LF (n.u) | 71.7 (57.5–87.2) | 17.7 (11.8–33.5) | 0.170 | −1.19 | 1.57 |
| SD1 | 17 (12.3–21.8) | 5.68 (3.84–10.7) | 0.181 | 0.09 | −2.19 |
| SD2 | 36.6 (25.6–48) | 13.5 (8.81–25.1) | 0.734 | 0.29 | 0.35 |
| RPP (mmHg/min) | 9330 (8210–10,400) | 1320 (893–2480) | 0.333 | 0.83 | 1.05 |
| MAP (mmHg) | 94.4 (88.6–99.9) | 6.98 (4.48–12.5) | 0.090 | 1.52 | 2.30 |
| VO2peak−67 | 123 (105–140) | 21.2 (13.6–38.6) | 0.167 | 0.37 | −1.75 |
| RER | 1.13 (1.06–1.19) | 0.08 (0.05–0.15) | 0.920 | −0.00 | −1.02 |
| BPM | 41.5 (34.4–48.4) | 8.49 (5.7–15.9) | 0.047 * (Nor. 0.097) | 1.26 (Nor. 1.10) | 0.28 (Nor. −0.18) |
| HRmax (bpm) | 170 (165–176) | 6.29 (4.17–11.7) | 0.791 | −0.01 | 1.01 |
| Time to exhaustion (s) | 842 (718–956) | 140 (91.3–261) | 0.192 | −0.89 | 0.19 |
| Variable | HF (ms2) | LF (ms2) | HF (n.u) | LF (n.u) | SD1 | SD2 | |
|---|---|---|---|---|---|---|---|
| MeanRR (ms) | rho | 0.357 | −0.465 | 0.324 | −0.332 | 0.231 | −0.393 |
| Upper 95% HDI | 0.793 | 0.284 | 0.802 | 0.394 | 0.799 | 0.32 | |
| Lower 95% HDI | −0.378 | −0.85 | −0.392 | −0.803 | −0.397 | −0.824 | |
| SDNN (ms) | rho | 0.651 | 0.9 | −0.702 | 0.653 | 0.765 | 0.918 |
| Upper 95% HDI | 0.923 | 0.982 | −0.074 | 0.932 | 0.954 | 0.987 | |
| Lower 95% HDI | 0.041 | 0.568 | −0.937 | 0.065 | 0.235 | 0.654 | |
| RMSSD (ms) | rho | 0.9 | 0.716 | −0.177 | 0.269 | 0.92 | 0.721 |
| Upper 95% HDI | 0.982 | 0.943 | 0.464 | 0.767 | 0.987 | 0.947 | |
| Lower 95% HDI | 0.567 | 0.093 | −0.752 | −0.464 | 0.665 | 0.141 | |
| pNN50 (%) | rho | 0.896 | 0.749 | −0.307 | 0.282 | 0.907 | 0.785 |
| Upper 95% HDI | 0.984 | 0.946 | 0.42 | 0.778 | 0.98 | 0.953 | |
| Lower 95% HDI | 0.574 | 0.146 | −0.781 | −0.416 | 0.588 | 0.23 | |
| SD1 | rho | 0.895 | 0.691 | −0.146 | 0.179 | ||
| Upper 95% HDI | 0.982 | 0.94 | 0.483 | 0.724 | |||
| Lower 95% HDI | 0.582 | 0.1 | −0.742 | −0.507 | |||
| SD2 | rho | 0.605 | 0.893 | −0.731 | 0.724 | ||
| Upper 95% HDI | 0.901 | 0.98 | −0.137 | 0.942 | |||
| Lower 95% HDI | −0.083 | 0.575 | −0.94 | 0.135 |
| Variable | RPP | MAP | PeakVO2 | BPM | HRmax | Time | |
|---|---|---|---|---|---|---|---|
| MeanRR (ms) | rho | −0.68 | 0.134 | 0.44 | 0.050 | −0.144 | 0.464 |
| Upper 95% HDI | −0.064 | 0.676 | 0.858 | 0.655 | 0.563 | 0.853 | |
| Lower 95% HDI | −0.935 | −0.564 | −0.251 | −0.601 | −0.682 | −0.249 | |
| SDNN (ms) | rho | 0.672 | 0.578 | 0.132 | −0.195 | 0.488 | −0.398 |
| Upper 95% HDI | 0.918 | 0.89 | 0.724 | 0.423 | 0.863 | 0.344 | |
| Lower 95% HDI | 0.001 | −0.12 | −0.521 | −0.778 | −0.216 | −0.819 | |
| RMSSD (ms) | rho | 0.366 | 0.605 | 0.419 | −0.209 | 0.668 | −0.221 |
| Upper 95% HDI | 0.83 | 0.912 | 0.843 | 0.488 | 0.91 | 0.415 | |
| Lower 95% HDI | −0.319 | −0.058 | −0.278 | −0.737 | −0.044 | −0.788 | |
| pNN50 (%) | rho | 0.376 | 0.671 | 0.423 | −0.237 | 0.629 | −0.37 |
| Upper 95% HDI | 0.827 | 0.928 | 0.833 | 0.432 | 0.907 | 0.36 | |
