Brain Activity during Different Throwing Games: EEG Exploratory Study
Abstract
:1. Introduction
1.1. Brain Wave Indicators
1.1.1. Delta Band (0.5–4 Hz)
1.1.2. Theta Band (4–7 Hz)
1.1.3. Alpha Band (7–13 Hz)
1.1.4. Beta Band (13–30 Hz)
1.1.5. Gamma Band (30–50 Hz)
1.2. Motricity and Cortical Records
1.3. Emotion and Cortical Records
1.4. Children’s Play
2. Materials and Methods
2.1. Participants
2.2. Procedure
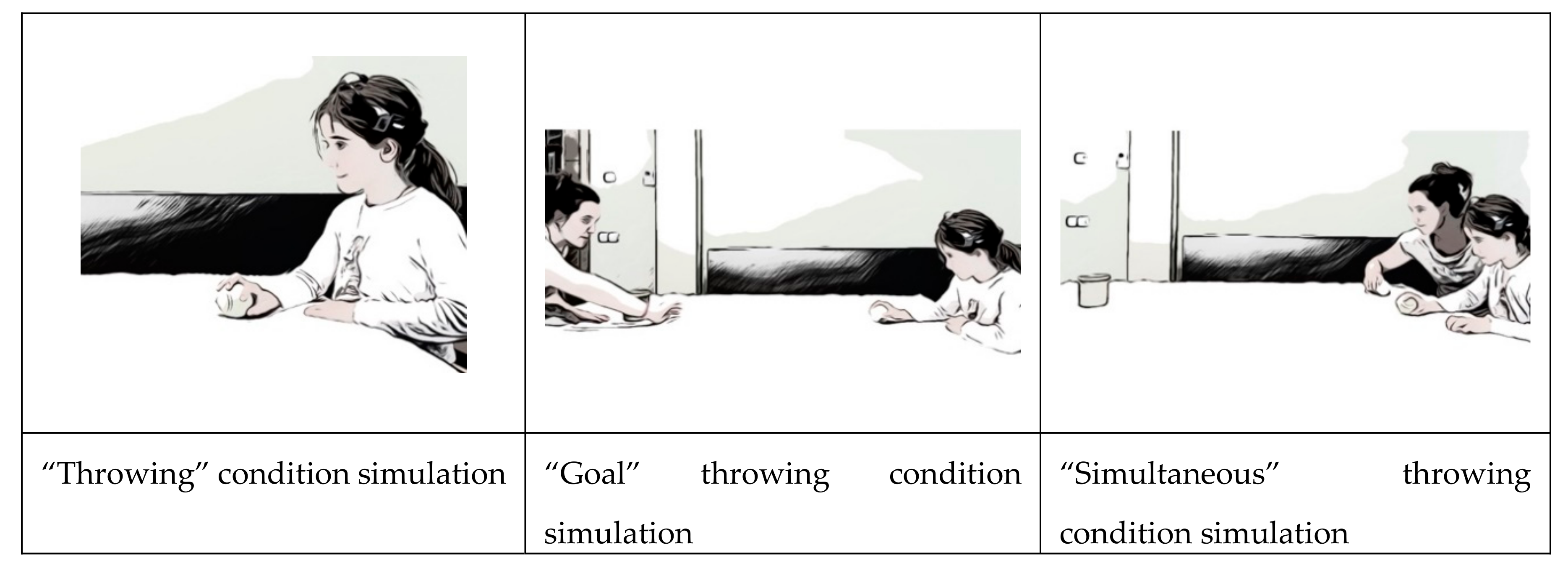
- First condition: “Throwing.” Participant had to throw tennis balls at 10 wooden pieces from 2.5m. In preliminary tests we had seen that it was an easy challenge for children of this age.
- Second condition: “Goal.” Participant had to throw, from a distance of 2.5m, tennis balls to a goal (of 80cm) defended by a dummy handled by a friend of the participant. This challenge increased the complexity of the throw as the target became changeable and a relational variable was introduced into the game.
- Third condition: “Simultaneous.” This consisted of a throw to 10 wooden blocks located 2.5 m away, simultaneously to another opponent who threw to the same targets. This challenge introduces a time factor (knocking down the blocks before the opponent) and therefore could increase the arousal.
