Fertility Deterioration in a Remediated Petroleum-Contaminated Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Control and Remediated Soil Collection
2.2. Soil Physical-Chemical Analysis
2.3. Field Capacity
2.4. Hydrocarbon Concentration
2.5. Acute Toxicity
2.6. Water Repellency Analyses
2.7. Soil Compaction Test
2.8. Data Analysis
3. Results
3.1. Control Soil Profile
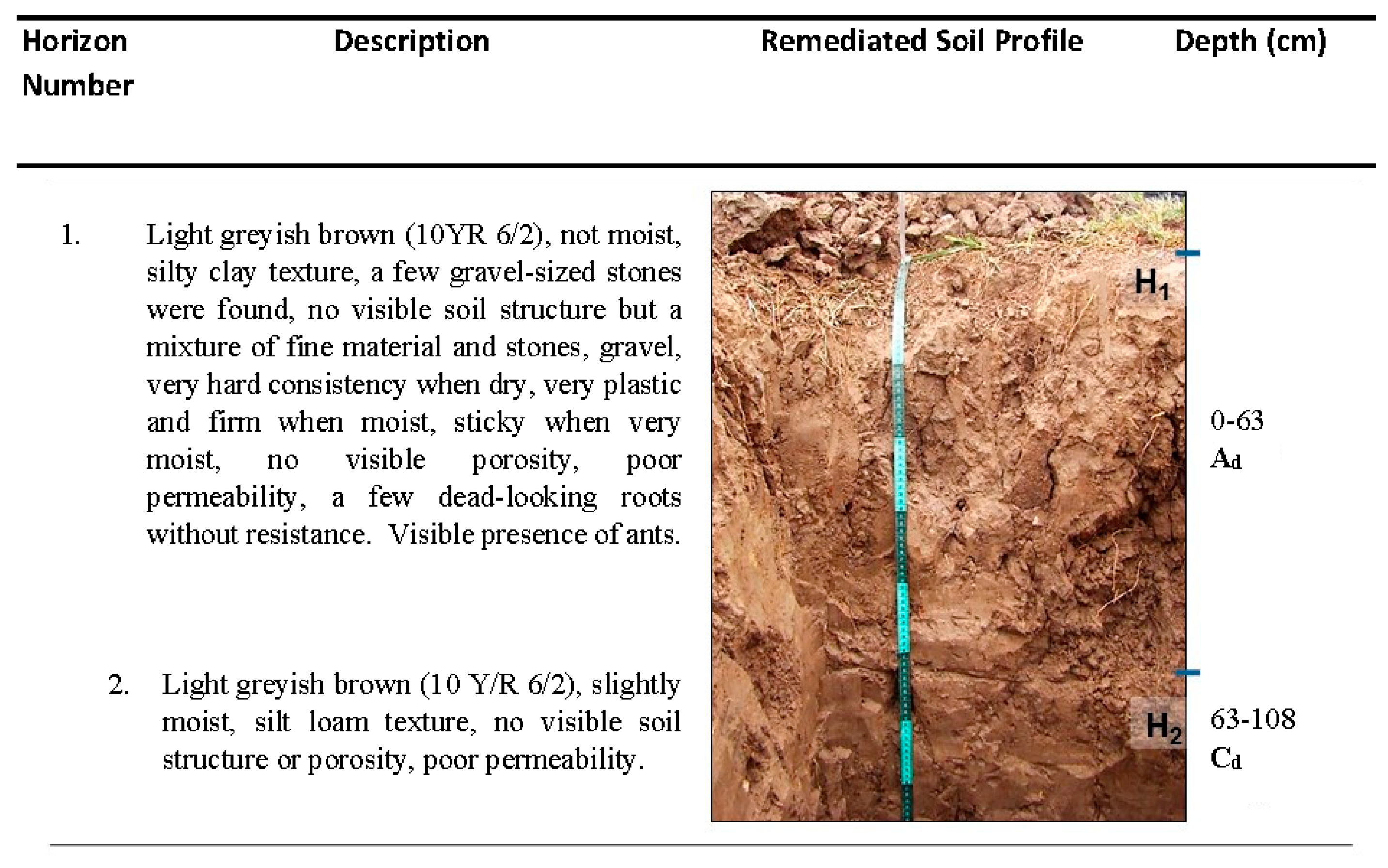
3.2. Remediated Soil Profile
3.3. Control Soil vs. Remediated Soil Profile
3.3.1. Physical-Chemical Properties
3.3.2. pH and Water Repellency
3.4. Control vs. Remediated Surface Soil
3.4.1. Physical-Chemical Properties
3.4.2. pH and Water Repellency
3.4.3. Acute Toxicity
3.5. Soil Penetration Resistance (Compaction)
3.6. Residual TPH Concentrations and Soil Physical-Chemical Properties Relation
4. Discussion
4.1. Probable Causes of Soil Fertility Deterioration at This Site
4.2. Recommendations to Avoid Soil Fertility Deterioration in Remediated Soils with Agricultural Land Use
- (1)
- Excavate all contaminated soil from the first 30 cm. Soil horizons (mainly A or O) within these depths should be treated as a whole.
