The Effect of Self-Reported Lactose Intolerance and Dairy Consumption on Bone Mineral Density among American Hip Arthroplasty Patients: A Cross-Sectional Study
Abstract
1. Introduction
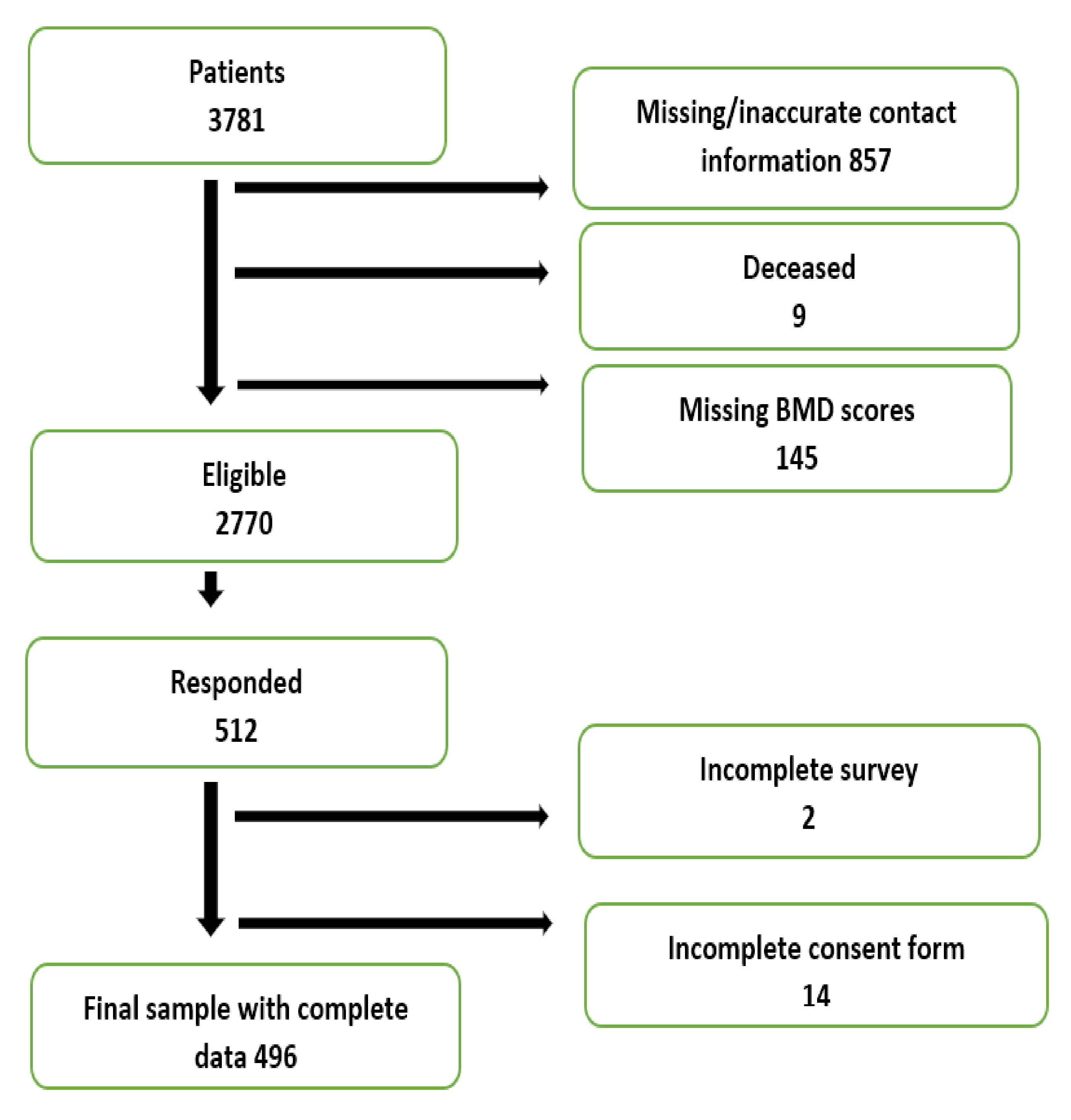
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Ethics
2.4. Statistical Analysis
3. Results
3.1. Analysis of the Lactose Intolerant Population
3.2. Predictors of Bone Mineral Density
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DALY | disability-adjusted life years |
| DXA | dual-energy X-ray absorptiometry |
| BMD | bone mineral density |
| SD | standard deviations |
| WHO | World Health Organization |
| LIIs | lactose intolerant individuals |
| FNOH | femoral neck of the operative hip |
| FNH | femoral neck of the non-operative hip |
| LS | lumbar spine |
| BMI | body mass index |
| ARL | at risk latitude |
| NRL | no risk latitude |
| PBH | poor bone health |
| ANOVA | one-way analysis of variance |
References
- Consensus development conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 1993, 94, 646–650. [CrossRef]
- Kanis, J.A.; Melton, L.J.; Christiansen, C.; Johnston, C.C.; Khaltaev, N. The diagnosis of osteoporosis. J. Bone Miner. Res. 1994, 9, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Pisani, P.; Renna, M.D.; Conversano, F.; Casciaro, E.; di Paola, M.; Quarta, E.; Muratore, M.; Casciaro, S. Major osteoporotic fragility fractures: Risk factor updates and societal impact. World J. Orthop. 2016, 7, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Gullberg, B.; Johnell, O.; Kanis, J.A. World-wide projections for hip fracture. Osteoporos. Int. 1997, 7, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, N.; Tsilidis, K.K.; Orfanos, P.; Benetou, V.; Ntzani, E.E.; Soerjomataram, I.; Künn-Nelen, A.; Pettersson-Kymmer, U.; Eriksson, S.; Brenner, H.; et al. Burden of hip fracture using disability-adjusted life-years: A pooled analysis of prospective cohorts in the CHANCES consortium. Lancet Public Health 2017, 2, e239–e246. [Google Scholar] [CrossRef]
