Relationship between Quality of Life and the Complexity of Default Mode Network in Resting State Functional Magnetic Resonance Image in Down Syndrome
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Instruments
2.2.1. Checklist for MRI-Scanner
2.2.2. Quality-of-Life Assessment
2.3. Procedure
2.3.1. Image Acquisition
2.3.2. Image Preprocessing and ROIs Extraction
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schalock, R.L.; Vergudo, M.A. Handbook on Quality of Life for Human Service Practitioners; American Association on Mental Retardation: Washington, DC, USA, 2002. [Google Scholar]
- Jenaro, C.; Verdugo, M.A.; Caballo, C.; Balboni, G.; Lachappele, Y.; Otrebski, W.; Schalock, R.L. Cross-cultural study of person-centered quality of life domains and indicators: A replication. J. Intell. Disabil. Res. 2005, 49, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Schalock, R.L.; Verdugo, M.A.; Jenaro, C.; Wang, M.; Wehmeyer, M.; Jiancheng, X.; Lachapelle, Y. Cross-cultural study of quality of life indicators. Am. J. Ment. Retard. 2005, 110, 298–311. [Google Scholar] [CrossRef]
- Wang, M.; Schalock, R.L.; Verdugo, M.A.; Jenaro, C. Examining the factor structure and hierarchical nature of the quality of life construct. AJIDD-Am. J. Intellect. 2010, 115, 218–233. [Google Scholar] [CrossRef] [PubMed]
- Schalock, R.L. Professional and Organizational Best Practices. Siglo. Cero. 2015, 253, 7–23. [Google Scholar] [CrossRef][Green Version]
- Gómez, L.E.; Peña, E.; Arias, B.; Verdugo, M.A. Impact of individual and organizational variables on quality of life. Soc. Indic. Res. 2016, 125, 649–664. [Google Scholar] [CrossRef]
- Schalock, R.L.; Verdugo, M.A.; Gómez, L.E.; Reinders, H.S. Moving us toward a theory of individual quality of life. AJIDD-Am. J. Intellect. 2016, 121, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Schalock, R.L.; Baker, A.; Claes, C.; Gonzalez, J.; Malatest, R.; van Loon, J.; Verdugo, M.A.; Wesley, G. The use of quality of life scores for monitoring and reporting, quality improvement, and research. J. Policy Pract. Intellect. Disabil. 2018, 15, 176–182. [Google Scholar] [CrossRef]
- Calvo-Lobo, C.; Ramos García, A.; Losa Iglesias, M.E.; López-López, D.; Rodríguez-Sanz, D.; Romero-Morales, C.; Becerro-de-Bengoa-Vallejo, R. The relationship between shoe fitting and foot health of persons with Down syndrome: A case control study. Int. J. Environ. Res. Public Health 2018, 15, 983. [Google Scholar] [CrossRef]
