Pseudomonas fluorescens: A Bioaugmentation Strategy for Oil-Contaminated and Nutrient-Poor Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of the Study Area and Soil Sample
2.2. Culture Medium and Growth Kinetics of P. Fluorescens
2.3. Physicochemical Characterization of the Soil
2.4. Experimental Design
2.5. Analytical Methods for the Measurement of the Response Variables
2.5.1. Extraction of Residual TPH from the Soil
2.5.2. Surface Tension
2.5.3. Respiratory Activity (CO2)
2.5.4. Microbial Count
2.6. Statistical Analysis
3. Results and Discussion
3.1. Soil Properties
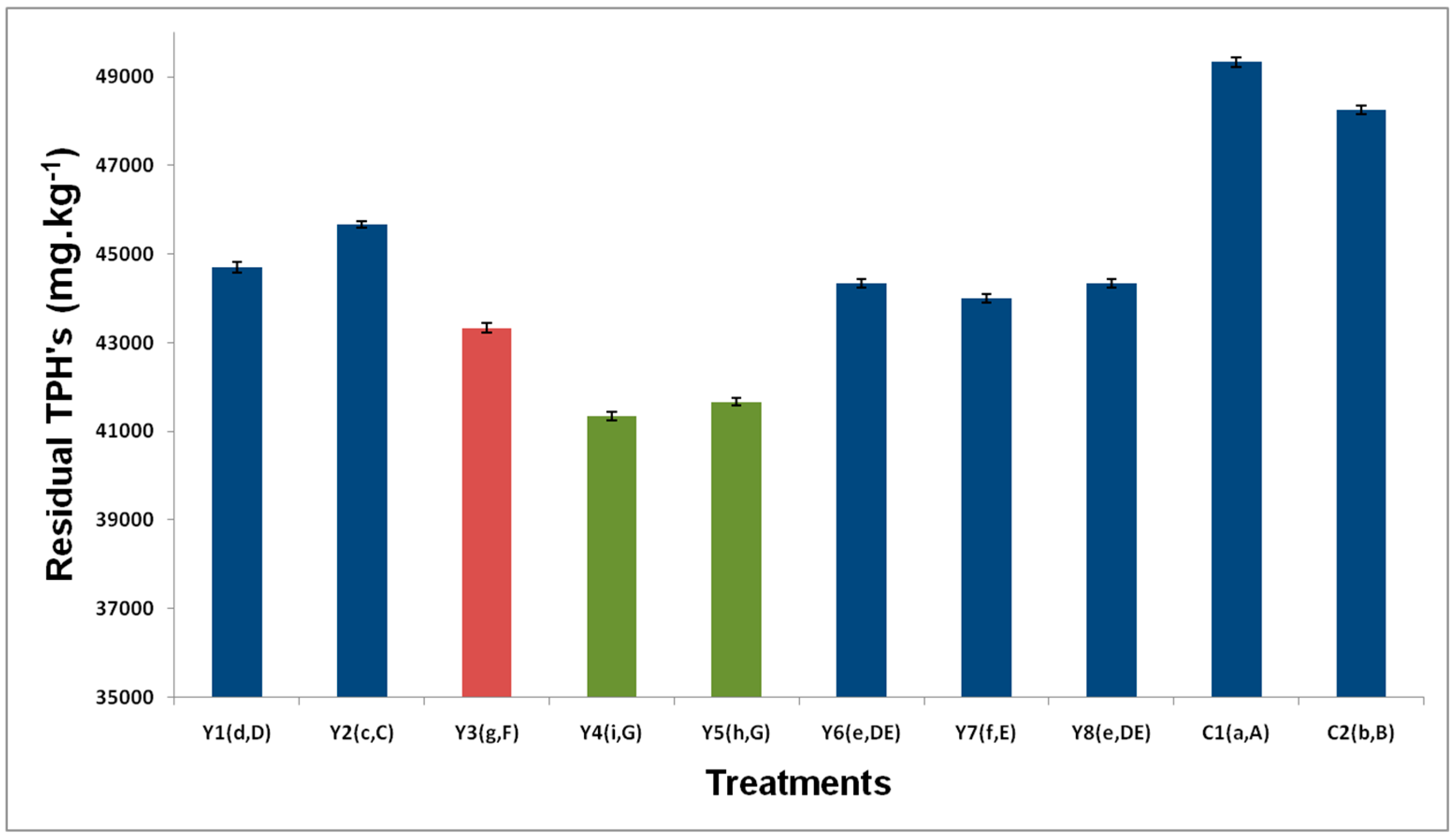
3.2. Residual TPH
3.3. Surface Tension
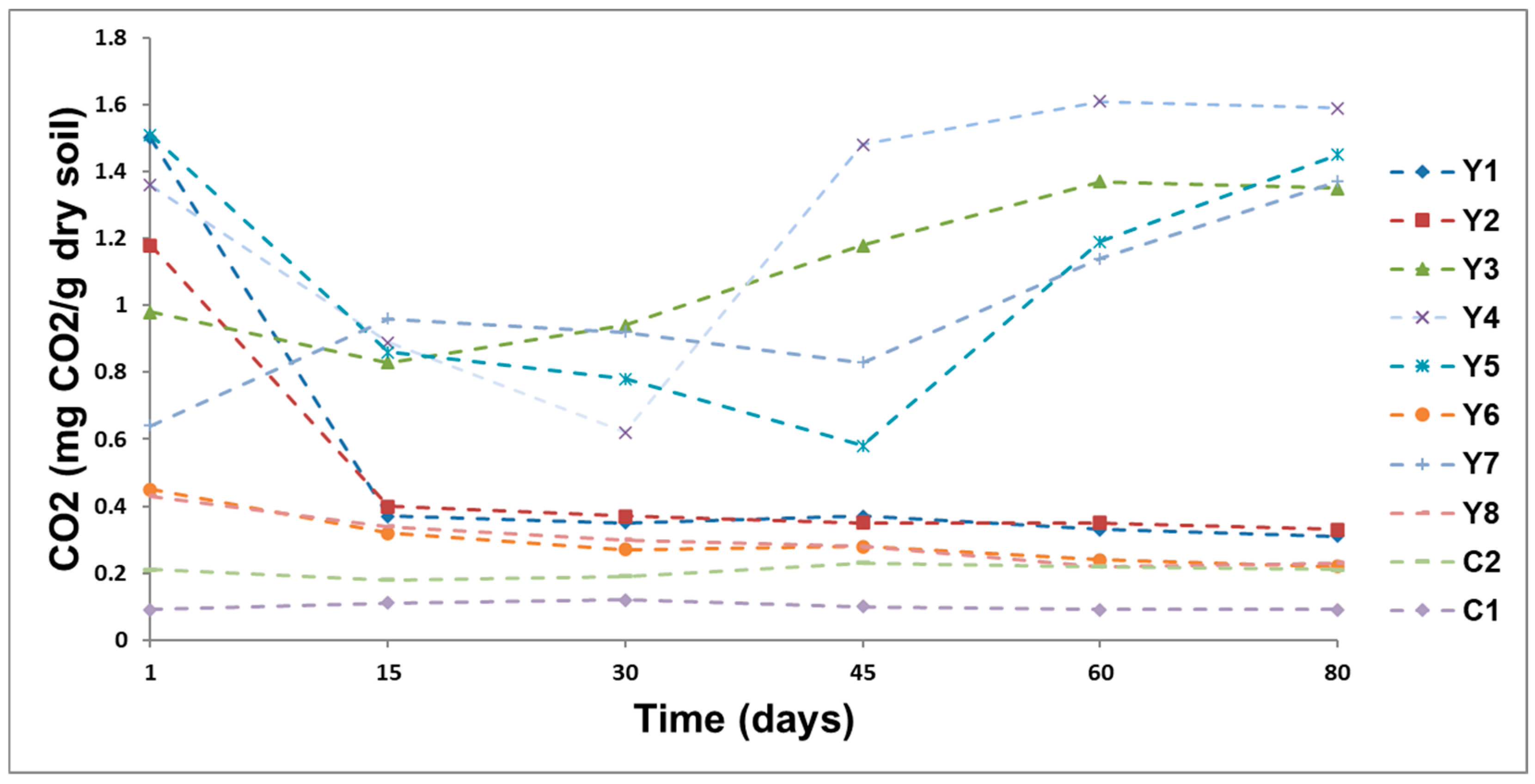
3.4. Soil Respiration
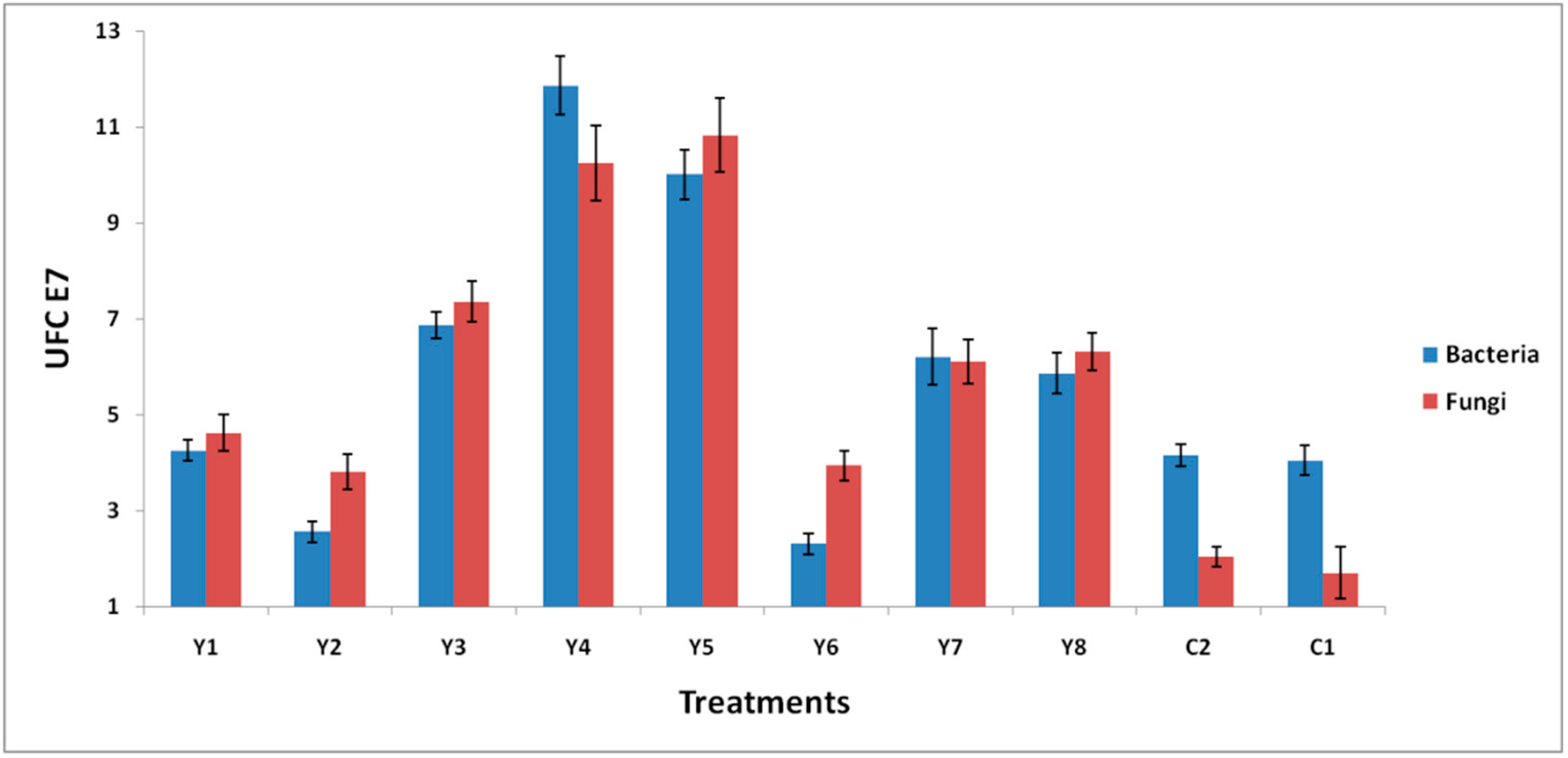
3.5. Population Growth of Bacteria and Fungi
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roy, A.; Dutta, A.; Pal, S.; Gupta, A.; Sarkar, J.; Chatterjee, A.; Saha, A.; Sarkar, P.; Sar, P.; Kazy, S.K. Biostimulation and bioaugmentation of native microbial community accelerated bioremediation of oil refinery sludge. Bioresour. Technol. 2018, 253, 22–32. [Google Scholar] [CrossRef]
- Maletić, S.; Dalmacija, B.D.; Rončević, S.D.; Agbaba, J.R.; Perović, S.U. Impact of hydrocarbon type, concentration and weathering on its biodegradability in soil. J. Environ. Sci. Health Part A 2011, 46, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry (ATSDR). ATSDR’s Substance Priority List. Available online: https://www.atsdr.cdc.gov/spl/ (accessed on 21 August 2018).