- Sànchez-Riera, L.; Carnahan, E.; Vos, T.; Veerman, L.; Norman, R.; Lim, S.S.; Hoy, D.; Smith, E.; Wilson, N.; Nolla, J.M.; et al. The global burden attributable to low bone mineral density. Annal. Rheum. Dis. 2014, 73, 1635–1645. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J. Dual-energy X-ray absorptiometry: Beyond bone mineral density determination. Endocrinol. Metab. 2016, 31, 25–30. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, B. Systematic review and meta-analysis for the association of bone mineral density and osteoporosis/osteopenia with vascular calcification in women. Int. J. Rheum. Dis. 2017, 20, 154–160. [Google Scholar] [CrossRef]
- Weaver, C.M.; Proulx, W.R.; Heaney, R. Choices for achieving adequate dietary calcium with a vegetarian diet. Am. J. Clin. Nutr. 1999, 70, 543s–548s. [Google Scholar] [CrossRef]
- Jackson, K.A.; Savaiano, D.A. Lactose maldigestion, calcium intake and osteoporosis in African-, Asian-, and Hispanic-Americans. J. Am. Coll. Nutr. 2001, 20, 198S–207S. [Google Scholar] [CrossRef]
- Campbell, T.C.; Campbell, T.M. The China Study: The Most Comprehensive Study of Nutrition Ever Conducted and the Startling Implications for Diet, Weight Loss and Long-Term Health; BenBella Books Inc.: Dallas, TX, USA, 2006. [Google Scholar]
- Hegsted, D.M. Calcium and osteoporosis. J. Nutr. 1986, 116, 2316–2319. [Google Scholar] [CrossRef] [PubMed]
- OECD. OECD-FAO Agricultural Outlook 2019–2028; OECD-FAO Agricultural Outlook; OECD Publishing: Paris, France, 2019; ISBN 9789264312456. [Google Scholar]
- Kanis, J.A.; Odén, A.; McCloskey, E.V.; Johansson, H.; Wahl, D.A.; Cooper, C. A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos. Int. 2012, 23, 2239–2256. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Recommendations for Preventing Osteoporosis. Available online: https://www.who.int/nutrition/topics/5_population_nutrient/en/index25.html (accessed on 7 March 2020).