- (2)
- Excavate all contaminated soil ranging from 30 cm to where visually affected soil is still found. Soil horizons of this second excavation (mainly B or C) should be treated as a whole, but not mixed with the previous mixture.
- (3)
- Assuming the soil remediation is completed, the second excavated soil layer should be returned first to the remediation site and finally, on top of this, the treated surface soil (0–30 cm).
- (4)
- Soil conditioners and a mid-term vegetative cover (2–3 years) should be incorporated at the remediation site. This setup can add organic matter and nutrients, improve soil structure and improve moisture retention capacity [23,30,33]. It may also replenish microbial populations and other soil biota if it was damaged by chemical treatments. Therefore, it is strongly recommended after physical-chemical or biological remediation [34,36].
4.3. Applicability of Recommendations to Other Sites
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rivera-Cruz, M.D.C.; Trujillo-Narcía, A. Estudio de toxicidad vegetal en suelos con petróleos nuevo e intemperizado. (Effect of vegetative toxicity in soils with new and weathered petroleums.). Interciencia 2004, 29, 369–376. [Google Scholar]
- Adams, R.H.; Osorio, F.G.; Cruz, J.Z. Water repellency in oil contaminated sandy and clayey soils. Int. J. Environ. Sci. Technol. 2008, 5, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Adams, R.H.; ZaVala-Cruz, J.; Morales-García, F. Concentración residual de hidrocarburos en suelo del trópico. II: Afectación a la fertilidad y su recuperación. (Residual concentration of hydrocarbons in soil in the tropics. II: Impacts to fertility and reclamation.). Interciencia 2008, 33, 483–489. [Google Scholar]
- Martínez, V.E. Efecto de hidrocarburos en las propiedades físicas y químicas de suelo arcilloso. (Effects of hydrocarbon pollutants on the physical and chemical properties of clay soil.). Terra Latinoam. 2001, 19, 9–17. [Google Scholar]
- Morales-Bautista, C.M.; Adams, R.H.; Hernández-Barajas, J.R.; Lobato-García, C.E.; Torres-Torres, J.G. Characterization of fresh and weathered petroleum for potential impacts to soil fertility. Int. J. Environ. Sci. Technol. 2016, 13, 2689–2696. [Google Scholar] [CrossRef]
- Secretaria de Recursos Naturalez (SEMARNAT). Norma Oficial Mexicana Nom-138-semarnat/ssa1-2012 que Establece los Límites Máximos Permisibles de Hidrocarburos en Suelos y Lineamientos Para el Muestreo en la Caracterización y Especificaciones Para la Remediación. (Oficial Mexicn Norm Nom-138-semarant/ssa1-2012 that Establishes the Maximum Permisible Limits of Hydrocarbons in Soils and Guidlines for Sampling during Characterization and Specifications for Remediation.); Diario Oficial de la Federación: Mexico City, Mexico, 2013; p. 14. [Google Scholar]
- Lim, M.W.; Von Lau, E.; Poh, P.E. A comprehensive guide of remediation technologies for oil contaminated soil—Present works and future directions. Mar. Pollut. Bull. 2016, 109, 14–45. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.L.; McGill, W.B. Characterization of disaggregated nonwettable surface soils found at old crude oil spill sites. Can. J. Soil Sci. 1998, 78, 331–344. [Google Scholar] [CrossRef]
- Chan-Quijano, J.G.; Jarquín-Sánchez, A.; Ochoa-Gaona, S.; Martínez-Zurimendi, P.; López-Jiménez, L.N.; Lázaro-Vázquez, A. Directrices para la remediación de suelos contaminados con hidrocarburos. (Guidelines for the remediation of contaminated soil with oil.). Teoría Y Praxis 2015, 17, 123–144. [Google Scholar] [CrossRef]