2.3. Signal Pre-processing
2.4. Statistical Analysis
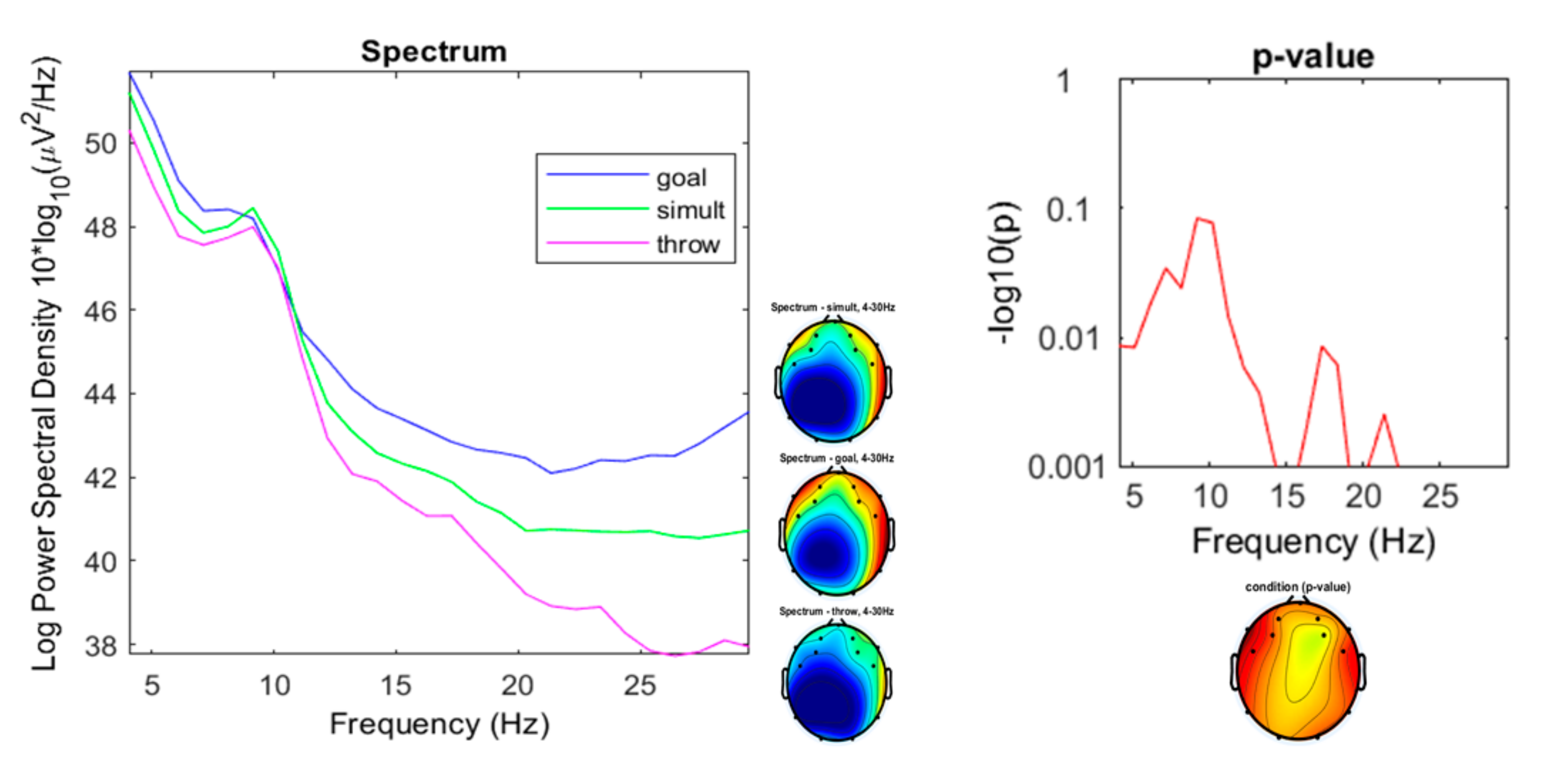
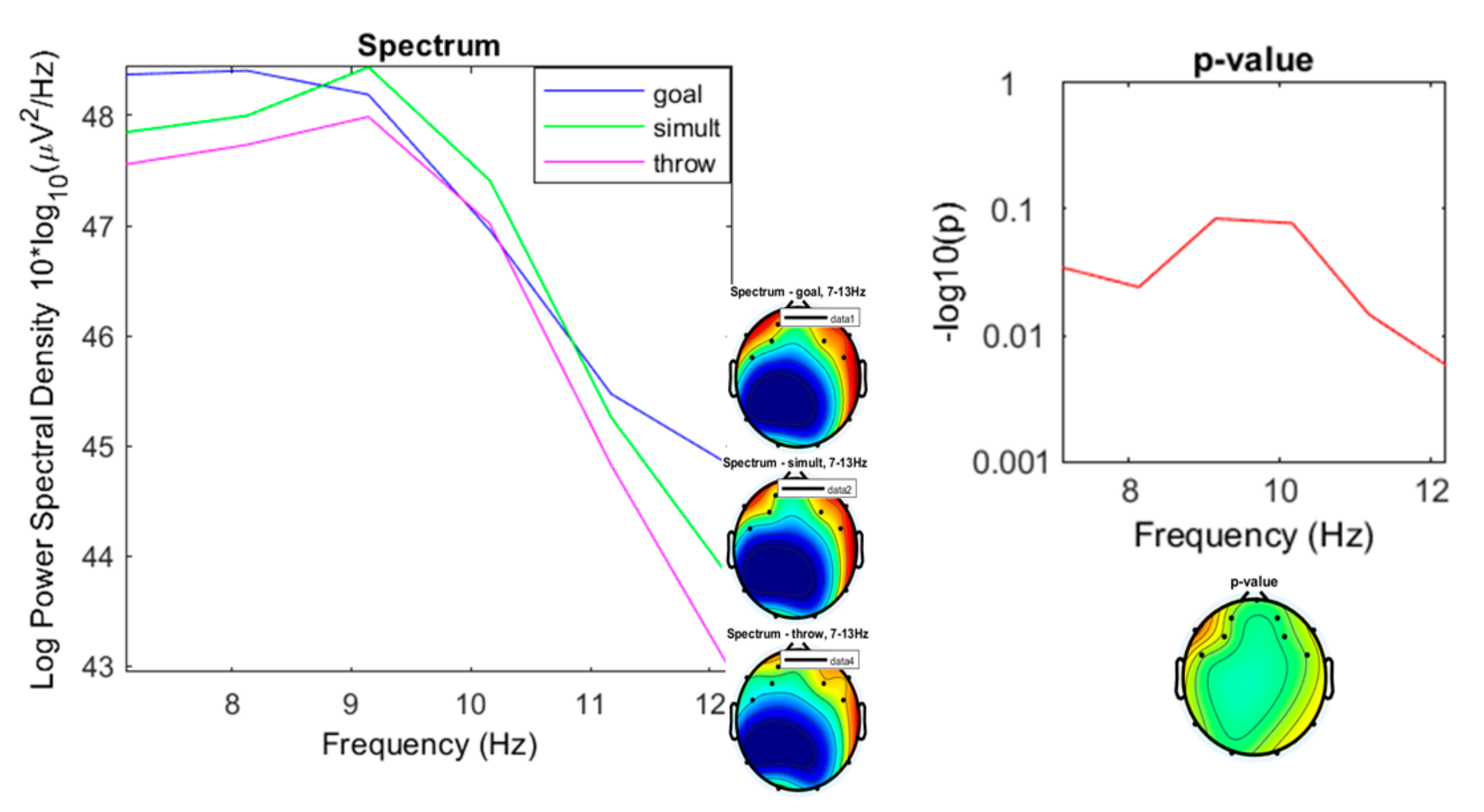
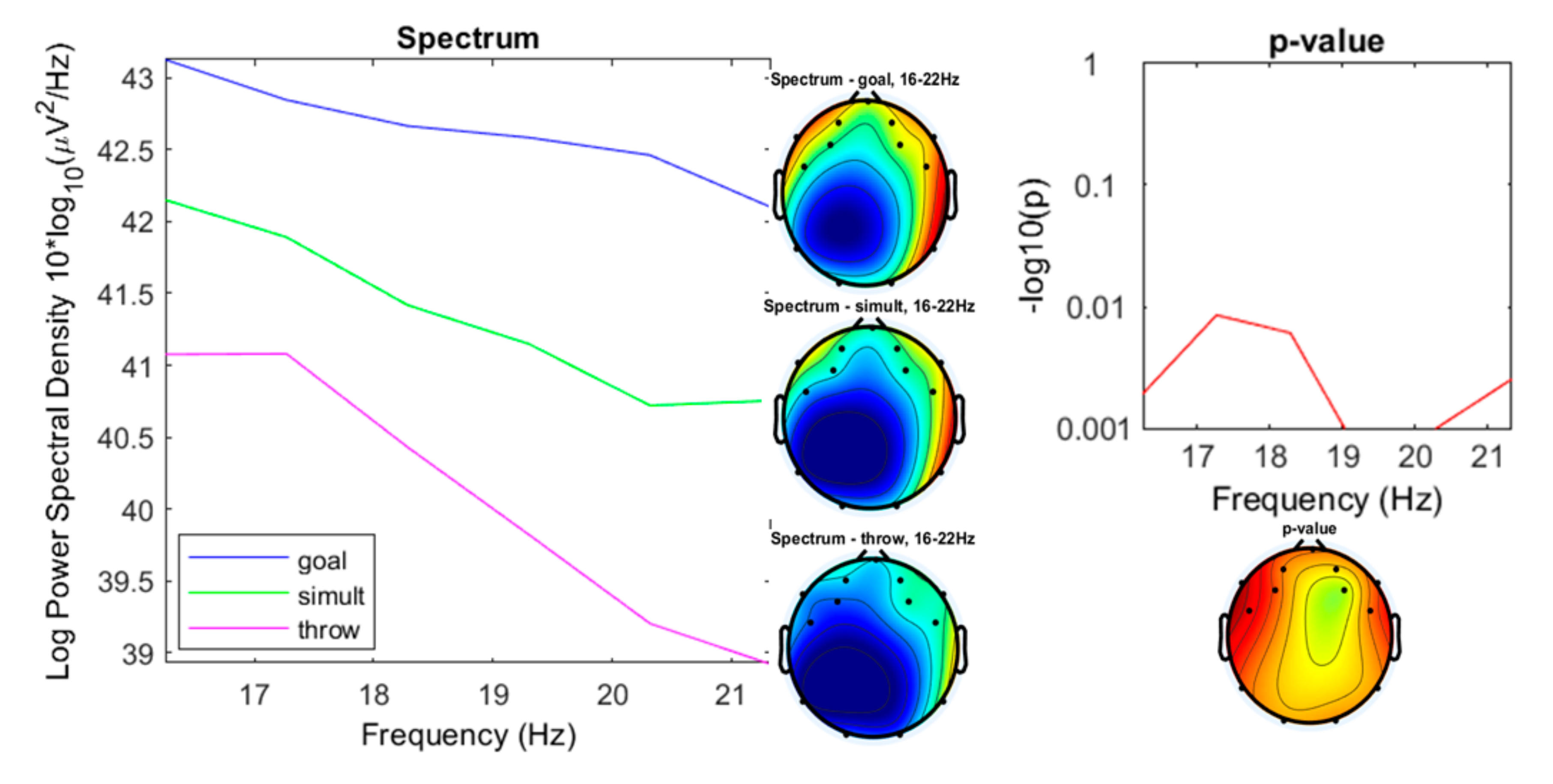
3. Results
3.1. Preliminary Data Inspection
3.2. Comparison of the Three Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Koeners, M.P.; Francis, J. The physiology of play: Potential relevance for higher education. Int. J. Play 2020, 9, 143–159. [Google Scholar] [CrossRef] [Green Version]
- Siviy, S.M. A Brain Motivated to Play: Insights into the Neurobiology of Playfulness. Behaviour 2016, 153, 819–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Monge, A.; Rodríguez-Navarro, H. Description of “logics” in motor games with rules in school physical education. Cult. Edu. 2013, 25, 35–47. [Google Scholar] [CrossRef]
- Casero-Carrillo, F.; García-Monge, A. Relationship between players personality and games structures: An exploratory study. Acc. Motriz 2017, 19, 47–58. [Google Scholar]
- Pizzagalli, D.A. Electroencephalography and High-Density Electrophysiological Source Localization. In Handbook of Psychophysiology; Cacioppo, J.T., Tassinary, L.G., Berntson, G., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 56–85. [Google Scholar]
- von Mohr, M.; Crowley, M.J.; Walthall, J. EEG captures affective touch: CT-optimal touch and neural oscillations. Cogn. Affect Behav. Neurosci. 2018, 18, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Tatum, W.O.; Husain, A.M.; Benbadis, S.L.; Kaplan, P. Handbook of EEG Interpretation; Demos: New York, NY, USA, 2008. [Google Scholar]
- Nácher, V.; Ledberg, A.; Deco, G.; Romo, R. Coherent delta-band oscillations between cortical areas correlate with decision making. Proc. Natl. Acad. Sci. USA 2013, 110, 15085–15090. [Google Scholar] [CrossRef] [Green Version]
- Güntekin, B.; Başar, E. Review of evoked and event-related delta responses in the human brain. Int. J. Psychophysiol. 2016, 103, 43–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnal, L.H.; Doelling, K.B.; Poeppel, D. Delta-Beta Coupled Oscillations Underlie Temporal Prediction Accuracy. Cereb Cortex. 2015, 25, 3077–3085. [Google Scholar] [CrossRef] [Green Version]
- Gruzelier, J. A theory of alpha/theta neurofeedback, creative performance enhancement, long distance functional connectivity and psychological integration. Cogn. Process. 2009, 10, 101–109. [Google Scholar] [CrossRef]