- National Institutes of Health Office of Dietary Supplements Calcium; Fact Sheet for Healthcare Professionals. Available online: https://ods.od.nih.gov/factsheets/Calcium-HealthProfessional/#h2 (accessed on 14 August 2020).
- National Institutes of Health Genetics of Bone Density. Available online: https://www.nih.gov/news-events/nih-research-matters/genetics-bone-density (accessed on 14 August 2020).
- Vicente-Rodríguez, G.; Ezquerra, J.; Mesana, M.I.; Fernández-Alvira, J.M.; Rey-López, J.P.; Casajus, J.A.; Moreno, L.A. Independent and combined effect of nutrition and exercise on bone mass development. J. Bone Miner. Metab. 2008, 26, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Frassetto, L.A.; Todd, K.M.; Morris, R.C.; Sebastian, A. Worldwide incidence of hip fracture in elderly women: Relation to consumption of animal and vegetable foods. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2000, 55, M585–M592. [Google Scholar] [CrossRef]
- Sellmeyer, D.E.; Stone, K.L.; Sebastian, A.; Cummings, S.R. A high ratio of dietary animal to vegetable protein increases the rate of bone loss and the risk of fracture in postmenopausal women. Am. J. Clin. Nutr. 2001, 73, 118–122. [Google Scholar]
- Itoh, R.; Nishiyama, N.; Suyama, Y. Dietary protein intake and urinary excretion of calcium: A cross-sectional study in a healthy Japanese population. Am. J. Clin. Nutr. 1998, 67, 438–444. [Google Scholar] [CrossRef]
- Calvez, J.; Poupin, N.; Chesneau, C.; Lassale, C.; Tomé, D. Protein intake, calcium balance and health consequences. Eur. J. Clin. Nutr. 2012, 66, 281–295. [Google Scholar] [CrossRef]
- Thorpe, M.P.; Evans, E.M. Dietary protein and bone health: Harmonizing conflicting theories. Nutr. Rev. 2011, 69, 215–230. [Google Scholar] [CrossRef]
- Sellmeyer, D.E.; Schloetter, M.; Sebastian, A. Potassium citrate prevents increased urine calcium excretion and bone resorption induced by a high sodium chloride diet. J. Clin. Endocrinol. Metab. 2002, 87, 2008–2012. [Google Scholar] [CrossRef]
- Kudlacek, S.; Freudenthaler, O.; Weissböeck, H.; Schneider, B.; Willvonseder, R. Lactose intolerance: A risk factor for reduced bone mineral density and vertebral fractures? J. Gastroenterol. 2002, 37, 1014–1019. [Google Scholar] [CrossRef]
- Scrimshaw, N.S.; Murray, E.B. The acceptability of milk and milk products in populations with a high prevalence of lactose intolerance. Am. J. Clin. Nutr. 1988, 48, 1079–1159. [Google Scholar] [CrossRef] [PubMed]
- Casellas, F.; Varela, E.; Aparici, A.; Casaus, M.; Rodríguez, P. Development, validation, and applicability of a symptoms questionnaire for lactose malabsorption screening. Digest. Dis. Sci. 2009, 54, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Ségurel, L.; Bon, C. On the Evolution of Lactase Persistence in Humans. Ann. Rev. Genom. Hum. Genet. 2017, 18, 297–319. [Google Scholar] [CrossRef] [PubMed]
- Scheindlin, B. Lactose intolerance and evolution: No use crying over undigested milk. Gastronomica 2007, 7, 59–63. [Google Scholar] [CrossRef]
