The Influence of Surgical Staff Behavior on Air Quality in a Conventionally Ventilated Operating Theatre during a Simulated Arthroplasty: A Case Study at the University Hospital of Parma
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Monitoring Programme
2.3. Sampling Points
2.4. Environmental Monitoring
2.4.1. Microbial Air Sampling
2.4.2. Particle Counting
2.4.3. Microclimatic Monitoring
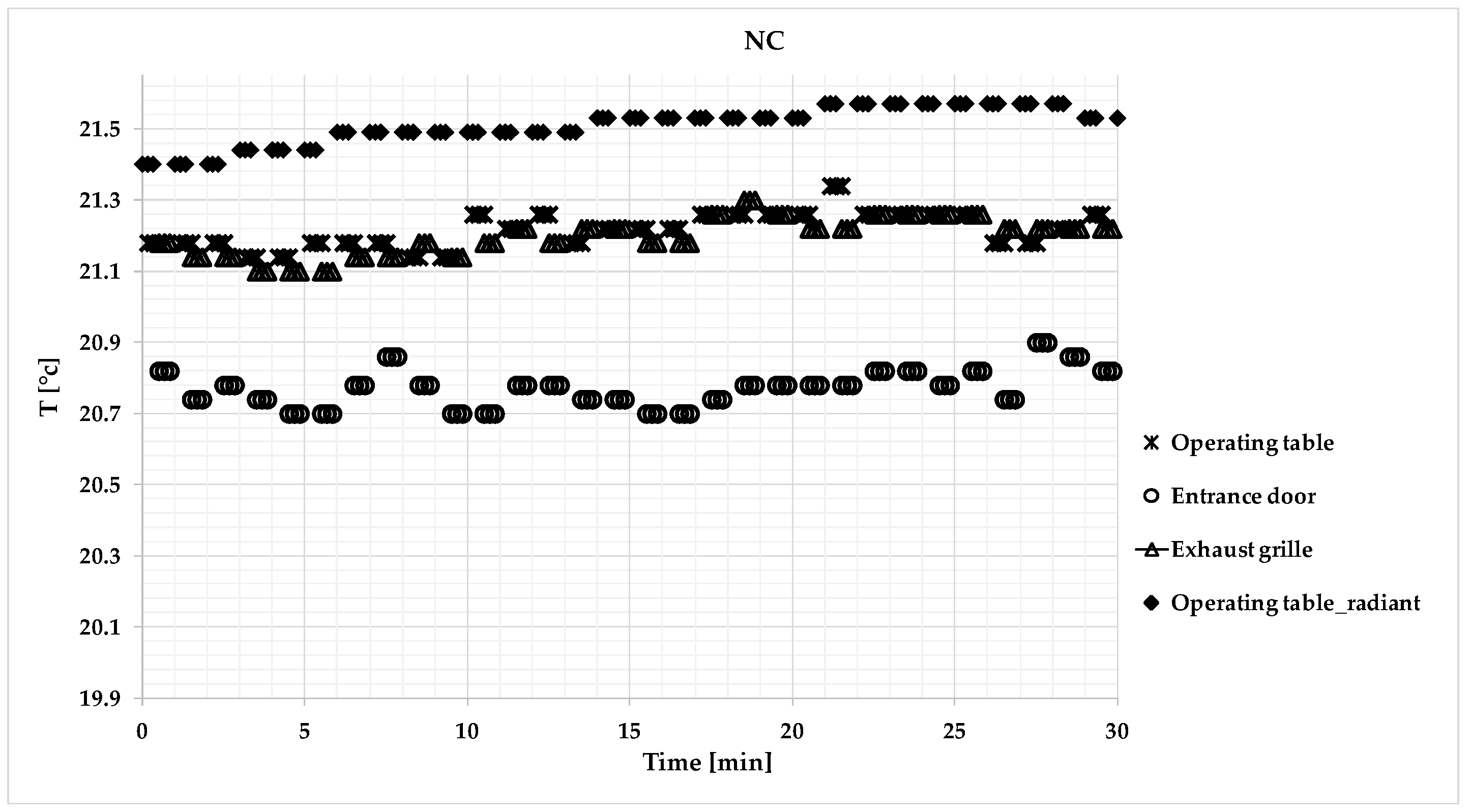
3. Results
3.1. Biological Sampling
3.2. Particle Counting
3.3. Microclimatic Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Global Guidelines for the Prevention of Surgical Site Infection; World Health Organization (WHO): Geneva, Switzerland, 2016. [Google Scholar]
- Mangram, A.J.; Horan, T.C.; Pearson, M.L.; Silver, L.C.; Jarvis, W.R. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am. J. Infect. Control. 1999, 27, 97–132. [Google Scholar] [CrossRef]
- Regione Emilia-Romagna. Prevenzione delle Infezioni del sito Chirurgico. Dossier 261-2017. Available online: https://assr.regione.emilia-romagna.it/pubblicazioni/dossier/doss261 (accessed on 13 June 2019).
- Italian Workers Compensation Authority. Linee Guida Sugli Standard di Sicurezza e di Igiene del Lavoro nel Reparto Operatorio; Istituto Superiore per la Prevenzione e la Sicurezza del Lavoro. Dipartimento Igiene del Lavoro (ISPESL): Rome, Italy, 2009.