- Abed, R.M.M.; Al-Kharusi, S.; Al-Hinai, M. Effect of biostimulation, temperature and salinity on respiration activities and bacterial community composition in an oil polluted desert soil. Int. Biodeterior. Biodegradation 2015, 98, 43–52. [Google Scholar] [CrossRef]
- Cerqueira, V.S.; Peralba, M.C.R.; Camargo, F.A.O.; Bento, F.M. Comparison of bioremediation strategies for soil impacted with petrochemical oily sludge. Int. Biodeterior. Biodegrad. 2014, 95, 338–345. [Google Scholar] [CrossRef]
- Coulon, F.; Brassington, K.J.; Bazin, R.; Linnet, P.E.; Thomas, K.A.; Mitchell, T.R.; Lethbridge, G.; Smith, J.W.N.; Pollard, S.J. Effect of fertilizer formulation and bioaugmentation on biodegradation and leaching of crude oils and refined products in soils. Environ. Technol. 2012, 33, 1879–1893. [Google Scholar] [CrossRef]
- Andreolli, M.; Lampis, S.; Brignoli, P.; Vallini, G. Bioaugmentation and biostimulation as strategies for the bioremediation of a burned woodland soil contaminated by toxic hydrocarbons: A comparative study. J. Environ. Manag. 2015, 153, 121–131. [Google Scholar] [CrossRef]
- Wu, M.; Chen, L.; Tian, Y.; Ding, Y.; Dick, W.A. Degradation of polycyclic aromatic hydrocarbons by microbial consortia enriched from three soils using two different culture media. Environ. Pollut. 2013, 178, 152–158. [Google Scholar] [CrossRef]
- Taccari, M.; Milanovic, V.; Comitini, F.; Casucci, C.; Ciani, M. Effects of biostimulation and bioaugmentation on diesel removal and bacterial community. Int. Biodeterior. Biodegrad. 2012, 66, 39–46. [Google Scholar] [CrossRef]
- Abed, R.M.M.; Al-Sabahi, J.; Al-Maqrashi, F.; Al-Habsi, A.; Al-Hinai, M. Characterization of hydrocarbon-degrading bacteria isolated from oil-contaminated sediments in the Sultanate of Oman and evaluation of bioaugmentation and biostimulation approaches in microcosm experiments. Int. Biodeterior. Biodegrad. 2014, 89, 58–66. [Google Scholar] [CrossRef]
- Sayara, T.; Borràs, E.; Caminal, G.; Sarrà, M.; Sánchez, A. Bioremediation of PAHs-contaminated soil through composting: Influence of bioaugmentation and biostimulation on contaminant biodegradation. Int. Biodeterior. Biodegrad. 2011, 65, 859–865. [Google Scholar] [CrossRef]
- Tahhan, R.A.; Ammari, T.G.; Goussous, S.J.; Al-Shdaifat, H.I. Enhancing the biodegradation of total petroleum hydrocarbons in oily sludge by a modified bioaugmentation strategy. Int. Biodeterior. Biodegrad. 2011, 65, 130–134. [Google Scholar] [CrossRef]
- Suja, F.; Rahim, F.; Taha, M.R.; Hambali, N.; Razali, M.R.; Khalid, A.; Hamzah, A. Effects of local microbial bioaugmentation and biostimulation on the bioremediation of total petroleum hydrocarbons (TPH) in crude oil contaminated soil based on laboratory and field observations. Int. Biodeterior. Biodegrad. 2014, 90, 115–122. [Google Scholar] [CrossRef]
- Mayumi, D.; Dolfing, J.; Sakata, S.; Maeda, H.; Miyagawa, Y.; Ikarashi, M.; Tamaki, H.; Takeuchi, M.; Nakatsu, C.H.; Kamagata, Y. Carbon dioxide concentration dictates alternative methanogenic pathways in oil reservoirs. Nat. Commun. 2013, 4, 1998. [Google Scholar] [CrossRef]
- Das, R.; Kazy, S.K. Microbial diversity, community composition and metabolic potential in hydrocarbon contaminated oily sludge: Prospects for in situ bioremediation. Environ. Sci. Pollut. Res. 2014, 21, 7369–7389. [Google Scholar] [CrossRef] [PubMed]
- Linn, D.M.; Doran, J.W. Effect of Water-Filled Pore Space on Carbon Dioxide and Nitrous Oxide Production in Tilled and Nontilled Soils. Soil Sci. Soc. Am. J. 1984, 48, 1267–1272. [Google Scholar] [CrossRef]
- Tan, B.; Fowler, S.J.; Abu Laban, N.; Dong, X.; Sensen, C.W.; Foght, J.; Gieg, L.M. Comparative analysis of metagenomes from three methanogenic hydrocarbon-degrading enrichment cultures with 41 environmental samples. ISME J. 2015, 9, 2028–2045. [Google Scholar] [CrossRef]
- Ghoreishi, G.; Alemzadeh, A.; Mojarrad, M.; Djavaheri, M. Bioremediation capability and characterization of bacteria isolated from petroleum contaminated soils in Iran. Sustain. Environ. Res. 2017, 27, 195–202. [Google Scholar] [CrossRef]