- Corazza, G.R.; Benati, G.; di Sario, A.; Tarozzi, C.; Strocchi, A.; Passeri, M.; Gasbarrini, G. Lactose intolerance and bone mass in postmenopausal Italian women. Br. J. Nutr. 1995, 73, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Honkanen, R.; Pulkkinen, P.; Jlirvinen, R.; Krijger, H.; Lindstedt, K.; Tuppurainen, M.; Uusitupa, M. Does lactose intolerance predispose to low bone density? A population-based study of perimenopausal finnish women. Bone 1996, 19, 23–28. [Google Scholar] [CrossRef]
- Salomão, N.A.; Silva, T.D.A.; Lima-Silva, A.E.; Geraldes, A.R.R. Ingestão de cálcio e densidade mineral óssea em mulheres adultas intolerantes à lactose|Calcium intake and bone mineral density in adult women with lactose intolerance. Revista de Nutrição 2012, 25, 587–595. [Google Scholar] [CrossRef]
- Phatama, K.Y.; Pradana, A.S.; Mustamsir, E.; Hidayat, M.; Sakti, S.W.; Pandiangan, R.A.H.; Muhammad, S.I.; Putera, M.A. Primary single stage Total Hip Arthroplasty in a patient 40 years post traumatic Hip dysplasia, a case report. Trauma Case Rep. 2019, 23. [Google Scholar] [CrossRef]
- Aspden, R.M. Subchondral bone architecture and quality in osteoarthritis. Arthritis Res. Ther. 2004, 6, S16–S17. [Google Scholar] [CrossRef]
- Sinha, R.; Bukhari, M. OP0292 The Diagnosis of osteoporosis using BMD and T score measurements at specific skeletal sites. Annal. Rheum. Dis. 2014, 73, 172. [Google Scholar] [CrossRef]
- National Institutes of Health Bone Mass Measurement: What the Numbers Mean. Available online: https://www.bones.nih.gov/health-info/bone/bone-health/bone-mass-measure (accessed on 14 August 2020).
- United States Postal Service Zip Code Database; United States Zip Codes. Available online: https://www.unitedstateszipcodes.org/zip-code-database/ (accessed on 5 June 2017).
- Harvard Medical School. Time for More Vitamin D. Available online: http://www.health.harvard.edu/staying-healthy/time-for-more-vitamin-d (accessed on 6 March 2017).
- Alhava, E.M.; Jussila, J.; Karjalainen, P.; Vuojolahti, P. Lactose malabsorption and bone mineral content. Acta Med. Scand. 1977, 201, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Slemenda, C.W.; Christian, J.C.; Hui, S.; Fitzgerald, J.; Johnston, C.C. No evidence for an effect of lactase deficiency on bone mass in pre-or postmenopausal women. J. Bone Miner. Res. 1991, 6, 1367–1371. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, M.; Veneto, G.; Malservisi, S.; Cecchetti, L.; Minguzzi, L.; Strocchi, A.; Corazza, G.R. Lactose malabsorption and intolerance and peak bone mass. Gastroenterology 2002, 122, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Nachshon, L.; Goldberg, M.R.; Schwartz, N.; Sinai, T.; Amitzur-Levy, R.; Elizur, A.; Eisenberg, E.; Katz, Y. Decreased bone mineral density in young adult IgE-mediated cow’s milk-allergic patients. J. Allergy Clin. Immunol. 2014, 134, 1108–1113. [Google Scholar] [CrossRef]