- Van Gameren-Oosterom, H.B.; Fekkes, M.; Buitendijk, S.E.; Mohangoo, A.D.; Bruil, J.; Van Wouwe, J.P. Development, problem behavior, and quality of life in a population based sample of eight-year-old children with Down syndrome. PLoS ONE 2011, 6, e21879. [Google Scholar] [CrossRef]
- Friston, K.J.; Frith, C.D.; Liddle, P.F.; Frackowiak, R.S.J. Functional connectivity: The principal-component analysis of large (PET) data sets. J. Cereb. Blood Flow Metab. 1993, 13, 5–14. [Google Scholar] [CrossRef]
- Biswal, B.; Yetkin, F.Z.; Haughton, V.M.; Hyde, J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995, 34, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Vidaurre, D.; Beckmann, C.F.; Glasser, M.F.; Jenkinson, M.; Miller, K.L.; Nichols, T.E.; Robinson, E.C.; Salimi-Khorshidi, G.; Woolrich, M.W.; et al. Functional connectomics from resting-state fMRI. Trends Cogn. Sci. 2013, 17, 666–682. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network-anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Shulman, G.L.; Fiez, J.A.; Corbetta, M.; Buckner, R.L.; Miezin, F.M.; Raichle, M.E.; Petersen, S.E. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J. Cogn. Neurosci. 1997, 9, 648–663. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Greicius, M.D.; Krasnow, B.; Reiss, A.L.; Menon, V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Carbó-Carreté, M.; Cañete-Massé, C.; Peró-Cebollero, M.; Guàrdia-Olmos, J. Using fMRI to Assess Brain Activity in People with Down Syndrome: A Systematic Review. Front. Hum. Neurosci. 2020, 14, 147. [Google Scholar] [CrossRef]
- Anderson, J.S.; Nielsen, J.A.; Ferguson, M.A.; Burback, M.C.; Cox, E.T.; Dai, L.; Gerig, G.; Edgin, J.O.; Korenberg, J.R. Abnormal brain synchrony in Down Syndrome. NeuroImage Clin. 2013, 2, 703–715. [Google Scholar] [CrossRef]
- Vega, J.N.; Hohman, T.J.; Pryweller, J.R.; Dykens, E.M.; Thornton-Wells, T.A. Resting-state functional connectivity in individuals with Down syndrome and Williams syndrome compared with typically developing controls. Brain Connect. 2015, 5, 461–475. [Google Scholar] [CrossRef]
- Wilson, L.R.; Vatansever, D.; Annus, T.; Williams, G.B.; Hong, Y.T.; Fryer, T.D.; Nestor, P.J.; Holland, A.J.; Zaman, S.H. Differential effects of Down’s syndrome and Alzheimer’s neuropathology on default mode connectivity. Hum. Brain Mapp. 2019, 40, 4551–4563. [Google Scholar] [CrossRef]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Corbetta, M.; Van Essen, D.C.; Raichle, M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. USA 2005, 102, 9673–9678. [Google Scholar] [CrossRef] [PubMed]