- Clarke, A.R.; Barry, R.J.; McCarthy, R.; Selikowitz, M. Age and sex effects in the EEG: Development of the normal child. Clin. Neurophysiol. 2001, 112, 806–814. [Google Scholar] [CrossRef]
- Baars, J.; Gage, N.M. Cognition, Brain and Consciousness; Elsevier: San Diego, CA, USA, 2010. [Google Scholar]
- Cavanagh, J.F.; Frank, M.J. Frontal theta as a mechanism for cognitive control. Tr. Cogn. Sci. 2014, 18, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Domic-Siede, M.; Irani, M.; Valdés, J.; Perrone-Bertolotti, M.; Ossandón, T. Frontal Midline Theta Reflects Cognitive Control during Planning. BioRxiv 2019, 648758. [Google Scholar] [CrossRef]
- Kao, S.C.; Huang, C.J.; Hung, T.M. Frontal midline theta is a specific indicator of optimal attentional engagement during skilled putting performance. J Sport Exerc Psychol. 2013, 35, 470–478. [Google Scholar] [CrossRef]
- Lopes da Silva, F. EEG and MEG: Relevance to neuroscience. Neuron 2013, 80, 1112–1128. [Google Scholar] [CrossRef] [Green Version]
- Malik, A.S.; Amin, H.U. Designing EEG Experiments for Studying the Brain: Design Code and Example Datasets; Elsevier: London, UK, 2017. [Google Scholar]
- Klimesch, W.; Doppelmayr, W.; Russegger, H.; Pachinger, T.; Schwaiger, J. Induced alpha band power changes in the human EEG and attention. Neurosc. Lett. 1998, 244, 73–76. [Google Scholar] [CrossRef]
- Güntekin, B.; Başar, E. A review of brain oscillations in perception of faces and emotional pictures. Neuropsychologia 2014, 58, 33–51. [Google Scholar] [CrossRef]
- Fries, P. Rhythms for Cognition: Communication through Coherence. Neuron 2015, 88, 220–235. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.J. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev. 2010, 90, 1195–1268. [Google Scholar] [CrossRef]
- Martini, N.; Menicucci, D.; Sebastiani, L.; Bedini, R.; Pingitore, A.; Vanello, N.; Milanesi, M.; Landini, L.; Gemignani, A. The dynamics of EEG gamma responses to unpleasant visual stimuli: From local activity to functional connectivity. NeuroImage 2012, 60, 922–932. [Google Scholar] [CrossRef]
- Klimesch, W. α-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 2012, 16, 606–617. [Google Scholar] [CrossRef] [Green Version]
- Knyazev, G.G. Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neurosci. Biobehav. Rev. 2007, 31, 377–395. [Google Scholar] [CrossRef]
- Tran, Y.; Craig, A.; McIsaac, P. Extraversion–introversion and 8–13 Hz waves in frontal cortical regions. Personal. Individ. Differ. 2001, 30, 205–215. [Google Scholar] [CrossRef]