- Rivera-Cruz, M.C. Biotecnología para descontaminar suelos con hidrocarburos del petróleo crudo. (Biotechnology for decontaminating soils with hydrocarbons from crude petrolem.). Tecnociencia Universitaria 2004, 3, 44–55. [Google Scholar]
- Trujillo-Narcía, A.; Rivera-Cruz, M.D.C.; Lagunes-Espinoza, L.D.C.; Palma-López, D.J.; Soto-Sánchez, S.; Ramírez-Valverde, G. Efecto de la restauración de un Fluvisol contaminado con petróleo crudo. (Effect of the restoratino of a Fluvisol contaminated with crude petroleum.). Revista Internacional de Contaminacion Ambiental 2012, 28, 361–374. [Google Scholar]
- Salvador Chablé, J.C. Evaluación de Restauración Comercial en Suelo Contaminado por Fuga y Derrame de Hidrocarburos en Oleogasoducto de 24″ Ø Batería Oxiacaque–Íride, Mediante Bioensayo con Planta de Rábano (Raphanus Sativus L.) en Cunduacán, Tabasco, México. (Evaluation of the Commercial Restoration of a Soil Contaminated by Pipeline Break and Spill in the Gas-Oil Pipeline of 24″ Ø from Oxiacaque–Íride, by Bioassay with Radish Plant (Rhaphanus Sativus L.) in Cunduacán, Tabasco, Mexico). Master’s Thesis, Universidad Juárez Autónoma de Tabasco, Tabasco, Mexico, 2010; p. 8. [Google Scholar]
- Gómez Rivera, P.; Geissen, V. Tecnologías de restauración en suelos de Tabasco: ¿realmente restauran? (Technologies for restoration of soils of Tabasco: Do the really restore?). Kuxulkab’ Rev. de Divulg. 2007, 12, 43–50. [Google Scholar]
- Cuanalo de la Cerda, H.E. Manual Para la Descripción de Perfiles de Suelo en el Campo. Tercera Edición. (Manual for Description of Soil Profiles in the Field.), 3rd ed.; Colegio de Postgraduados, Centro de Edafología: Estado de México, Mexico, 1990. [Google Scholar]
- Porta, J.; Lopez Acevedo, M.; Poch, R. Edafología: Uso y Protección de Suelos (Edaphology: Use and Protection of Soils.), 3rd ed.; Mundi-Prensa: Madrid, Spain, 2014. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining organic carbon in soils: Effect of variation in digestion conditions and of inorganic soil constituents. Soil Sci. 1934, 63, 251–263. [Google Scholar] [CrossRef]
- Secretaria de Medio Ambiente y Recurso Naturales (SEMARNAT). Norma Oficial Mexicana NOM-021-SEMARNAT-2000 que Establece las Especificaciones de Fertilidad, Salinidad y Clasificación de Suelos, Estudio, Muestreo y Análisis. (Oficial Mexican Norm NOM-021-SEMARNAT-2000, that Establishes Specifications of Fertility, Salinity and Clasification of Soils, Study, Sampling and Analysis.); Diario Oficial de la Federación: Mexico City, Mexico, 2002; p. 85. [Google Scholar]
- Domínguez, V.I.; Aguilera, H. Metodología de Análisis Físico-Químicos de Suelos (Methodology for Phyisical-Chemical Analysis of Soils.), 1st ed.; Universidad Nacional Autónoma de México: Mexico City, Mexico, 1989. [Google Scholar]
- Kirkham, M.B. Principles of Soil and Plant Water Relations; Elsevier Science: San Diego, CA, USA, 2005. [Google Scholar]
- Lal, R.; Shukla, M.K. Principles of Soil Physics; Marcel Dekker: Columbus, OH, USA, 2004. [Google Scholar]
- US Environmental Protection Agency. Test Methods for Evaluating Solid Waste: Physical/Chemical Methods; EPA 530/SW-846; Environmental Protection Agency Office of Land and Emergency Management: Washington, DC, USA, 1997.