- Sporns, O. The human connectome: Origins and challenges. Neuroimage 2013, 80, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Farahani, F.V.; Karwowski, W.; Lighthall, N.R. Application of Graph Theory for Identifying Connectivity Patterns in Human Brain Networks: A Systematic Review. Front. Neurosci. 2019, 13, 585. [Google Scholar] [CrossRef] [PubMed]
- Bullmore, E.; Sporns, O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Butts, C.T. Revisiting the Foundations of Network Analysis. Science 2009, 325, 414–416. [Google Scholar] [CrossRef]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses andinterpretations. Neuroimage 2010, 52, 1059–1069. [Google Scholar] [CrossRef]
- Supekar, K.; Menon, V.; Rubin, D.; Musen, M.; Greicius, M.D. Network analysis of intrinsic functional brain connectivity in Alzheimer’s disease. PLoS Comput. Biol. 2008, 4, e1000100. [Google Scholar] [CrossRef]
- Sporns, O.; Honey, C.J. Small worlds inside big brains. Proc. Natl. Acad. Sci. USA 2006, 103, 19219–19220. [Google Scholar] [CrossRef]
- Luo, Y.; Huang, X.; Yang, Z.; Li, B.; Liu, J.; Wei, D. Regional homogeneity of intrinsic brain activity in happy and unhappy individuals. PLoS ONE 2014, 9, e85181. [Google Scholar] [CrossRef]
- Luo, Y.; Kong, F.; Qi, S.; You, X.; Huang, X. Resting-state functional connectivity of the default mode network associated with happiness. Soc. Cogn. Affect. Neur. 2016, 11, 516–524. [Google Scholar] [CrossRef]
- Kraft, I.; Balardin, J.B.; Sato, J.R.; Sommer, J.; Tobo, P.; Barrichello, C.; Amaro, E., Jr.; Kozasa, E.H. Quality of life is related to the functional connectivity of the default mode network at rest. Brain Imaging Behav. 2019, 13, 1418–1426. [Google Scholar] [CrossRef] [PubMed]
- Nawani, H.; Smith, M.L.; Wheeler, A.L.; Widjaja, E. Functional Connectivity Associated with Health-Related Quality of Life in Children with Focal Epilepsy. AJNR Am. J. Neuroradiol. 2019, 40, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Friston, K. Ten ironic rules for non-statistical reviewers. Neuroimage 2012, 61, 1300–1310. [Google Scholar] [CrossRef] [PubMed]
