A Comprehensive Assessment of the Associations Between Season of Conception and Birth Defects, Texas, 1999–2015
Abstract
:1. Introduction
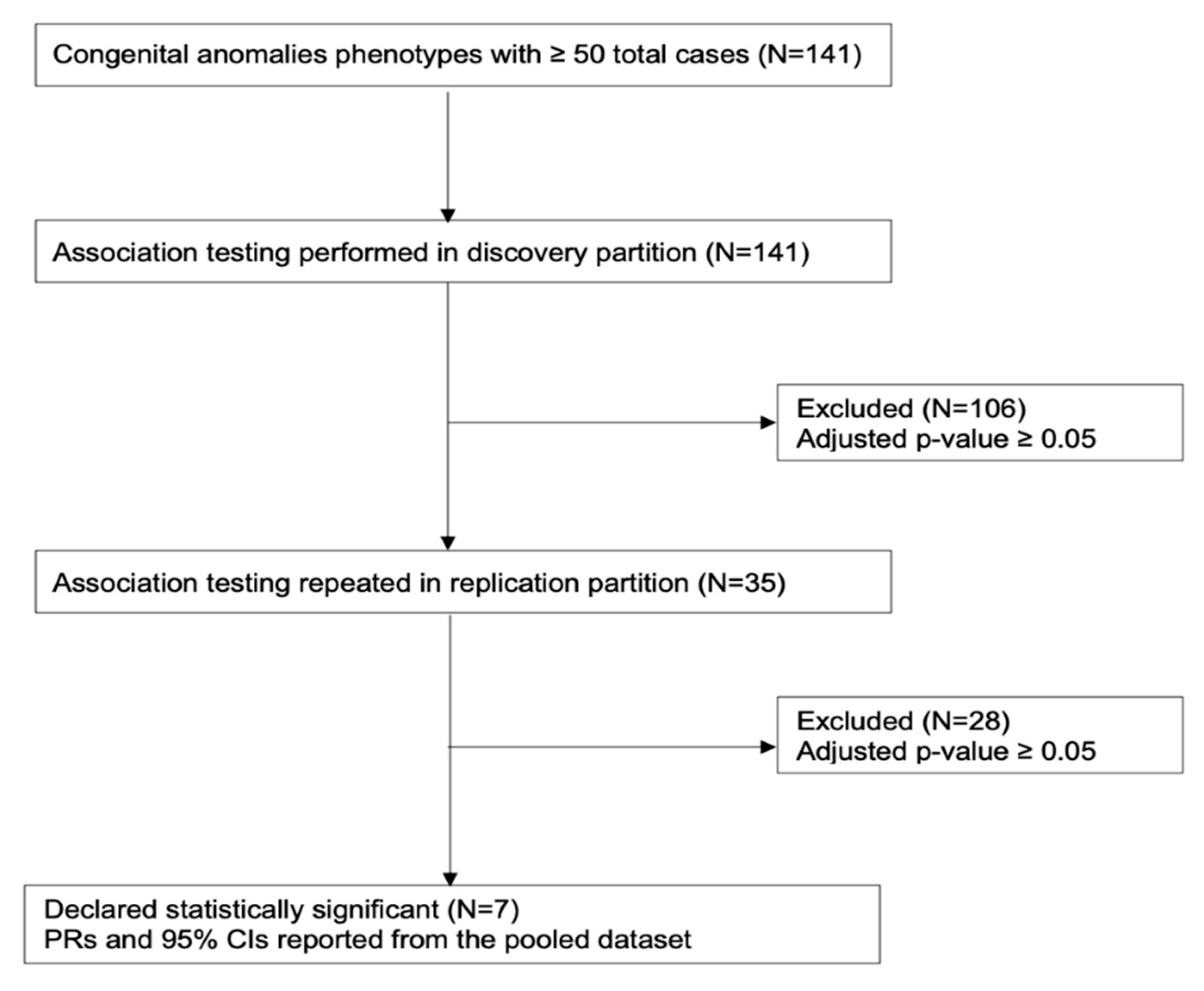
2. Methods
2.1. Birth Defect Ascertainment and Classification
2.2. Statistical Analysis
3. Results
3.1. Prevalence of Any Monitored Birth Defect According to Month of Conception
3.2. Prevalence of Specific Birth Defects According to Season of Conception
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Centers for Disease Control and Prevention (CDC). Data and Statistics on Birth Defects. Available online: https://www.cdc.gov/ncbddd/birthdefects/data.html (accessed on 19 June 2020).