- Adams, R.H.; Guzmán-Osorio, F.J.; Domínguez-Rodríguez, V.I. Field-scale evaluation of the chemical–biological stabilization process for the remediation of hydrocarbon-contaminated soil. Int. J. Environ. Sci. Technol. 2014, 11, 1343–1352. [Google Scholar] [CrossRef] [Green Version]
- Secrataria de Comercio y Fomento Industrial (SECOFI). Norma Mexicana NMX-AA-112-1995-SCFI. Análisis de Agua y Sedimento-Evaluación de Toxicidad Aguda con Photobacterium Phosphoreum-Método de Prueba. (Meixcan Norm NNX-AA-112-1995-SCFI. Analysis of Water and Sediment—Evaluation of Acute Toxicity with Photobacterium Phosphoreum—Test Method.); Dirección General de Normas: Mexico City, Mexico, 1996; p. 33. [Google Scholar]
- Adams, R.H.; Dominguez-Rodriguez, V.I.; Zavala-Cruz, J.O.E.L. Vibrio fischeri bioassay for determination of toxicity in petroleum contaminated soils from tropical southeast Mexico. Sains Malaysiana 2015, 44, 337–346. [Google Scholar] [CrossRef]
- Watson, C.L.; Letey, J. Indices for characterizing soil-water repellency based upon contact angle-surface tension relationships. Soil Sci. Soc. Am. J. 1970, 34, 841–844. [Google Scholar] [CrossRef]
- Dekker, L.W.; Jungeris, P.D. Water repellency in the dunes with special reference to The Netherlands. Catena Suppl. 1990, 18, 173–183. [Google Scholar]
- Duiker, S.W. Diagnosing soil compaction using a penetrometer (soil compaction tester). Agron. Facts 2002, 63, 1–4. [Google Scholar]
- Zavala-Cruz, J.; Jiménez Ramírez, R.; Palma-López, D.J.; Bautista Zúñiga, F.; Gavi Reyes, F. Paisajes geomorfológicos: Base para el levantamiento de suelos en Tabasco, México. (Geomorphological landscapes: Framework for soil surveys in Tabasco, Mexico.). Ecosistemas y Recursos Agropecuarios 2016, 3, 161–171. [Google Scholar]
- Julca-Otiniano, A.; Meneses-Florián, L.; Blas-Sevillano, R.; Bello-Amez, S. La materia orgánica, importancia y experiencia de su uso en la agricultura. (Organic matter, importance, experiences and its role in agriculture.). Idesia 2006, 24, 49–61. [Google Scholar] [CrossRef] [Green Version]
- Ortega, T.E. Química de Suelos (Soil Chemistry), 1st ed.; Universidad Autónoma Chapingo: Mexico City, Mexico, 1981. [Google Scholar]
- Navarro Blaya, S.; Navarro García, G. Química agrícola, el suelo y los elementos químicos esenciales para la vida vegetal (Agricultural chemistry, soil and esential chemical elements for vegetable life.), 2nd ed.; Mundi-Prensa: Madrid, Spain, 2003. [Google Scholar]