- Klados, M.A.; Konstantinidi, P.; Dacosta-Aguayo, R.; Kostaridou, V.D.; Vinciarelli, A.; Zervakis, M. Automatic Recognition of Personality Profiles Using EEG Functional Connectivity During Emotional Processing. Brain Sci. 2020, 10, 278. [Google Scholar] [CrossRef] [PubMed]
- Park, J.L.; Fairweather, M.M.; Donaldson, D.I. Making the case for mobile cognition: EEG and sports performance. Neurosci. Biobehav. Rev. 2015, 52, 117–130. [Google Scholar] [CrossRef] [Green Version]
- Babiloni, C.; Marzano, N.; Iacoboni, M.; Infarinato, F.; Aschieri, P.; Buffo, P.; Del Percio, C. Resting state cortical rhythms in athletes: A high-resolution EEG study. Brain Res. Bull. 2010, 81, 149–156. [Google Scholar] [CrossRef]
- Del Percio, C.; Babiloni, C.; Marzano, N. Neural efficiency of athletes’ brain for upright standing: A high-resolution EEG study. Brain Res Bull. 2009, 79, 193–200. [Google Scholar] [CrossRef]
- Janelle, C.M.; Hatfield, B.D. Visual attention and brain processes that underlie expert performance: Implications for sport and military psychology. Military Psychology 2008, 20, 39–69. [Google Scholar] [CrossRef]
- Baumeister, J.; Reinecke, K.; Liesen, H.; Weiss, M. Cortical activity of skilled performance in a complex sports related motor task. Eur. J. Appl. Physiol. 2008, 104, 625–631. [Google Scholar] [CrossRef]
- Deeny, S.P.; Hillman, C.H.; Janelle, C.M.; Hatfield, B.D. Cortico-cortical communication and superior performance in skilled marksmen: An EEG coherence analysis. J. Sport Exer. Psycho. 2003, 25, 188–204. [Google Scholar] [CrossRef]
- Grabner, R.H.; Neubauer, A.C.; Stern, E. Superior performance and neural efficiency: The impact of intelligence and expertise. Brain Res. Bull. 2006, 69, 422–439. [Google Scholar] [CrossRef]
- Neubauer, A.C.; Grabner, R.H.; Freudenthaler, H.H.; Beckmann, J.F.; Guthke, J. Intelligence and individual differences in becoming neurally efficient. Acta Psychol. 2004, 116, 55–74. [Google Scholar] [CrossRef]
- Chang, C.Y.; Hung, T.M. Understanding and Controlling Cortical Activity for Superior Performance. Kinesi. Rev. 2020, 9, 41–50. [Google Scholar] [CrossRef]
- Johnson, B.; Jobst, C.; Al-Loos, R.; He, W.; Cheyne, D. Developmental Changes in Movement Related Brain Activity in Early Childhood. BioRxiv 2019, 531905. [Google Scholar] [CrossRef] [Green Version]
- Durston, S.; Casey, B.J. What have we learned about cognitive development from neuroimaging? Neuropsychologia 2006, 44, 2149–2157. [Google Scholar] [CrossRef]