- Newcomer, A.D.; Stephen, F.A.C.P.; Hodgson, F.; Mcgill, D.B.; Thomas, P.J.; Rochester, M. Lactase deficiency: Prevalence in osteoporosis. Ann. Intern. Med. 1978, 89, 218–220. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kawai, S.; Ohbe, Y.; Nagashima, Y. Effects of dietary lactose and a lactase preparation on the intestinal absorption of calcium and magnesium in normal infants. Am. J. Clin. Nutr. 1975, 28, 681–683. [Google Scholar] [CrossRef]
- Goulding, A.; Taylor, R.W.; Keil, D.; Gold, E.; Lewis-Barned, N.J.; Williams, S.M. Lactose malabsorption and rate of bone loss in older women. Age Ageing 1999, 28, 175–180. [Google Scholar] [CrossRef]
- Hunter, P. Nutrition: More than the sum of its parts. the modern craze for dietary supplements is under increasing scrutiny, while biofortified crops look promising in the quest to deliver nutrition in developing countries. EMBO Rep. 2011, 12, 307–310. [Google Scholar] [CrossRef][Green Version]
- Keller, J.L.; Lanou, A.J.; Barnard, N. The consumer cost of calcium from food and supplements. J. Am. Diet. Assoc. 2002, 102, 1669–1671. [Google Scholar] [CrossRef]
- Chen, Y.M.; Ho, S.C.; Woo, J.L.F. Greater fruit and vegetable intake is associated with increased bone mass among postmenopausal Chinese women. Br. J. Nutr. 2006, 96, 745–751. [Google Scholar] [CrossRef]
- McGartland, C.P.; Robson, P.J.; Murray, L.J.; Cran, G.W.; Savage, M.J.; Watkins, D.C.; Rooney, M.M.; Boreham, C.A. Fruit and vegetable consumption and bone mineral density: The Northern Ireland Young Hearts Project. Am. J. Clin. Nutr. 2004, 80, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.L.; Hannan, M.T.; Chen, H.; Cupples, L.A.; Wilson, P.W.; Kiel, D.P. Potassium, magnesium, and fruit and vegetable intakes are associated with greater bone mineral density in elderly men and women. Am. J. Clin. Nutr. 1999, 69, 727–736. [Google Scholar] [CrossRef]
- Michaëlsson, K.; Wolk, A.; Langenskiöld, S.; Basu, S.; Lemming, E.W.; Melhus, H.; Byberg, L. Milk intake and risk of mortality and fractures in women and men: Cohort studies. BMJ (Online) 2014, 349. [Google Scholar] [CrossRef] [PubMed]
- Feskanich, D.; Bischoff-Ferrari, H.A.; Frazier, A.L.; Willett, W.C. Milk consumption during teenage years and risk of hip fractures in older adults. JAMA Pediatr. 2014, 168, 54–60. [Google Scholar] [CrossRef]
- Facioni, M.S.; Raspini, B.; Pivari, F.; Dogliotti, E.; Cena, H. Nutritional management of lactose intolerance: The importance of diet and food labelling. J. Transl. Med. 2020, 18, 260. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; Baron, J.A.; Burckhardt, P.; Li, R.; Spiegelman, D.; Specker, B.; Orav, J.E.; Wong, J.B.; Staehelin, H.B.; et al. Calcium intake and hip fracture risk in men and women: A meta-analysis of prospective cohort studies and randomized controlled trials 1–3. Am. J. Clin. Nutr. 2007, 86, 1780–1790. [Google Scholar] [CrossRef]