- Carbó-Carreté, M.; Guàrdia-Olmos, J.; Giné, C. Psychometric properties of the Spanish version of the Personal Outcomes Scale. Int. J. Clin. Health. Psychology 2015, 15, 236–252. [Google Scholar] [CrossRef]
- Diez, I.; Bonifazi, P.; Escudero, I.; Mateos, B.; Munñoz, M.A.; Stramaglia, S.; Cortes, J.M. A novel brain partition highlights the modular skeleton shared by structure and function. Sci. Rep. 2015, 5, 10532. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Nonlinear spatial normalization using basis functions. Hum. Brain Mapp. 1999, 7, 254–266. [Google Scholar] [CrossRef]
- Cordes, D.; Haughton, V.M.; Arfanakis, K.; Carew, J.D.; Turski, P.A.; Moritz, C.H.; Quigley, M.A.; Meyerand, M.E. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. Am. J. Neuroradiol. 2001, 22, 1326–1333. [Google Scholar]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. Neuroimage 2002, 15, 273–289. [Google Scholar] [CrossRef]
- Huang, C.C.; Hsieh, W.J.; Lee, P.L.; Peng, L.N.; Liu, L.K.; Lee, W.J.; Huang, J.K.; Chen, L.K.; Lin, C.P. Age-related changes in resting-state networks of a large sample size of healthy elderly. CNS Neurosci. Ther. 2015, 21, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Yeo, B.T.; Krienen, F.M.; Sepulcre, J.; Sabuncu, M.R.; Lashkari, D.; Hollinshead, M.; Roffman, J.L.; Smoller, J.W.; Zöllei, L.; Polimeni, J.R.; et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011, 106, 1125–1165. [Google Scholar] [CrossRef]
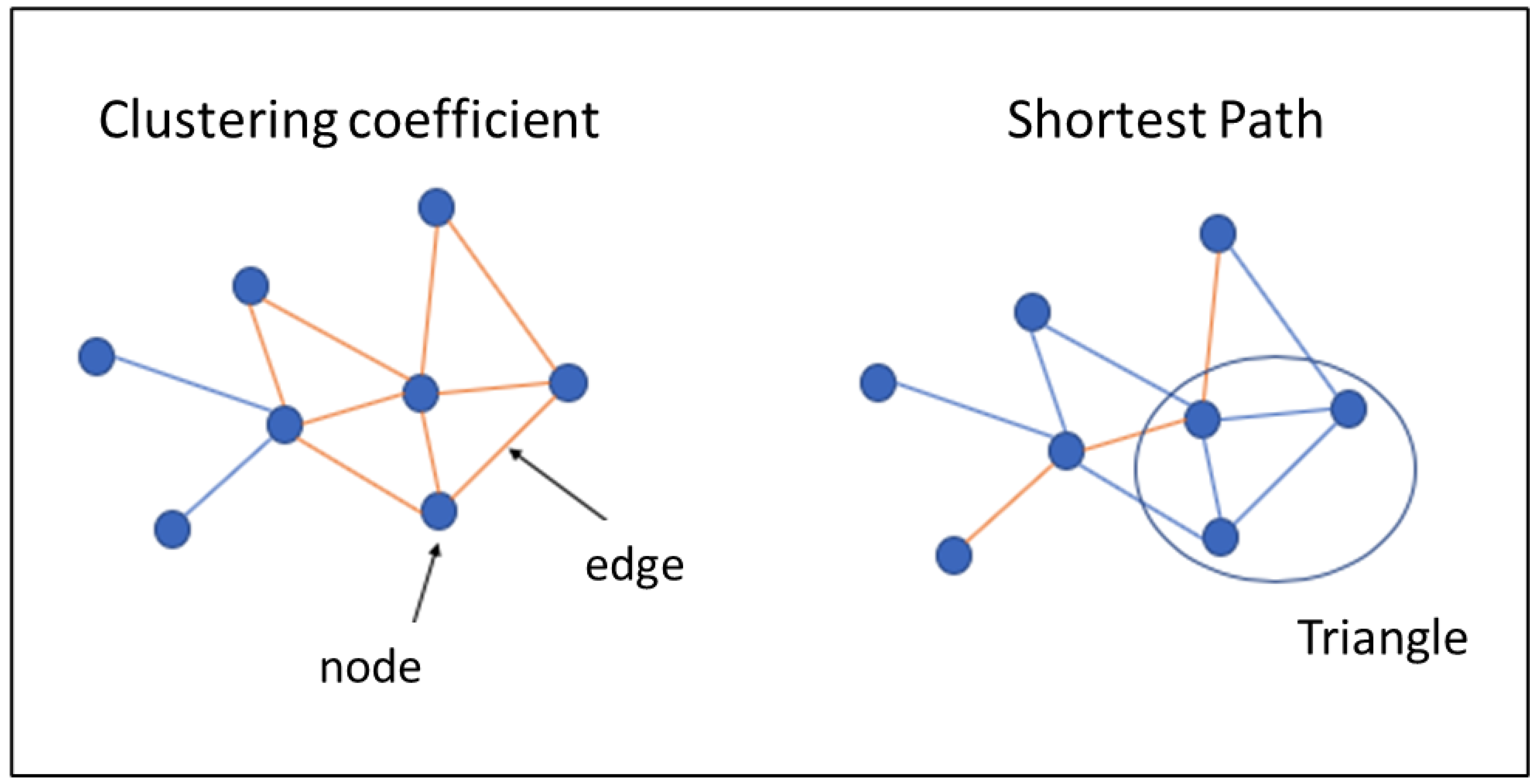

| DMN | DMN Anterior | DMN Ventral | |||
|---|---|---|---|---|---|
| ROI | Region Name | ROI | Region Name | ROI | Region Name |
| 59 | Parietal_Sup_L | 29 | Insula_L | 35 | Cingulum_Post_L |
| 60 | Parietal_Sup_R | 30 | Insula_R | 36 | Cingulum_Post_R |
| 61 | Parietal_Inf_L | 31 | Cingulum_Ant_L | 37 | Hippocampus_L |
| 62 | Parietal_Inf_R | 32 | Cingulum_Ant_R | 38 | Hippocampus_R |
| 85 | Temporal_Mid_L | 87 | Temporal_Pole_Mid_L | 39 | ParaHippocampal_L |
| 86 | Temporal_Mid_R | 88 | Temporal_Pole_Mid_R | 40 | ParaHippocampal_R |
| 55 | Fusiform_L | ||||
| 56 | Fusiform_R | ||||
| 65 | Angular_L | ||||
| 66 | Angular_R | ||||
| 67 | Precuneus_L | ||||
| 68 | Precuneus_R | ||||
| Indicators | Description | Calculations |
|---|---|---|
| Degree | Number of links connected to all nodes. Important marker of network development and resilience. | N is the set of all nodes in the network, (i,j) is a link between nodes i and j (i,j∈N). aij is the connection status between i and j: aij = 1 when link (i, j) exists (when i and j are neighbors); aij = 0 otherwise (aii = 0 for all i). |