- Bekkedal, M.Y.; Rossi, J.; Panksepp, J. Human brain EEG indices of emotions: Delineating responses to affective vocalizations by measuring frontal theta event-related synchronization. Neur. Biobeh. Rev. 2011, 35, 1959–1970. [Google Scholar] [CrossRef]
- Lewis, M.D. Bridging emotion theory and neurobiology through dynamic systems modeling. Behav. Brain Sci. 2005, 28, 169–194. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Xie, J.; Yang, M.; Li, Z.; Li, Z.; Liao, D.; Xu, X.; Yang, X. A Review of Emotion Recognition Using Physiological Signals. Sensors 2018, 18, 2074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dzedzickis, A.; Kaklauskas, A.; Bucinskas, V. Human Emotion Recognition: Review of Sensors and Methods. Sensors 2020, 20, 592. [Google Scholar] [CrossRef] [Green Version]
- Güntekin, B.; Başar, E. Event-related beta oscillations are affected by emotional eliciting stimuli. Neurosci Lett. 2010, 483, 173–178. [Google Scholar] [CrossRef]
- Balconi, M.; Lucchiari, C. Consciousness and arousal effects on emotional face processing as revealed by brain oscillations. A gamma band analysis. Int. J. Psychophysiol. 2008, 67, 41–46. [Google Scholar] [CrossRef]
- Li, M.; Lu, B.L. Emotion classification based on gamma-band EEG. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009, 2009, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Ichikawa, Y.; Kanayama, N.; Ohira, H.; Iidaka, T. Gamma band activity and its synchronization reflect the dysfunctional emotional processing in alexithymic persons. Psychophysiology 2006, 43, 533–540. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Y.; Wang, J.; Tong, S.; Li, H.; Yan, J. Induced gamma activity in EEG represents cognitive control during detecting emotional expressions. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2011, 2011, 1717–1720. [Google Scholar] [CrossRef]
- Abhang, P.A.; Gawali, B.W.; Mehrotra, S.C. Technical aspects of brain rhythms and speech parameters. In Introduction to EEG- and Speech-Based Emotion Recognition; Abhang, P.A., Gawali, B.W., Mehrotra, S.C., Eds.; Academic Press: Cambridge, MA, USA; Cambridge, UK, 2016; pp. 51–79. [Google Scholar] [CrossRef]
- Wei, L.; Duan, X.; Zheng, C. Specific frequency bands of amplitude low-frequency oscillation encodes personality. Hum Brain Mapp. 2014, 35, 331–339. [Google Scholar] [CrossRef]
- Stevens, P. Yes, we need a neuroscience of play. Int. J. Play 2020, 1–10. [Google Scholar] [CrossRef]