- Liu, C.; You, Y.; Zhao, R.; Sun, D.; Zhang, P.; Jiang, J.; Zhu, A.; Liu, W. Biosurfactant production from Pseudomonas taiwanensis L1011 and its application in accelerating the chemical and biological decolorization of azo dyes. Ecotoxicol. Environ. Saf. 2017, 145, 8–15. [Google Scholar] [CrossRef]
- Aparna, A.; Srinikethan, G.; Smitha, H. Production and characterization of biosurfactant produced by a novel Pseudomonas sp. 2B. Colloids Surf. B Biointerfaces 2012, 95, 23–29. [Google Scholar] [CrossRef]
- Al-Wahaibi, Y.M.; Joshi, S.; Al-Bahry, S.; Elshafie, A.; Al-Bemani, A.; Shibulal, B. Biosurfactant production by Bacillus subtilis B30 and its application in enhancing oil recovery. Colloids Surf. B Biointerfaces 2014, 114, 324–333. [Google Scholar] [CrossRef]
- Sha, R.; Meng, Q. Antifungal activity of rhamnolipids against dimorphic fungi. J. Gen. Appl. Microbiol. 2016, 62, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Pantsyrnaya, T.; Blanchard, F.; Delaunay, S.; Goergen, J.-L.; Guedon, E.; Guseva, E.; Boudrant, J. Effect of surfactants, dispersion and temperature on solubility and biodegradation of phenanthrene in aqueous media. Chemosphere 2011, 83, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Wyrwas, B.; Chrzanowski, Ł.; Ławniczak, Ł.; Szulc, A.; Cyplik, P.; Białas, W.; Szymański, A. Utilization of Triton X-100 and polyethylene glycols during surfactant-mediated biodegradation of diesel fuel. J. Hazard. Mater. 2011, 197, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Szulc, A.; Ambrożewicz, D.; Sydow, M.; Ławniczak, Ł.; Piotrowska-Cyplik, A.; Marecik, R.; Chrzanowski, Ł. The influence of bioaugmentation and biosurfactant addition on bioremediation efficiency of diesel-oil contaminated soil: Feasibility during field studies. J. Environ. Manag. 2014, 132, 121–128. [Google Scholar] [CrossRef]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L. Biosurfactants: Multifunctional Biomolecules of the 21st Century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef]
- Sarubbo, L.; Rocha, R.; Luna, J.; Rufino, R.; Santos, V.; Banat, I.M. Some aspects of heavy metals contamination remediation and role of biosurfactants. Chem. Ecol. 2015, 31, 707–723. [Google Scholar] [CrossRef]
- Peng, F.; Wang, Y.; Sun, F.; Liu, Z.; Lai, Q.; Shao, Z. A novel lipopeptide produced by a Pacific Ocean deep-sea bacterium, Rhodococcussp. TW53. J. Appl. Microbiol. 2008, 105, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Prieto, L.; Michelon, M.; Burkert, J.; Kalil, S.J.; Burkert, C. The production of rhamnolipid by a Pseudomonas aeruginosa strain isolated from a southern coastal zone in Brazil. Chemosphere 2008, 71, 1781–1785. [Google Scholar] [CrossRef]
- Kitamoto, D.; Isoda, H.; Nakahara, T. Functions and potential applications of glycolipid biosurfactants — from energy-saving materials to gene delivery carriers. J. Biosci. Bioeng. 2002, 94, 187–201. [Google Scholar] [CrossRef]
- Chayabutra, C.; Wu, J.; Ju, L.W. Rhamnolipid production by Pseudomonas aeruginosa under denitrificacion: Effects of limiting nutrients and carbón substrates. Biotechnol. Bioeng. 2001, 72, 25–33. [Google Scholar] [CrossRef]
- Haftka, J.; Hammer, J.; Hermens, J.L.M. Mechanisms of Neutral and Anionic Surfactant Sorption to Solid-Phase Microextraction Fibers. Environ. Sci. Technol. 2015, 49, 11053–11061. [Google Scholar] [CrossRef]
- Marchant, R.; Banat, I.M. Biosurfactants: A sustainable replacement for chemical surfactants? Biotechnol. Lett. 2012, 34, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Jyot, J.; Kuhad, R.; Lal, B. In situ bioremediation potential of an oily sludge-degrading bacterial consortium. Curr. Microbiol. 2001, 43, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.D.; Neeson, M.J.; Dagastine, R.R.; Chan, D.; Tabor, R.; Berry, J.D. Measurement of surface and interfacial tension using pendant drop tensiometry. J. Colloid Interface Sci. 2015, 454, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Romero, H.; Acaro, J.; Camacho, A.; Castillo, A.; Vega, C.; Dávila, K.; Gadvay, K. Reliability of a method for determining CO2 by gas chromatography. J. Cumbres 2017, 3, 41–46. [Google Scholar]