- Mayo-López, T.M.; Adams, R.H.; Domínguez-Rodríguez, V.I.; Guzmán-Osorio, F.J. Organic amendment optimization for treatment of hydrocarbon contaminated soil using the chemical-biological stabilization process. Afr. J. Biotechnol. 2010, 9, 7079–7085. [Google Scholar]
- Trujillo-Narcía, A.; Rivera-Cruz, M.; del Carmen Lagunes-Espinoza, L.; Palma-López, D.J.; Sánchez-Soto, S.; Ramírez-Valverde, G. Uso de fertilizantes orgánicos en la enmendación de un fluvisol restaurado tras la contaminación con petróleo. (Use of organic fertilizers in remediation of a fluvisol restored after oil contamination.). Interciencia 2014, 39, 266–273. [Google Scholar]
- De León González, F.; Zelaya, F.P.; Jerónimo, G.P.; Rodríguez, V.N.; Aime, M.A. Área foliar, longitud de raíces y producción de grano de Amaranthus hypocondriacus L. en un suelo compactado. (Leaf area, root lenght and grain yield of Amaranthus hypocondriacus L. grown on a highly compacted soil.). Agro Sur 1997, 25, 170–179. [Google Scholar] [CrossRef]
- Claassens, S.; Van Rensburg, L.; Riedel, K.J.; Bezuidenhout, J.J.; Van Rensburg, P.J. Evaluation of the efficiency of various commercial products for the bioremediation of hydrocarbon contaminated soil. Environmentalist 2006, 26, 51–62. [Google Scholar] [CrossRef]
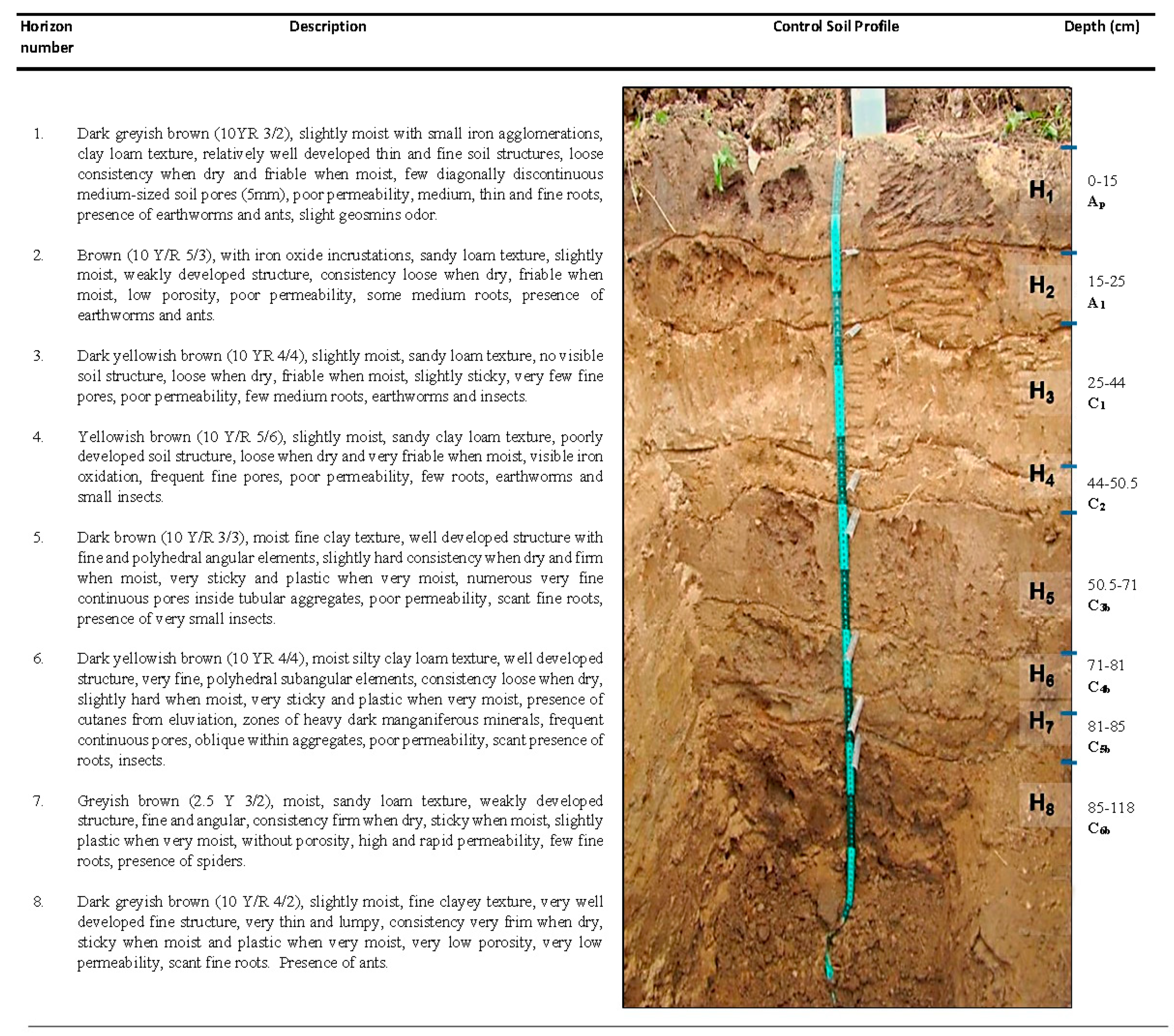





| Ho | BD (g cm−3) | SD (g cm−3) | Po (%) | FC (%) | H (%) | Sand (%) | Clay (%) | Silt (%) | Texture (USDA) | OM (%) | pH | WDPT (s) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H1 (Ap) | 1.06 | 2.38 | 55.31 | 35.88 | 24.69 | 50.46 | 9.34 | 40.20 | Loam | 2.80 | 7.08 | 0.59 |
| H2 (A1) | 1.16 | 2.50 | 53.76 | 32.44 | 16.31 | 42.96 | 17.41 | 39.63 | Loam | 0.97 | 7.98 | 0.48 |
| H3 (C1) | 1.20 | 2.63 | 54.40 | 31.23 | 11.17 | 49.04 | 17.34 | 33.62 | Loam | 0.16 | 8.01 | 0.52 |
| H4 (C2) | 1.28 | 2.63 | 51.51 | 28.30 | 18.87 | 67.38 | 13.12 | 19.50 | Sandy loam | 0.09 | 8.11 | 0.52 |
| H5 (C3b) | 1.05 | 2.50 | 58.08 | 34.19 | 26.08 | 9.60 | 47.41 | 42.99 | Silty clay | 0.16 | 8.14 | 2.36 |
| H6 (C4b) | 1.03 | 2.78 | 62.99 | 36.10 | 27.64 | 7.02 | 45.62 | 47.35 | Silty clay | 0.29 | 8.16 | 2.33 |
| H7 (C5b) | 1.22 | 2.38 | 48.59 | 31.51 | 24.33 | 57.08 | 15.64 | 27.28 | Sandy clay loam | 0.42 | 8.40 | 0.76 |
| H8 (C6b) | 1.04 | 2.63 | 60.63 | 35.97 | 29.06 | 15.08 | 37.64 | 47.28 | Silty clay loam | 0.43 | 7.91 | 2.27 |