- Moyá, J. El juego como función neurofisiológica. In Juegos, Juguetes y Ludotecas; Tripero, T.A., Ed.; Publicaciones Pablo Montesino: Madrid, Spain, 1990; pp. 165–167. [Google Scholar]
- Pellis, S.M.; Pellis, V.C.; Whishaw, I.Q. The role of the cortex in play fighting by rats: Developmental and evolutionary implications. Brain Behav. Evol. 1992, 39, 270–284. [Google Scholar] [CrossRef]
- Liu, C.; Solis, S.; Jensen, H.; Hopkins, E.; Neale, D.; Zosh, J.; Whitebread, D. Neuroscience and Learning through Play: A Review of the Evidence (Research Summary); The LEGO Foundation: Billund, Denmark, 2019. [Google Scholar]
- Sutton-Smith, B. Ambiguity of Play; Harvard University Press: Cambdridge, UK, 2001. [Google Scholar]
- Lee, P.F.; Kan, P.X. A pilot study on theta frequency of preschool children with different playing activities at prefrontal cortex. Biomedical Engineering: Applications. Basis Commun. 2017, 29, 1750004. [Google Scholar] [CrossRef]
- Yamada, F. Frontal midline theta rhythm and eyeblinking activity during a VDT task and a video game: Useful tools for psychophysiology in ergonomics. Ergonomics 1998, 41, 678–688. [Google Scholar] [CrossRef]
- Charan, J.; Biswas, T. How to calculate sample size for different study designs in medical research? Indian J. Psychol. Med. 2013, 35, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Arambula, P.; Peper, E.; Kawakami, M.; Gibney, K.H. The physiological correlates of Kundalini Yoga meditation: A study of a Yoga master. Appl. Psychophys. Biofeed. 2001, 26, 147–153. [Google Scholar] [CrossRef]
- Tsai, J.F.; Jou, S.H.; Cho, W.C.; Lin, C.M. Electroencephalography when meditation advances: A case-based time-series analysis. Cogn. Proc. 2013, 14, 371–376. [Google Scholar] [CrossRef]
- Kjaer, T.W.; Bertelsen, C.; Piccini, P.; Brooks, D.; Alving, J.; Lou, H.C. Increased dopamine tone during meditation-induced change of consciousness. Cogn. Brain Res. 2002, 13, 255–259. [Google Scholar] [CrossRef]
- Barnhofer, T.; Chittka, T.; Nightingale, H.; Visser, C.; Crane, C. State tate effects of two forms of meditation on prefrontal EEG asymmetry in previously depressed individuals. Mindfulness 2010, 1, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Vialatte, F.B.; Bakardjian, H.; Prasad, R.; Cichocki, A. EEG paroxysmal gamma waves during bhramari pranayama: A yoga breathing technique. Consc. Cogn. 2009, 18, 977–988. [Google Scholar] [CrossRef]