- Zhai, G.; Hart, D.J.; Valdes, A.M.; Kato, B.S.; Richards, J.B.; Hakim, A.; Spector, T.D. Natural history and risk factors for bone loss in postmenopausal Caucasian women: A 15-year follow-up population-based study. Osteoporos. Int. 2008, 19, 1211–1217. [Google Scholar] [CrossRef]
- Bauer, D.C. Calcium supplements and fracture prevention. N. Engl. J. Med. 2013, 369, 1537–1543. [Google Scholar] [CrossRef]
- Chapuy, M.C.; Arlot, M.E.; Duboeuf, F.; Brun, J.; Crouzet, B.; Arnaud, S.; Delmas, P.D.; Meunier, P.J. Vitamin D3 and calcium to prevent hip fractures in elderly women. N. Engl. J. Med. 1992, 327, 1637–1642. [Google Scholar] [CrossRef]
- Larsen, E.R.; Mosekilde, L.; Foldspang, A. Vitamin D and calcium supplementation prevents osteoporotic fractures in elderly community dwelling residents: A pragmatic population-based 3-year intervention study. J. Bone Miner. Res. 2004, 19, 370–378. [Google Scholar] [CrossRef]
- Dawson-Hughes, B.; Harris, S.S.; Krall, E.A.; Dallal, G.E. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N. Engl. J. Med. 1997, 337, 670–676. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, H.M.; Wood, A.D.; Aucott, L.S.; Black, A.J.; Fraser, W.D.; Mavroeidi, A.; Reid, D.M.; Secombes, K.R.; Simpson, W.G.; Thies, F. Hip bone loss is attenuated with 1000 IU but not 400 IU daily vitamin D3: A 1-year double-blind RCT in postmenopausal women. J. Bone Miner. Res. 2013, 28, 2202–2213. [Google Scholar] [CrossRef] [PubMed]
- Gregory, K.E.; Radovinsky, L. Research strategies that result in optimal data collection from the patient medical record. Appl. Nurs. Res. 2012, 25, 108–116. [Google Scholar] [CrossRef] [PubMed]
| Categorical Variables a | All Participants n = 496 | With Lactose Intolerance n = 46 | Without Lactose Intolerance n = 450 | p-Value |
|---|---|---|---|---|
| Gender | ||||
| Male | 342 (68.9%) | 30 (65.2%) | 312 (69.3%) | 0.566 b |
| Female | 154 (21.1%) | 16 (34.8%) | 138 (30.7%) | |
| At-risk latitude | ||||
| Yes | 145 (29.2%) | 13 (28.3%) | 132 (29.3%) | 0.879 b |
| No | 351 (70.8%) | 33 (71.7%) | 318 (70.7%) | |
| Smoking Status | ||||
| Smokers | 7 (1.4%) | 0 (0.0%) | 7 (1.6%) | 0.386 c |
| Non-smokers | 489 (98.6%) | 46 (100.0%) | 443 (98.4%) | |
| BMI categories d | 0.773 c | |||
| Underweight | 7 (1.5%) | 0 (0.0%) | 7 (1.7%) | |
| Healthy weight | 173 (37.5%) | 19 (45.2%) | 154 (36.7%) | |
| Overweight | 186 (40.3%) | 15 (35.7%) | 171 (40.8%) | |
| Obese | 95 (20.6%) | 8 (19.1%) | 87 (20.8%) | |
| Menopausal status of women e | 0.672 b | |||
| Pre or perimenopausal | 59 (38.3%) | 9 (56.2%) | 50 (36.2%) | |
| Postmenopausal | 95 (61.7%) | 7 (43.7%) | 88 (63.8%) | |
| Vitamin D Supplementation | 0.015 b | |||
| Yes | 117 (23.6%) | 6 (13.0%) | 111 (24.7%) | |
| No | 379 (76.4%) | 40 (67.0%) | 339 (75.3%) | |
| Calcium supplementation | 0.484 b | |||
| Yes | 142 (29.0%) | 9 (24.3%) | 133 (43.0%) | |
| No | 354 (71.0%) | 37 (75.7%) | 317 (57%) | |
| Consumption of food fortified with calcium >1 time/week | <0.0001 b | |||
| Yes | 437 (88.1%) | 20 (43.5%) | 417 (92.7%) | |
| No | 59 (11.9%) | 26 (56.5%) | 33 (7.3%) | |