- Abouseoud, M.; Maachi, R.; Amrane, A.; Boudergua, S.; Nabi, A. Evaluation of different carbon and nitrogen sources in production of biosurfactant by Pseudomonas fluorescens. Desalination 2008, 223, 143–151. [Google Scholar] [CrossRef]
- Jiang, Y.; Brassington, K.J.; Prpich, G.P.; Paton, G.I.; Semple, K.T.; Pollard, S.J.; Coulon, F. Insights into the biodegradation of weathered hydrocarbons in contaminated soils by bioaugmentation and nutrient stimulation. Chemosphere 2016, 161, 300–307. [Google Scholar] [CrossRef]
- Silva, R.D.C.F.S.D.; Almeida, D.G.; Meira, H.M.; Silva, E.J.; Farias, C.B.; Rufino, R.D.; Luna, J.M.; Sarubbo, L. Production and characterization of a new biosurfactant from Pseudomonas cepacia grown in low-cost fermentative medium and its application in the oil industry. Biocatal. Agric. Biotechnol. 2017, 12, 206–215. [Google Scholar] [CrossRef]
- Kumari, S.; Wati, L.; Kant, R.; Singh, U. Effect of Mixed Bioinocula on Growth and Efficiency of Azotobacter Species. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1–13. [Google Scholar] [CrossRef]
- Atlas, R.M. Petroleum biodegradation and oil spill bioremediation. Mar. Pollut. Bull. 1995, 31, 178–182. [Google Scholar] [CrossRef]
- Dibble, J.T.; Bartha, R. Effect of environmental parameters on the biodegradation of oil sludge. Appl. Environ. Microbiol. 1979, 37, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Farber, R.; Dabush-Busheri, I.; Chaniel, G.; Rozenfeld, S.; Bormashenko, E.; Multanen, V.; Cahan, R. Biofilm grown on wood waste pretreated with cold low-pressure nitrogen plasma: Utilization for toluene remediation. Int. Biodeterior. Biodegrad. 2019, 139, 62–69. [Google Scholar] [CrossRef]
- Farber, R.; Rosenberg, A.; Rozenfeld, S.; Banet, G.; Cahan, R. Bioremediation of Artificial Diesel-Contaminated Soil Using Bacterial Consortium Immobilized to Plasma-Pretreated Wood Waste. Microorganisms 2019, 7, 497. [Google Scholar] [CrossRef]
- Liu, P.-W.G.; Chang, T.C.; Chen, C.-H.; Wang, M.-Z.; Hsu, H.-W. Effects of soil organic matter and bacterial community shift on bioremediation of diesel-contaminated soil. Int. Biodeterior. Biodegrad. 2013, 85, 661–670. [Google Scholar] [CrossRef]
- Hassan, I.A.; Mohamedelhassan, E.; Yanful, E.K.; Weselowski, B.; Yuan, Z.-C. Isolation and characterization of novel bacterial strains for integrated solar-bioelectrokinetic of soil contaminated with heavy petroleum hydrocarbons. Chemosphere 2019, 237, 124514. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-A.; Liu, P.-W.G.; Whang, L.-M.; Wu, Y.-J.; Cheng, S.-S. Effect of soil organic matter on petroleum hydrocarbon degradation in diesel/fuel oil-contaminated soil. J. Biosci. Bioeng. 2020, 129, 603–612. [Google Scholar] [CrossRef]
- Millioli, V.S.; Servulo, E.L.C.; Sobral, L.G.S.; De Carvalho, D.D. Bioremediation of crude oil bearing soil: Evaluating the effect of rhamnolipid addition to soil toxicity and to crude oil biodegradation efficiency. Glob. Nest J. 2009, 11, 181–188. [Google Scholar]
- Wu, M.; Dick, W.A.; Li, W.; Wang, X.C.; Yang, Q.; Wang, T.; Xu, L.; Zhang, M.; Chen, L. Bioaugmentation and biostimulation of hydrocarbon degradation and the microbial community in a petroleum-contaminated soil. Int. Biodeterior. Biodegrad. 2016, 107, 158–164. [Google Scholar] [CrossRef]
- Atlas, R.M.; Hazen, T.C. Oil Biodegradation and Bioremediation: A Tale of the Two Worst Spills in U.S. History. Environ. Sci. Technol. 2011, 45, 6709–6715. [Google Scholar] [CrossRef]
- Kauppi, S.; Sinkkonen, A.; Romantschuk, M. Enhancing bioremediation of diesel-fuel-contaminated soil in a boreal climate: Comparison of biostimulation and bioaugmentation. Int. Biodeterior. Biodegrad. 2011, 65, 359–368. [Google Scholar] [CrossRef]
- Mariano, A.; Kataoka, A.P.D.A.G.; Angelis, D.D.F.D.; Bonotto, D.M. Laboratory study on the bioremediation of diesel oil contaminated soil from a petrol station. Braz. J. Microbiol. 2007, 38, 346–353. [Google Scholar] [CrossRef]