- Brooker, R.J.; Bates, J.E.; Buss, K.A.; Canen, M.J.; Dennis-Tiwary, T.A.; Gatzke-Kopp, L.M.; Hoyniak, C.P.; Klein, D.N.; Kujawa, A.; Lahat, A.; et al. Conducting Event-Related Potential (ERP) Research with Young Children: A Review of Components, Special Considerations, and Recommendations for Research on Cognition and Emotion. J. Psychophys. 2020, 34, 137–158. [Google Scholar] [CrossRef]
- Miranda-Correa, J.A.; Abadi, M.K.; Sebe, N.; Patras, I. AMIGOS: A Dataset for Affect, Personality and Mood Research on Individuals and Groups. IEEE Trans. Aff. Comp. 2018. [Google Scholar] [CrossRef] [Green Version]
- Mehmood, R.; Lee, H. Towards Building a Computer Aided Education System for Special Students Using Wearable Sensor Technologies. Sensors 2017, 17, 317. [Google Scholar] [CrossRef]
- Pham, T.D.; Tran, D. Emotion Recognition Using the Emotiv EPOC Device. In Neural Information Processing; Huang, T., Zeng, Z., Li, C., Leung, C.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Aspinall, P.; Mavros, P.; Coyne, R.; Roe, J. The urban brain: Analysing outdoor physical activity with mobile EEG. Br. J. Sp. Med. 2015, 49, 272–276. [Google Scholar] [CrossRef] [Green Version]
- Maureira, F.; Flores, E.; Díaz, H.; Navarro, B.; Gavotto, O.; Matheu, A. Efectos de una sesión de ejercicio físico sobre la actividad neurofisiológica durante la resolución de una prueba de atención selectiva. Retos 2019, 36, 391–397. [Google Scholar]
- Sheikholeslami, C.; Yuan, H.; He, E.J.; Bai, X.; Yang, L.; He, B. A high resolution EEG study of dynamic brain activity during video game play. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2007, 2007, 2489–2491. [Google Scholar] [CrossRef]
- Reuderink, B.; Mühl, C.; Poel, M. Valence, arousal and dominance in the EGG during game play. Int. J. Aut. Adap. Commun. Syst. 2013, 6, 45–62. [Google Scholar] [CrossRef]
- Jahng, J.; Kralik, J.D.; Hwang, D.U. Neural dynamics of two players when using nonverbal cues to gauge intentions to cooperate during the Prisoner’s Dilemma Game. Neuroimage 2017, 157, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.N. Oscillatory interactions between sensorimotor cortex and the periphery. Curr. Opin. Neurobiol. 2007, 17, 649–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engel, A.K.; Fries, P. Beta-band oscillations—Signalling the status quo? Curr. Opin. Neurobiol. 2010, 20, 156–165. [Google Scholar] [CrossRef]