| Lower 95% HDI | −0.31 | 0.004 | −0.323 | −0.765 | −0.056 | −0.805 | |
| HF (ms2) | rho | 0.303 | 0.639 | 0.461 | −0.153 | 0.626 | −0.295 |
| Upper 95% HDI | 0.782 | 0.924 | 0.858 | 0.481 | 0.917 | 0.412 | |
| Lower 95% HDI | −0.426 | −0.017 | −0.257 | −0.74 | −0.06 | −0.787 | |
| LF (ms2) | rho | 0.733 | 0.629 | 0.031 | −0.227 | 0.41 | −0.323 |
| Upper 95% HDI | 0.935 | 0.904 | 0.624 | 0.434 | 0.819 | 0.371 | |
| Lower 95% HDI | 0.118 | −0.095 | −0.624 | −0.772 | −0.328 | −0.81 | |
| HF (n.u) | rho | −0.346 | −0.177 | −0.157 | −0.061 | −0.089 | −0.023 |
| Upper 95% HDI | 0.388 | 0.477 | 0.496 | 0.641 | 0.558 | 0.613 | |
| Lower 95% HDI | −0.8 | −0.757 | −0.729 | −0.615 | −0.702 | −0.647 | |
| LF (n.u) | rho | 0.345 | 0.246 | 0.16 | 0.011 | 0.064 | −0.009 |
| Upper 95% HDI | 0.804 | 0.748 | 0.719 | 0.633 | 0.683 | 0.616 | |
| Lower 95% HDI | −0.379 | −0.471 | −0.528 | −0.624 | −0.559 | −0.638 | |
| SD1 | rho | 0.362 | 0.599 | 0.473 | −0.217 | 0.652 | −0.303 |
| Upper 95% HDI | 0.825 | 0.909 | 0.856 | 0.497 | 0.916 | 0.426 | |
| Lower 95% HDI | −0.344 | −0.064 | −0.28 | −0.747 | −0.055 | −0.782 | |
| SD2 | rho | 0.692 | 0.551 | 0.078 | −0.242 | 0.481 | −0.403 |
| Upper 95% HDI | 0.939 | 0.906 | 0.672 | 0.42 | 0.852 | 0.326 | |
| Lower 95% HDI | 0.055 | −0.134 | −0.578 | −0.785 | −0.265 | −0.824 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shalfawi, S.A.I. Bayesian Estimation of Correlation between Measures of Blood Pressure Indices, Aerobic Capacity and Resting Heart Rate Variability Using Markov Chain Monte Carlo Simulation and 95% High Density Interval in Female School Teachers. Int. J. Environ. Res. Public Health 2020, 17, 6750. https://doi.org/10.3390/ijerph17186750
Shalfawi SAI. Bayesian Estimation of Correlation between Measures of Blood Pressure Indices, Aerobic Capacity and Resting Heart Rate Variability Using Markov Chain Monte Carlo Simulation and 95% High Density Interval in Female School Teachers. International Journal of Environmental Research and Public Health. 2020; 17(18):6750. https://doi.org/10.3390/ijerph17186750
Chicago/Turabian StyleShalfawi, Shaher A. I. 2020. "Bayesian Estimation of Correlation between Measures of Blood Pressure Indices, Aerobic Capacity and Resting Heart Rate Variability Using Markov Chain Monte Carlo Simulation and 95% High Density Interval in Female School Teachers" International Journal of Environmental Research and Public Health 17, no. 18: 6750. https://doi.org/10.3390/ijerph17186750
APA StyleShalfawi, S. A. I. (2020). Bayesian Estimation of Correlation between Measures of Blood Pressure Indices, Aerobic Capacity and Resting Heart Rate Variability Using Markov Chain Monte Carlo Simulation and 95% High Density Interval in Female School Teachers. International Journal of Environmental Research and Public Health, 17(18), 6750. https://doi.org/10.3390/ijerph17186750