| Number of Triangles | A basis for measuring segregation. Number of triangles around a node i. | |
| Global Clustering Coefficient | Prevalence of clustered connectivity around individual nodes. | where Ci is the clustering coefficient of node I (Ci = 0 for ki < 2). |
| Characteristic Path Length | The characteristic path length is a global measure of the network, i.e., there is only one value for the entire network. It consists of the average path length of each node in the network. | where n is the number of nodes involved and dij is the shortest path length between node i and j. |
| Modularity | Where the network is fully subdivided into a set of non-overlapping modules M, and euv is the proportion of all links that connect nodes in module u with nodes in module v. | |
| Small-Worldness | Measures an optimal balance of functional integration and segregation on the networks. | where C and Crand are the clustering coefficients, and L and Lrand are the characteristic path lengths of the respective tested network and a random network. |
| Indicators | Group | n | Mean | Mean Rank | SD | Significance |
|---|---|---|---|---|---|---|
| Global Clustering Coefficient | Down * | 10 | 0.9349 | 21.80 | 1.39454 | 0.015 |
| Control | 22 | 0.4579 | 14.09 | 0.04490 | - | |
| Number of Triangles | Down | 22 | 858.68 | 20.77 | 701.804 | 0.186 |
| Control | 22 | 1049.50 | 24.23 | 837.193 | - | |
| Modularity | Down | 22 | 0.3792 | 21.41 | 0.27243 | 0.286 |
| Control | 22 | 0.4467 | 23.59 | 0.03244 | - | |
| Characteristic Path Length | Down | 22 | 1.1342 | 14.73 | 6.32448 | <0.001 |
| Control | 22 | 3.8778 | 30.27 | 0.48123 | - | |
| Mean Path Length | Down | 22 | 0.5830 | 20.18 | 0.15949 | 0.115 |
| Control | 22 | 0.6471 | 24.82 | 0.06442 | - | |
| SD Path Length | Down | 22 | 0.2909 | 26.73 | 0.17160 | 0.014 |
| Control | 22 | 0.1511 | 18.27 | 0.02040 | - | |
| Complexity | Down | 22 | 0.5867 | 27.14 | 0.05813 | 0.008 |
| Control | 22 | 0.5272 | 17.86 | 0.10270 | - | |
| Small-Worldness | Down | 22 | 1.6735 | 19.23 | 0.12892 | 0.045 |
| Control | 22 | 1.7235 | 25.77 | 0.11505 | - | |
| Degree | Down | 22 | 4.1976 | 23.23 | 0.17693 | 0.353 |
| Control | 22 | 4.1490 | 21.77 | 0.14788 | - | |
| Dunn Index | Down | 22 | 0.4945 | 32.50 | 0.10255 | <0.001 |
| Control | 22 | 0.1811 | 12.50 | 0.22281 | - |