- Centers for Disease Control and Prevention (CDC). What are Birth Defects? Available online: https://www.cdc.gov/ncbddd/birthdefects/facts.html (accessed on 25 September 2020).
- Stanford Children’s Health. Birth Defects in Children. Available online: https://www.stanfordchildrens.org/en/topic/default?id=overview-of-birth-defects-90-P02113 (accessed on 25 September 2020).
- United States Environmental Protection Agency (EPA). Supplementary Topics: Birth Defects. Available online: https://www.epa.gov/sites/production/files/2015-06/documents/supplementary-topics-birth-defects.pdf (accessed on 29 July 2020).
- Øyen, N.; Diaz, L.J.; Leirgul, E.; Boyd, H.A.; Priest, J.; Mathiesen, E.R.; Quertermous, T.; Wohlfahrt, J.; Melbye, M. Prepregnancy diabetes and offspring risk of congenital heart disease: A nationwide cohort study. Circulation 2016, 133, 2243–2253. [Google Scholar] [CrossRef] [PubMed]
- Siebold, B.; Heike, C.L.; Leroux, B.G.; Speltz, M.L.; Drake, A.F.; Johns, A.L.; Kapp-Simon, K.A.; Magee, L.; Luquetti, D.V. Evaluation of prenatal diabetes mellitus and other risk factors for craniofacial microsomia. Birth Defects Res. 2019, 111, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Vinceti, M.; Malagoli, C.; Rothman, K.J.; Rodolfi, R.; Astolfi, G.; Calzolari, E.; Puccini, A.; Bertolotti, M.; Lunt, M.; Paterlini, L.; et al. Risk of birth defects associated with maternal pregestational diabetes. Eur. J. Epidemiol. 2014, 29, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Tomson, T.; Battino, D.; Perucca, E. Teratogenicity of Antiepileptic Drugs. Curr. Opin. Neurol. 2019, 32, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Kashif, T.; Fathima, N.; Usman, N.; Qaseem, A.; Jayaraj, J.S. Women with epilepsy: Anti-epileptic drugs and perinatal outcomes. Cureus 2019, 11, e5642. [Google Scholar] [CrossRef] [Green Version]
- Caton, A.R. Exploring the seasonality of birth defects in the New York state congenital malformations registry. Birth Defects Res. Part A-Clin. Mol. Teratol. 2012, 94, 424–437. [Google Scholar] [CrossRef]
- Luteijn, J.M.; Dolk, H.; Addor, M.C.; Arriola, L.; Barisic, I.; Bianchi, F.; Calzolari, E.; Draper, E.; Garne, E.; Gatt, M.; et al. Seasonality of congenital anomalies in Europe. Birth Defects Res. Part A-Clin. Mol. Teratol. 2014, 100, 260–269. [Google Scholar] [CrossRef] [Green Version]
- Peterka, M.; Likovsky, Z.; Panczak, A.; Peterkova, R. Long-term significant seasonal differences in the numbers of new-borns with an orofacial cleft in the Czech Republic—A retrospective study. BMC Pregnancy Childbirth 2018, 18, 348. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). State-Based Birth Defects Tracking Systems. Available online: https://www.cdc.gov/ncbddd/birthdefects/states/index.html (accessed on 19 June 2020).
- Roden, D.M. Phenome-wide association studies: A new method for functional genomics in humans. J. Physiol. 2017, 595, 4109–4115. [Google Scholar] [CrossRef] [Green Version]
- Hebbring, S.J. The challenges, advantages and future of phenome-wide association studies. Immunology 2014, 141, 157–165. [Google Scholar] [CrossRef]
- Schraw, J.M.; Langlois, P.H.; Lupo, P.J. Comprehensize assessment of the assoications between maternal diabetes and structural birth defects in offspring: A phenome-wide association study. Ann. Epidemiol. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The strengthening the reporting of observational studies in Epidemiology (STROBE) statement: Guidelines for reporting observational atudies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Centers for Environmental Information (NCEI); National Oceanic and Atmospheric Administration (NOAA). Meteorological Versus Astronomical Seasons. Available online: https://www.ncei.noaa.gov/news/meteorological-versus-astronomical-seasons (accessed on 19 June 2020).