| Ho | BD (g cm−3) | SD (g cm−3) | Po (%) | FC (%) | H (%) | Sand (%) | Clay (%) | Silt (%) | Texture (USDA) | OM (%) | pH | WDPT (s) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H1 (Ad) | 1.25 | 2.50 | 49.92 | 30.93 | 10.05 | 30.36 | 32.64 | 37.00 | Clay loam | 0.49 | 7.50 | 1.26 |
| H2 (Ad) | 1.18 | 2.50 | 52.80 | 30.23 | 15.77 | 20.36 | 37.64 | 42.00 | Clay loam | 0.02 | 7.80 | 1.97 |
| Parameters | Control Soil | Remediated Soil |
|---|---|---|
| BD (g cm−3) | 0.8100 | 0.3721 |
| SD (g cm−3) | 0.7500 | 0.5329 |
| H (%) | 0.3400 | 0.3969 |
| FC (%) | 0.0049 | 0.2209 |
| Po (%) | 0.6400 | 0.1936 |
| OM (%) | 0.0900 | 0.0081 |
| pH | 0.0841 | 0.4489 |
| WDPT (s) | 0.1024 | 0.6889 |
| Sand (%) | 0.1521 | 0.0036 |
| Silt (%) | 0.1764 | 0.0001 |
| Clay (%) | 0.0001 | 0.0324 |
| Parameters | Control Soil | Remediated Soil | Importance |
|---|---|---|---|
| Parameters very likely related to soil degradation from contamination or remediation technique | |||
| Proportion fine to coarse particles (clay + silt):sand | 62:38 | 77:23 | May cause compaction, reduced infiltration, reduced root penetration, reduced gas exchange |
| OM (%) | 0.81 | 0.26 | Reduces CEC, CIC, may reduce moisture content and availability of soil nutrients |
| FC (%) | 35.0 | 30.0 | Reduces moisture retention, may cause water stress, wilting |
| Po (%) | 54.1 | 49.8 | May cause compaction, reduced infiltration, reduced root penetration, reduced gas exchange |
| BD (g cm−3) | 11.1 | 12.3 | May cause compaction, reduced infiltration, reduced root penetration, reduced gas exchange |
| Compaction (MPa) | 0.84–1.10 | 1.32–3.04 | Reduces water infiltration, root penetration, free gas exchange (respiration of soil organisms); about two to three times greater in remediated soil |
| H (%) | 22.9 | 12.5 | May cause water stress and wilting; about half as much moisture in remediated soil |
| Depth of roots (soil profile, cm) | 118 | 3–10 | Sign of unfertile conditions for plant growth, possible due to compaction |
| Presence of insects and spiders (soil profile, cm) | 0–118 | 0–63 | Sign of poor conditions, possibly due to poor plant growth (root penetration, primary productivity) and less food available |
| Parameters very likely not related to soil degradation from contamination or remediation technique | |||