| Vegan or vegetarian | 0.147 c | |||
| Yes | 480 (97%) | 3 (6.5%) | 12 (2.7%) | |
| No | 15 (3.0%) | 43 (93.5%) | 437 (97.3%) | |
| Cardiovascular exercise at least once a week for 30 min | 0.372 b | |||
| Yes | 382 (77.0%) | 33 (71.7%) | 349 (77.7%) | |
| No | 114 (23%) | 13 (28.3%) | 101 (22.4%) | |
| Surgery Type | 0.724 b | |||
| Hip resurfacing surgery | 481 (97.0%) | 45 (97.8%) | 436 (96.9%) | |
| Total hip replacement surgery | 15 (3.0%) | 1 (2.2%) | 14 (3.1%) | |
| Osteoporosis or osteopenia | 0.123 b | |||
| Yes | 165 (33.3%) | 20 (43.5%) | 145 (32.2%) | |
| No | 331 (66.7%) | 26 (56.5%) | 305 (67.8%) | |
| Continuous Variables f | p-Value g | |||
| Age (years) | 60.0 (0.40) | 58.7 (2.27) | 60.20 (0.38) | 0.295 |
| Sum of all dairy consumption | 6.82 (0.81) | 5.65 (0.28) | 6.94 (0.08) | <0.0001 |
| Sum of all protein consumption | 12.01 (0.11) | 11.04 (0.34) | 12.11 (0.11) | 0.004 |
| BMI (kg/m2) | 26.23 (0.21) | 25.57 (0.63) | 26.29 (0.23) | 0.295 |
| Variables | BMD: Femoral Neck of the Operative Hip | BMD: Femoral Neck of the Non-Operative Hip | BMD: Lumbar Spine | |||
|---|---|---|---|---|---|---|
| All Study Participants (n = 496) | Mean (SE) | p-Value | Mean (SE) | p-Value | Mean (SE) | p-Value |
| Gender | 0.062 | 0.076 | 0.017 | |||
| Males | −0.16 (0.07) | −0.10 (0.06) | 1.31 (0.11) | |||
| Females | −0.42 (0.11) | −0.30 (0.10) | 0.84 (0.15) | |||
| Calcium supplementation | 0.202 | 0.707 | 0.325 | |||
| Yes | −0.36 (1.00) | −0.13 (0.87) | 1.04 (1.76) | |||
| No | −0.18 (0.80) | −0.17 (0.64) | 1.24 (1.07) | |||
| Vitamin D supplementation | 0.005 | 0.085 | 0.613 | |||
| Yes | −0.27 (1.00) | −0.12 (1.06) | 0.97 (1.91) | |||
| No | −0.85 (1.19) | −0.55 (1.12) | 0.57 (1.93) | |||
| Consumption of food fortified with calcium >1 time/week | 0.070 | 0.181 | 0.973 | |||
| Yes | −0.19 (0.07) | −0.14 (0.05) | 1.17 (0.10) | |||
| No | −0.56 (0.18) | −0.35 (0.15) | 1.16 (0.26) | |||
| Consumption of milk >1 time/week | 0.942 | 0.486 | 0.624 | |||
| Yes | −0.23 (0.07) | −0.18 (0.05) | 1.19 (0.10) | |||
| No | −0.25 (0.14) | −0.07 (0.14) | 1.06 (0.25) | |||
| Consumption of cheese >1 time/week | 0.434 | 0.700 | 0.274 | |||
| Yes | −0.22 (0.07) | −0.16 (0.05) | 1.13 (0.09) | |||
| No | −0.38 (0.20) | −0.22 (0.15) | 1.44 (0.33) | |||
| Consumption of yogurt >1 time/week | 0.015 | 0.269 | 0.853 | |||
| Yes | −0.11 (0.08) | −0.12 (0.70) | 1.16 (0.12) | |||
| No | −0.42 (0.93) | −0.23 (0.07) | 1.19 (0.14) | |||
| Vegan or vegetarian | 0.049 | 0.068 | <0.001 | |||
| Yes | −1.0 (0.41) | −0.69 (0.23) | −0.75 (0.52) | |||
| No | −0.21 (0.06) | −0.15 (0.05) | 1.22 (0.9) | |||
| At-risk Latitude | 0.103 | 0.652 | 0.643 | |||
| Yes | −0.05 (0.11) | −0.13 (0.10) | 1.10 (1.63) | |||
| No | −0.29 (0.08) | −0.18 (0.61) | 1.19 (1.07) | |||
| Menopausal status of women only a | 0.0036 | <0.001 | 0.005 | |||
| Pre or perimenopausal | −0.15 (0.07) | −0.07 (0.06) | 1.30 (1.65) | |||
| Postmenopausal | −0.61 (0.13) | −0.55 (0.100) | 1.540 (1.58) | |||