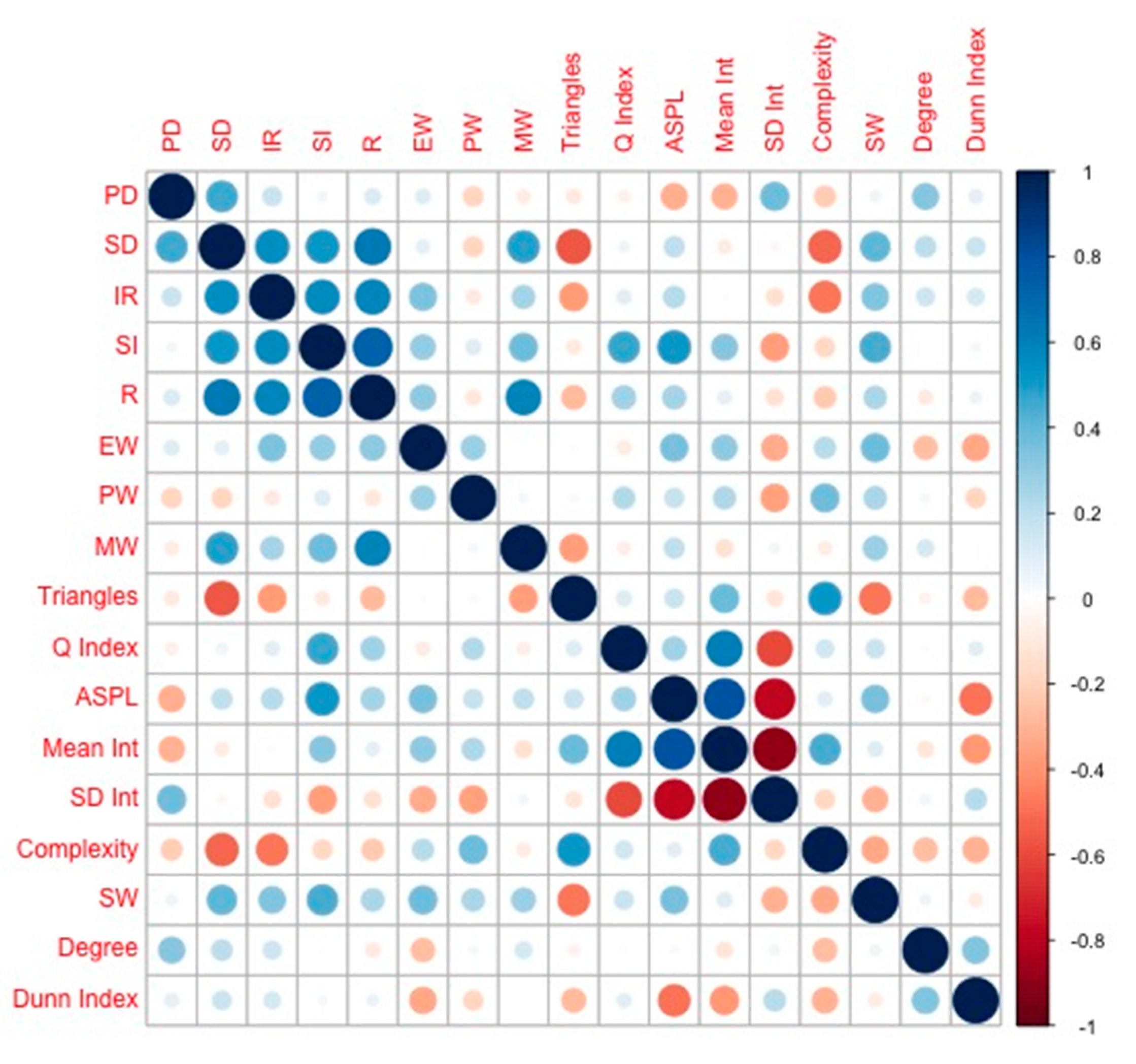
| PD | SD | IR | SI | R | EW | PW | MW | Trian-Gles | Q Index | ASPL | Mean Int. | SD Int. | Com-Plexity | SW | Degree | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PD | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| SD | 0.454 * | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| IR | 0.168 | 0.543 ** | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| SI | 0.041 | 0.515 * | 0.554 ** | - | - | - | - | - | - | - | - | - | - | - | - | - |
| R | 0.116 | 0.621 ** | 0.577 ** | 0.722 ** | - | - | - | - | - | - | - | - | - | - | - | - |
| EW | 0.101 | 0.089 | 0.348 | 0.293 | 0.307 | - | - | - | - | - | - | - | - | - | - | - |
| PW | −0.188 | −0.185 | −0.091 | 0.106 | −0.109 | 0.276 | - | - | - | - | - | - | - | - | - | - |
| MW | −0.080 | 0.483 * | 0.251 | 0.370 | 0.586 ** | −0.005 | 0.038 | - | - | - | - | - | - | - | - | - |
| Triangles | −0.093 | −0.558 ** | −0.372 | −0.090 | −0.273 | −0.014 | 0.029 | −0.364 | - | - | - | - | - | - | - | - |
| Q Index | −0.069 | 0.052 | 0.094 | 0.460 * | 0.266 | −0.088 | 0.222 | −0.075 | 0.106 | - | - | - | - | - | - | - |
| ASPL | −0.320 | 0.196 | 0.219 | 0.519 * | 0.260 | 0.357 | 0.174 | 0.192 | 0.165 | 0.265 | - | - | - | - | - | - |
| Mean Int. | −0.305 | −0.087 | −0.017 | 0.322 | 0.085 | 0.302 | 0.233 | −0.136 | 0.385 | 0.609 ** | 0.785 ** | - | - | - | - | - |
| SD Int. | 0.378 | −0.032 | −0.135 | −0.363 | −0.131 | −0.322 | −0.359 | 0.042 | −0.116 | −0.598 ** | −0.773 ** | −0.882 ** | - | - | - | - |
| Complexity | −0.212 | −0.511 * | −0.475 * | −0.177 | −0.223 | 0.213 | 0.375 | −0.080 | 0.511 * | 0.148 | 0.094 | 0.449 * | −0.176 | - | - | - |
| Entropy | 0.132 | 0.293 | 0.053 | −0.177 | −0.109 | −0.274 | −0.083 | 0.140 | −0.291 | −0.401 | −0.047 | −0.323 | 0.198 | −0.454 * | - | - |
| SW | 0.055 | 0.409 | 0.336 | 0.442 * | 0.242 | 0.373 | 0.240 | 0.272 | −0.473 * | 0.166 | 0.352 | 0.100 | −0.301 | −0.330 | - | - |
| Degree | 0.324 | 0.204 | 0.152 | 0.007 | −0.091 | −0.264 | 0-037 | 0.131 | −0.055 | 0.018 | −0.030 | −0.118 | 0.047 | −0.269 | 0.051 | - |
| Dunn Index | 0.089 | 0.165 | 0.131 | 0.039 | 0.061 | −0.333 | −0.181 | −0.006 | −0.275 | 0.095 | −0.483 * | −0.385 | 0.218 | −0.309 | −0.084 | 0.334 |
| Complexity Indicators | Personal Development | Self Determination | Interpersonal Relations | Social Inclusion | Rights | EmotionalWell-Being | PhysicalWell-Being | MaterialWell-Being |