- Zhang, W.; Spero, T.L.; Nolte, C.G.; Garcia, V.C.; Lin, Z.; Romitti, P.A.; Shaw, G.M.; Sheridan, S.C.; Feldkamp, M.L.; Woomert, A.; et al. Projected changes in maternal heat exposure during early pregnancy and the associated congenital heart defect burden in the United States. J. Am. Heart Assoc. 2019, 8, e010995. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Lin, Z.; Ou, Y.; Soim, A.; Shrestha, S.; Lu, Y.; Sheridan, S.; Luben, T.J.; Fitzgerald, E.; Bell, E.; et al. Maternal ambient heat exposure during early pregnancy in summer and spring and congenital heart defects—A large US population-based, case-control study. Environ. Int. 2018, 118, 211–221. [Google Scholar] [CrossRef] [PubMed]
- De la Vega, A.; Martinez, E. Seasonal variation in the incidence of cleft lip and palate based on the age of conception. Puerto Rico Health Sci. J. 2006, 25, 343–346. [Google Scholar]
- Siffel, C.; Alverson, C.J.; Correa, A. Analysis of seasonal variation of birth defects in Atlanta. Birth Defects Res. Part A Clin. Mol. Teratol. 2005, 73, 655–662. [Google Scholar] [CrossRef]
- Rogerson, P.A. A generalization of Hewitt’s Test for seasonality. Int. J. Epidemiol. 1996, 25, 644–648. [Google Scholar] [CrossRef]
- Walter, S.D.; Elwood, J.M. A test for seasonality of events with a variable population at risk. J. Epidemiol. Community Health 1975, 29, 18–21. [Google Scholar] [CrossRef] [Green Version]
- Weather Atlas. Texas, USA—Climate Data and Average Monthly Weather. Available online: https://www.weather-us.com/en/texas-usa-climate#climate_text_1 (accessed on 19 June 2020).
- Stingone, J.A.; Luben, T.J.; Sheridan, S.C.; Langlois, P.H.; Shaw, G.M.; Reefhuis, J.; Romitti, P.A.; Feldkamp, M.L.; Nembhard, W.N.; Browne, M.L.; et al. Associations between fine particulate matter, extreme heat events, and congenital heart defects. Environ. Epidemiol. 2019, 3, e071. [Google Scholar] [CrossRef] [Green Version]
- Bennett, G.D. Hyperthermia: Malformations to chaperones. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2010, 89, 279–288. [Google Scholar] [CrossRef]
- Bekkar, B.; Pacheco, S.; Basu, R.; DeNicola, N. Association of air pollution and heat exposure with preterm birth, low birth weight, and stillbirth in the US. JAMA Netw. Open 2020, 3, e208243. [Google Scholar] [CrossRef] [PubMed]
- Kirkbride, J.B.; Susser, E.; Kundakovic, M.; Kresovich, J.K.; Davey Smith, G.; Relton, C.L. Prenatal nutrition, epigenetics and schizophrenia risk: Can we test causal effects? Epigenomics 2012, 4, 303–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdoux, H.; Takei, N.; De Saint-Mathurin, R.C.; Bourgeois, M. Analysis of the seasonal variation of schizophrenic births using a Kolmogorov-Smirnov Type statistic. Eur. Psychiatry 1997, 12, 111–116. [Google Scholar] [CrossRef]
- Dowell, S.F. Seasonal variation in host susceptibility and cycles of certain infectious diseases. Emerg. Infect. Dis. 2001, 7, 369–374. [Google Scholar] [CrossRef]


| All Livebirths, N (%) | Birth Defects Cases, N (%) | |
|---|---|---|
| Maternal age (years) | ||
| 10–19 | 831,365 (12.7) | 36,245 (11.9) |
| 20–24 | 1,769,279 (27.0) | 77,171 (25.3) |
| 25–29 | 1,784,385 (27.3) | 79,903 (26.2) |
| 30–34 | 1,387,169 (21.2) | 66,081 (21.7) |
| 35–39 | 632,649 (9.7) | 35,202 (11.6) |