- Patowary, R.; Patowary, K.; Kalita, M.C.; Deka, S. Application of biosurfactant for enhancement of bioremediation process of crude oil contaminated soil. Int. Biodeterior. Biodegrad. 2018, 129, 50–60. [Google Scholar] [CrossRef]
- Cha, M.; Lee, N.; Kim, M.; Kim, M.; Lee, S.-J. Heterologous production of Pseudomonas aeruginosa EMS1 biosurfactant in Pseudomonas putida. Bioresour. Technol. 2008, 99, 2192–2199. [Google Scholar] [CrossRef] [PubMed]
- Ramadass, K.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Bioavailability of weathered hydrocarbons in engine oil-contaminated soil: Impact of bioaugmentation mediated by Pseudomonas spp. on bioremediation. Sci. Total Environ. 2018, 636, 968–974. [Google Scholar] [CrossRef]
- Agnello, A.; Bagard, M.; Van Hullebusch, E.D.; Esposito, G.; Huguenot, D. Comparative bioremediation of heavy metals and petroleum hydrocarbons co-contaminated soil by natural attenuation, phytoremediation, bioaugmentation and bioaugmentation-assisted phytoremediation. Sci. Total Environ. 2016, 563, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Mao, G.; Wang, Y.; Bartlam, M. Structural insights into diversity and n-alkane biodegradation mechanisms of alkane hydroxylases. Front. Microbiol. 2013, 4, 131. [Google Scholar] [CrossRef]
- Liu, T.; Wang, F.; Guo, L.; Li, X.; Yang, X.; Lin, A.-J. Biodegradation of n-hexadecane by bacterial strains B1 and B2 isolated from petroleum-contaminated soil. Sci. China Chem. 2012, 55, 1968–1975. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, D.; Zhu, C.; Lundaa, T.; Scherr, K.E. Isolation and identification of biosurfactant producing and crude oil degrading Pseudomonas aeruginosa strains. Chem. Eng. J. 2012, 209, 138–146. [Google Scholar] [CrossRef]
- Sarkar, D.; Ferguson, M.; Datta, R.; Birnbaum, S. Bioremediation of petroleum hydrocarbons in contaminated soils: Comparison of biosolids addition, carbon supplementation, and monitored natural attenuation. Environ. Pollut. 2005, 136, 187–195. [Google Scholar] [CrossRef]
- Hejazi, R.F.; Husain, T. Landfarm Performance under Arid Conditions. 2. Evaluation of Parameters. Environ. Sci. Technol. 2004, 38, 2457–2469. [Google Scholar] [CrossRef]
- Almansoory, A.F.; Abu Hasan, H.; Abdullah, S.R.S.; Idris, M.; Anuar, N.; Al-Adiwish, W.M. Biosurfactant produced by the hydrocarbon-degrading bacteria: Characterization, activity and applications in removing TPH from contaminated soil. Environ. Technol. Innov. 2019, 14, 100347. [Google Scholar] [CrossRef]
- Shekhar, S.; Sundaramanickam, A.; Balasubramanian, T. Biosurfactant Producing Microbes and their Potential Applications: A Review. Crit. Rev. Environ. Sci. Technol. 2014, 45, 1522–1554. [Google Scholar] [CrossRef]
- Pourfadakari, S.; Moghadam, M.A.; Jaafarzadeh, N.; Takdastan, A.; Neisi, A.A.; Ghafari, S.; Jorfi, S.; Jaafarzadeh, N. Remediation of PAHs contaminated soil using a sequence of soil washing with biosurfactant produced by Pseudomonas aeruginosa strain PF2 and electrokinetic oxidation of desorbed solution, effect of electrode modification with Fe3O4 nanoparticles. J. Hazard. Mater. 2019, 379, 120839. [Google Scholar] [CrossRef] [PubMed]
- Fanaei, F.; Moussavi, G.; Shekoohiyan, S. Enhanced treatment of the oil-contaminated soil using biosurfactant-assisted washing operation combined with H2O2-stimulated biotreatment of the effluent. J. Environ. Manag. 2020, 271, 110941. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-C.; Huang, Y.-C.; Wei, Y.-H.; Chang, J.-S. Biosurfactant-enhanced removal of total petroleum hydrocarbons from contaminated soil. J. Hazard. Mater. 2009, 167, 609–614. [Google Scholar] [CrossRef]
- Das, N.; Chandran, P. Microbial Degradation of Petroleum Hydrocarbon Contaminants: An Overview. Biotechnol. Res. Int. 2011, 2011, 941810. [Google Scholar] [CrossRef]
- Diplock, E.E.; Mardlin, D.; Killham, K.; Paton, G. Predicting bioremediation of hydrocarbons: Laboratory to field scale. Environ. Pollut. 2009, 157, 1831–1840. [Google Scholar] [CrossRef]