|---|---|---|---|---|---|---|---|---|
| R2 = 0.630 AIC = 19.93 | R2 = 0.641 AIC = 21.49 | R2 = 0.251 AIC = 24.13 | R2 = 0.820 AIC = 35.61 | R2 = 0.391 AIC = −4.26 | R2 = 0.443 AIC = 16.95 | R2 = 0.317 AIC = 42.38 | ||
| Global Clustering Coefficient | - | - | - | - | - | - | - | - |
| Number of Triangles | 0.002 | - | - | - | - | - | - | - |
| Modularity | - | - | - | 10.122 | - | −1.734 | - | - |
| Characteristic Path Length | −0.569 | - | - | 0.522 | - | - | - | 0.396 |
| Mean Path Length | 51.340 | 18.665 | - | - | - | 3.289 | −12.852 | −14.821 |
| SD Path Length | 22.493 | - | - | 14.314 | - | - | −12.605 | - |
| Complexity | −75.633 | −42.367 | −18.081 | −49.936 | - | - | 19.136 | - |
| Small-Worldness | - | - | - | - | - | - | - | - |
| Degree | - | - | - | −6.374 | - | - | - | - |
| Dunn Index | - | - | - | - | - | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbó-Carreté, M.; Cañete-Massé, C.; Figueroa-Jiménez, M.D.; Peró-Cebollero, M.; Guàrdia-Olmos, J. Relationship between Quality of Life and the Complexity of Default Mode Network in Resting State Functional Magnetic Resonance Image in Down Syndrome. Int. J. Environ. Res. Public Health 2020, 17, 7127. https://doi.org/10.3390/ijerph17197127
Carbó-Carreté M, Cañete-Massé C, Figueroa-Jiménez MD, Peró-Cebollero M, Guàrdia-Olmos J. Relationship between Quality of Life and the Complexity of Default Mode Network in Resting State Functional Magnetic Resonance Image in Down Syndrome. International Journal of Environmental Research and Public Health. 2020; 17(19):7127. https://doi.org/10.3390/ijerph17197127
Chicago/Turabian StyleCarbó-Carreté, Maria, Cristina Cañete-Massé, María D. Figueroa-Jiménez, Maribel Peró-Cebollero, and Joan Guàrdia-Olmos. 2020. "Relationship between Quality of Life and the Complexity of Default Mode Network in Resting State Functional Magnetic Resonance Image in Down Syndrome" International Journal of Environmental Research and Public Health 17, no. 19: 7127. https://doi.org/10.3390/ijerph17197127
APA StyleCarbó-Carreté, M., Cañete-Massé, C., Figueroa-Jiménez, M. D., Peró-Cebollero, M., & Guàrdia-Olmos, J. (2020). Relationship between Quality of Life and the Complexity of Default Mode Network in Resting State Functional Magnetic Resonance Image in Down Syndrome. International Journal of Environmental Research and Public Health, 17(19), 7127. https://doi.org/10.3390/ijerph17197127