| TPH (mg kg−1) | 182 | 969 | Low levels, no significant correlation found between TPH and other factors (R2 < 0.7) in remediated soil |
| pH | 7.1–8.0 | 7.4–8.5 | Mildly alkaline but in same range as subsurface of control soil (7.9–8.4); probably not detrimental to soil fertility |
| WDPT (s) | 0.48–0.59 | 1.26 | Levels classified as “null” |
| Toxicity | NA | NA | No relationship was found between the soil concentration in the bioassay and response of the test organisms (all samples considered non-toxic) |
| Parameters | Importance |
|---|---|
| CEC (meq kg−1) | Low levels reduce availability of soil nutrients, especially related to low OM |
| Salinity (dS/m) | Some remediation agents could increase salinity; probably not a factor in this study considering neutral—mildly alkaline conditions (high salinity is usually associated with high pH) |
| Microbial biomass/respiration (CFU g−1; mg CO2 h−1 kg−1) | Extreme pH, oxidizing conditions, and high surfactant concentrations may reduce microbial biomass, activity and important soil functions; this may not be a factor at this site considering the five year time span since remediation, humid tropical climate, and Fluvisol conditions (generally optimal for soil recovery) |
| Plant bioassay | This is a true confirmation of successful site remediation; a previous in situ study at this site with radish did not show reduced emergence, establishment or vigor, but bulb diameter was much less in the remediated soil; possibly due to soil compaction |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez-Rodríguez, V.I.; Adams, R.H.; Vargas-Almeida, M.; Zavala-Cruz, J.; Romero-Frasca, E. Fertility Deterioration in a Remediated Petroleum-Contaminated Soil. Int. J. Environ. Res. Public Health 2020, 17, 382. https://doi.org/10.3390/ijerph17020382
Domínguez-Rodríguez VI, Adams RH, Vargas-Almeida M, Zavala-Cruz J, Romero-Frasca E. Fertility Deterioration in a Remediated Petroleum-Contaminated Soil. International Journal of Environmental Research and Public Health. 2020; 17(2):382. https://doi.org/10.3390/ijerph17020382
Chicago/Turabian StyleDomínguez-Rodríguez, Verónica Isidra, Randy H. Adams, Mariloli Vargas-Almeida, Joel Zavala-Cruz, and Enrique Romero-Frasca. 2020. "Fertility Deterioration in a Remediated Petroleum-Contaminated Soil" International Journal of Environmental Research and Public Health 17, no. 2: 382. https://doi.org/10.3390/ijerph17020382