- Covino, S.; Čvančarová, M.; Muzikář, M.; Svobodová, K.; D’Annibale, A.; Petruccioli, M.; Federici, F.; Křesinová, Z.; Cajthaml, T. An efficient PAH-degrading Lentinus (Panus) tigrinus strain: Effect of inoculum formulation and pollutant bioavailability in solid matrices. J. Hazard. Mater. 2010, 183, 669–676. [Google Scholar] [CrossRef]
- Sarkar, P.; Roy, A.; Pal, S.; Mohapatra, B.; Kazy, S.K.; Maiti, M.K.; Sar, P. Enrichment and characterization of hydrocarbon-degrading bacteria from petroleum refinery waste as potent bioaugmentation agent for in situ bioremediation. Bioresour. Technol. 2017, 242, 15–27. [Google Scholar] [CrossRef]
- Sarkar, J.; Kazy, S.K.; Gupta, A.; Dutta, A.; Mohapatra, B.; Roy, A.; Bera, P.; Mitra, A.; Sar, P. Biostimulation of Indigenous Microbial Community for Bioremediation of Petroleum Refinery Sludge. Front. Microbiol. 2016, 7, 1407. [Google Scholar] [CrossRef]
- Kostka, J.E.; Prakash, O.; Overholt, W.A.; Green, S.J.; Freyer, G.; Canion, A.; Delgardio, J.; Norton, N.; Hazen, T.C.; Huettel, M. Hydrocarbon-Degrading Bacteria and the Bacterial Community Response in Gulf of Mexico Beach Sands Impacted by the Deepwater Horizon Oil Spill. Appl. Environ. Microbiol. 2011, 77, 7962–7974. [Google Scholar] [CrossRef] [PubMed]

| Factors (Independent Variables) | Levels | ||||||
|---|---|---|---|---|---|---|---|
| Sources of | −1 (g·kg−1 of soil) | +1 (g·kg−1 of soil) | |||||
| X1: Source of carbon | 13.6 (Glucose) | 0.01 (Yeast extract) | |||||
| X2: Source of nitrogen | 5.0 (NaNO3) | 1.0 (NH4Cl) | |||||
| X3: Source of phosphorous | 2.0 (K2HPO4) | 0.2 (K3PO4) | |||||
| Experiment matrix | Experimentation plan | Response | |||||
| Treatment | X1 | X2 | X3 | Source of carbon | Source of nitrogen | Source of phosphorous | |
| 1 | − | − | − | Glucose | NaNO3 | K2HPO4 | Y1 |
| 2 | + | − | − | Yeast extract | NaNO3 | K2HPO4 | Y2 |
| 3 | − | + | − | Glucose | NH4Cl | K2HPO4 | Y3 |
| 4 | + | + | − | Yeast extract | NH4Cl | K2HPO4 | Y4 |
| 5 | − | − | + | Glucose | NaNO3 | K3PO4 | Y5 |
| 6 | + | − | + | Yeast extract | NaNO3 | K3PO4 | Y6 |
| 7 | − | + | + | Glucose | NH4Cl | K3PO4 | Y7 |
| 8 | + | + | + | Yeast extract | NH4Cl | K3PO4 | Y8 |
| Parameter | Value | Method |
|---|---|---|
| Moisture (%) | 32.64 ± 0.46 | Gravimetry |
| pH | 7.85 ± 0.01 | Potentiometric |
| Density (kg.cm3) | 1.09 ± 0.03 | Pycnometer |
| Total nitrogen (%) | 0.25 ± 0.00 | Micro-Kjeldahl |
| Total phosphorous (mg·kg−1) | n/d | Bray I |
| Organic matter (%) | 11.14 ± 0.26 | Oxidation |
| Texture | Sandy-clay | Hydrometer |
| TPH (mg·kg−1) | 50,000 ± 852 | Gravimetry |
| Total bacteria (CFUb) | 1.04 × 104 ± 3.21 × 102 | Plate count |
| Total fungi (CFUf) | 1.06 × 103 ± 3.06 × 101 | Plate count |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez, E.J.; Abraham, M.d.R.; Baltazar, J.C.; Vázquez, G.; Delgadillo, E.; Tirado, D. Pseudomonas fluorescens: A Bioaugmentation Strategy for Oil-Contaminated and Nutrient-Poor Soil. Int. J. Environ. Res. Public Health 2020, 17, 6959. https://doi.org/10.3390/ijerph17196959
Gutiérrez EJ, Abraham MdR, Baltazar JC, Vázquez G, Delgadillo E, Tirado D. Pseudomonas fluorescens: A Bioaugmentation Strategy for Oil-Contaminated and Nutrient-Poor Soil. International Journal of Environmental Research and Public Health. 2020; 17(19):6959. https://doi.org/10.3390/ijerph17196959
Chicago/Turabian StyleGutiérrez, Eduardo Jahir, María del Rosario Abraham, Juan Carlos Baltazar, Guadalupe Vázquez, Eladio Delgadillo, and David Tirado. 2020. "Pseudomonas fluorescens: A Bioaugmentation Strategy for Oil-Contaminated and Nutrient-Poor Soil" International Journal of Environmental Research and Public Health 17, no. 19: 6959. https://doi.org/10.3390/ijerph17196959
APA StyleGutiérrez, E. J., Abraham, M. d. R., Baltazar, J. C., Vázquez, G., Delgadillo, E., & Tirado, D. (2020). Pseudomonas fluorescens: A Bioaugmentation Strategy for Oil-Contaminated and Nutrient-Poor Soil. International Journal of Environmental Research and Public Health, 17(19), 6959. https://doi.org/10.3390/ijerph17196959