| ≥40 | 138,010 (2.1) | 9997 (3.3) |
| Maternal race/ethnicity | ||
| Hispanic | 3,165,219 (48.4) | 144,452 (47.5) |
| Non-Hispanic white | 2,328,963 (35.6) | 112,837 (37.1) |
| Non-Hispanic black | 738,796 (11.3) | 33,733 (11.1) |
| Other | 302,756 (4.6) | 13,385 (4.4) |
| Maternal education | ||
| <High school | 1,841,020 (28.3) | 85,824 (28.8) |
| High school | 1,809,367 (27.8) | 79,841 (26.8) |
| >High school | 2,849,877 (43.8) | 132,471 (44.4) |
| Previous livebirths | ||
| 0 | 2,490,880 (38.6) | 120,918 (40.8) |
| 1 | 2,007,439 (31.1) | 86,992 (29.3) |
| 2 | 1,165,476 (18.1) | 51,358 (17.3) |
| ≥3 | 786,727 (12.2) | 37,387 (12.6) |
| Season of Conception | ||
| Winter | 1,686,752 (25.8) | 77,603 (25.5) |
| Spring | 1,634,191 (25.0) | 75,312 (24.7) |
| Summer | 1,565,866 (23.9) | 74,181 (24.4) |
| Fall | 1,642,220 (25.1) | 76,733 (25.2) |
| BPA4 Code | Birth Defect Name | Season of Conception | PR (95% CI) 1 |
|---|---|---|---|
| Any monitored birth defect | Spring | 1.00 (0.99, 1.01) | |
| Summer | 1.03 (1.02, 1.04) | ||
| Fall | 1.02 (1.01. 1.03) | ||
| 742.2 | Reduction anomalies of brain | Spring | 1.07 (0.99, 1.15) |
| Summer | 1.14 (1.05, 1.23) | ||
| Fall | 1.05 (0.98, 1.14) | ||
| 750.5 | Congenital hypertrophic pyloric stenosis | Spring | 0.89 (0.84, 0.94) |
| Summer | 1.02 (0.96, 1.07) | ||
| Fall | 1.02 (0.97, 1.08) | ||
| 751.3 | Hirschsprung’s disease and other congenital functional disorders of the colon | Spring | 1.03 (0.85, 1.24) |
| Summer | 1.46 (1.22, 1.75) | ||
| Fall | 1.06 (0.88, 1.28) | ||
| 754.5 | Varus (inward) deformities of feet | Spring | 1.04 (0.97, 1.11) |
| Summer | 1.14 (1.06, 1.22) | ||
| Fall | 1.03 (0.96, 1.11) | ||
| 755.6 | Other anomalies of lower limb, including pelvic girdle | Spring | 1.04 (0.99, 1.09) |
| Summer | 1.10 (1.04, 1.15) | ||
| Fall | 1.04 (0.99, 1.09) | ||
| 756.0 | Anomalies of skull and face bones | Spring | 1.04 (0.99, 1.08) |
| Summer | 1.10 (1.05, 1.15) | ||
| Fall | 1.05 (1.01, 1.10) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benavides, E.; Lupo, P.J.; Langlois, P.H.; Schraw, J.M. A Comprehensive Assessment of the Associations Between Season of Conception and Birth Defects, Texas, 1999–2015. Int. J. Environ. Res. Public Health 2020, 17, 7120. https://doi.org/10.3390/ijerph17197120
Benavides E, Lupo PJ, Langlois PH, Schraw JM. A Comprehensive Assessment of the Associations Between Season of Conception and Birth Defects, Texas, 1999–2015. International Journal of Environmental Research and Public Health. 2020; 17(19):7120. https://doi.org/10.3390/ijerph17197120
Chicago/Turabian StyleBenavides, Elisa, Philip J. Lupo, Peter H. Langlois, and Jeremy M. Schraw. 2020. "A Comprehensive Assessment of the Associations Between Season of Conception and Birth Defects, Texas, 1999–2015" International Journal of Environmental Research and Public Health 17, no. 19: 7120. https://doi.org/10.3390/ijerph17197120
APA StyleBenavides, E., Lupo, P. J., Langlois, P. H., & Schraw, J. M. (2020). A Comprehensive Assessment of the Associations Between Season of Conception and Birth Defects, Texas, 1999–2015. International Journal of Environmental Research and Public Health, 17(19), 7120. https://doi.org/10.3390/ijerph17197120