| BMI (kg/m2) | <0.0001 | <0.0001 | <0.001 | |||
| Underweight | −1.4 (0.98) | −1.30 (1.34) | −1.14 (1.35) | |||
| Healthy | −0.54 (1.16) | −0.46 (1.07) | 0.85 (1.75) | |||
| Overweight | −0.16 (1.16) | −0.11 (1.11) | 1.23 (1.79 | |||
| Obese | 0.28 (1.08) | 0.29 (1.09) | 1.624 (1.56) | |||
| Cardiovascular exercise at least once a week for 30 min | 0.546 | 0.133 | 0.753 | |||
| Yes | −0.21 (0.07) | −0.12 (0.06) | 1.15 (0.10) | |||
| No | −0.30 (0.14) | −0.31 (0.11) | 1.22 (0.17) | |||
| BMD (T-Scores) | All Participants n = 496 Mean (SE) | With Lactose Intolerance n = 46 Mean (SE) | Without Lactose Intolerance n = 450 Mean (SE) | p-Value |
|---|---|---|---|---|
| BMD: Femoral neck of the operative hip | −0.23 (0.63) | −0.30 (0.22) | −0.23 (0.65) | 0.766 |
| BMD: Femoral neck of the non-operative hip | −0.16 (0.05) | −0.23 (0.17) | −0.16 (0.05) | 0.698 |
| BMD: Lumbar spine | 1.17 (0.09) | 1.19 (0.33) | 1.17 (0.09) | 0.954 |
| Variables | BMD: Femoral Neck of the Operative Hip | BMD: Femoral Neck of the Non-Operative Hip | BMD: Lumbar Spine | |||
|---|---|---|---|---|---|---|
| β (SE) | p-Value | β (SE) | p-Value | β (SE) | p-Value | |
| BMI (kg/m2) | 0.06 (0.01) | <0.0001 | 0.07 (0.11) | <0.0001 | 0.06 (0.02) | 0.008 |
| Age (years) | −0.16 (.01) | 0.047 | −0.02 (.01) | 0.001 | −0.03 (0.01) | 0.004 |
| Yogurt >1 time/week | 0.33 (0.13) | 0.014 | ||||
| Cardiovascular exercise >1 time/week | 0.35 (0.12) | 0.005 | ||||
| Gender (males) | 0.43 (0.21) | 0.04 | ||||
| Vegan or Vegetarian | −1.26 (0.59) | 0.33 | ||||
| Adjusted R2 | 7.4% | 10.3% | 7.3% | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamilton, N.K.; Ojo, O.; Adegboye, A.R.A. The Effect of Self-Reported Lactose Intolerance and Dairy Consumption on Bone Mineral Density among American Hip Arthroplasty Patients: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2020, 17, 7182. https://doi.org/10.3390/ijerph17197182
Hamilton NK, Ojo O, Adegboye ARA. The Effect of Self-Reported Lactose Intolerance and Dairy Consumption on Bone Mineral Density among American Hip Arthroplasty Patients: A Cross-Sectional Study. International Journal of Environmental Research and Public Health. 2020; 17(19):7182. https://doi.org/10.3390/ijerph17197182
Chicago/Turabian StyleHamilton, Nikola K., Omorogieva Ojo, and Amanda Rodrigues Amorim Adegboye. 2020. "The Effect of Self-Reported Lactose Intolerance and Dairy Consumption on Bone Mineral Density among American Hip Arthroplasty Patients: A Cross-Sectional Study" International Journal of Environmental Research and Public Health 17, no. 19: 7182. https://doi.org/10.3390/ijerph17197182
APA StyleHamilton, N. K., Ojo, O., & Adegboye, A. R. A. (2020). The Effect of Self-Reported Lactose Intolerance and Dairy Consumption on Bone Mineral Density among American Hip Arthroplasty Patients: A Cross-Sectional Study. International Journal of Environmental Research and Public Health, 17(19), 7182. https://doi.org/10.3390/ijerph17197182