- Wang, S.; Aamodt, S. Play, stress, and the learning brain. Cerebrum 2012, 2012, 12. [Google Scholar]
- Wimmer, K.; Ramon, M.; Pasternak, T.; Compte, A. Transitions between Multiband Oscillatory Patterns Characterize Memory-Guided Perceptual Decisions in Prefrontal Circuits. J. Neurosci. 2016, 36, 489–505. [Google Scholar] [CrossRef] [Green Version]
- Wong, Y.T.; Fabiszak, M.M.; Novikov, Y.; Daw, N.D.; Pesaran, B. Coherent neuronal ensembles are rapidly recruited when making a look-reach decision. Nat. Neurosci. 2016, 19, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Spitzer, B.; Haegens, S. Beyond the Status Quo: A Role for Beta Oscillations in Endogenous Content (Re) Activation. eNeuro, 2017; 4, ENEURO.0170-17.2017. [Google Scholar] [CrossRef] [Green Version]
- Berta, R.; Bellotti, F.; De Gloria, A.; Pranantha, D.; Schatten, C. Electroencephalogram and Physiological Signal Analysis for Assessing Flow in Games. IEEE Trans. Comp. Int. AI Games 2013, 5, 164–175. [Google Scholar] [CrossRef]
- Marco-Pallarés, J.; Münte, T.F.; Rodríguez-Fornells, A. The role of high-frequency oscillatory activity in reward processing and learning. Neurosci. Biobehav. Rev. 2015, 49, 1–7. [Google Scholar] [CrossRef]
- Parlebas, P. Elementos de Sociología del Deporte; Junta de Andalucía: Málaga, Spain, 1988. [Google Scholar]












© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Monge, A.; Rodríguez-Navarro, H.; González-Calvo, G.; Bores-García, D. Brain Activity during Different Throwing Games: EEG Exploratory Study. Int. J. Environ. Res. Public Health 2020, 17, 6796. https://doi.org/10.3390/ijerph17186796
García-Monge A, Rodríguez-Navarro H, González-Calvo G, Bores-García D. Brain Activity during Different Throwing Games: EEG Exploratory Study. International Journal of Environmental Research and Public Health. 2020; 17(18):6796. https://doi.org/10.3390/ijerph17186796
Chicago/Turabian StyleGarcía-Monge, Alfonso, Henar Rodríguez-Navarro, Gustavo González-Calvo, and Daniel Bores-García. 2020. "Brain Activity during Different Throwing Games: EEG Exploratory Study" International Journal of Environmental Research and Public Health 17, no. 18: 6796. https://doi.org/10.3390/ijerph17186796
APA StyleGarcía-Monge, A., Rodríguez-Navarro, H., González-Calvo, G., & Bores-García, D. (2020). Brain Activity during Different Throwing Games: EEG Exploratory Study. International Journal of Environmental Research and Public Health, 17(18), 6796. https://doi.org/10.3390/ijerph17186796