- Whyte, W.; Hodgson, R.; Tinkler, J. The importance of airborne bacterial contamination of wounds. J. Hosp. Infect. 1982, 3, 123–135. [Google Scholar] [CrossRef]
- Lidwell, O.M.; Lowbury, E.J.L.; Whyte, W.; Blowers, R.; Stanley, S.J.; Lowe, D. Effect of ultraclean air in operating rooms on deep sepsis in the joint after total hip or knee replacement: A randomised study. Br. Med. J. 1982, 285, 10–14. [Google Scholar] [CrossRef]
- Lidwell, O.M. Air, antibiotics and sepsis in replacement joints. J. Hosp. Infect. 1988, 11, 18–40. [Google Scholar] [CrossRef]
- Whyte, W.; Lidwell, O.M.; Lowbury, E.J.; Blowers, R. Suggested bacteriological standards for air ultraclean operating rooms. J. Hosp. Infect. 1983, 4, 133–139. [Google Scholar] [CrossRef]
- Estates NHS. Health Technical Memorandum 2025; Ventilation in Healthcare Premises. Part 3. Validation and Verification; National Health Service: London, UK, 1994. [Google Scholar]
- Heating and Ventilation Systems Health Technical Memorandum 03-01: Specialised Ventilation for Healthcare Premises Part A: Design and Validation; HTM-03-01; TSO: Edinburgh, UK, 2007.
- Friberg, B.; Friberg, S.; Burman, L.G. Inconsistent correlation between aerobic bacterial surface and air counts in operating rooms with ultraclean laminar air flows: Proposal of a new bacteriological standard for surface contamination. J. Hosp. Infect. 1999, 42, 287–293. [Google Scholar] [CrossRef]
- Pitzurra, M.; Savino, A.; Pasquarella, C. Microbiological environment monitoring (MEM). Ann. Ig. 1997, 9, 439–454. [Google Scholar]
- Pasquarella, C.; Pitzurra, O.; Savino, A. The index of microbial air contamination. J. Hosp. Infect. 2000, 46, 241–256. [Google Scholar] [CrossRef]
- H+ Die Spitäler der Schweiz. Klassifizierung und Technisce Anfordungen an Spitälraume; H+ Die Spitäler der Schweiz: Bern, Switzerland, 2007. [Google Scholar]
- UNI-11425:2011. Surgery Operating Theatre, Ventilation and Air-Conditioning System for Contamination Control (VCCC); Design, Construction, Commissioning, Qualification, Management and Maintenance; UNI: Milano, Italy, 2011. [Google Scholar]
- ISO 14644-1:2015. Cleanrooms and Associated Controlled Environments—Part 1: Classification of Air Cleanliness by Particle Concentration; ISO: Geneva, Switzerland, 2015. [Google Scholar]
- Brandt, C.; Hott, U.; Sohr, D.; Daschner, F.; Gastmeier, P.; Rüden, H. Operating room ventilation with laminar airflow shows no protective effect on surgical site infection rate in orthopaedic and abdominal surgery. Ann. Surg. 2008, 248, 695–700. [Google Scholar] [CrossRef]
- Bischoff, P.; Kubilay, N.Z.; Allegranzi, B.; Egger, M.; Gastmeier, P. Effect of laminar airflow ventilation on surgical site infections: A systematic review and meta-analysis. Lancet Infect. Dis. 2017, 17, 553–561. [Google Scholar] [CrossRef]
- Pasquarella, C.; Agodi, A.; Auxilia, F.; Lytsy, B.; Mura, I.; Parneix, P.; Popp, W.; Brusaferro, S. Air Quality in the Operating Theatre: A Perspective. Aerobiologia 2019. [Google Scholar] [CrossRef]
- Lytsy, B.; Whyte, W. Ultraclean air systems and the claim that laminar airflow systems fail to prevent deep infections after total joint arthroplasty. J. Hosp. Infect. 2019, 103, e9–e15. [Google Scholar]
- German Society of Hospital Hygiene (DGKH). Air Quality in the Operating Room: Surgical Site Infections, HVAC Systems and Discipline; German Society of Hospital Hygiene (DGKH): Berlin, German, 2018. [Google Scholar]
- Agodi, A.; Auxilia, F.; Barchitta, M.; Cristina, M.L.; D’Alessandro, D.; Mura, I.; Nobile, M.; Pasquarella, C.; Italian Study Group of Hospital Hygiene. Operating theatre ventilation systems and microbial air contamination in total joint replacement surgery: Results of the GISIO-ISChIA study. J. Hosp. Infect. 2015, 90, 213–219. [Google Scholar] [CrossRef]
- Andersson, A.E.; Bergh, I.; Karlsson, J.; Eriksson, B.I.; Nilsson, K. Traffic flow in the operating room: An explorative and descriptive study on air quality during orthopedic trauma implant surgery. Am. J. Infect. Control. 2012, 40, 750–755. [Google Scholar] [CrossRef]
- Andersson, E.; Petzold, M.; Bergh, I.; Karlsson, J.; Eriksson, B.I.; Nilsson, K. Comparison between mixed and laminar airflow systems in operating rooms and the influence of human factors: Experiences from a Swedish orthopedic center. Am. J. Infect. Control. 2014, 42, 665–669. [Google Scholar] [CrossRef]
- Balocco, C.; Petrone, G.; Cammarata, G. Assessing the effects of sliding doors on an operating theatre climate. Build. Simul. 2012, 5, 73–83. [Google Scholar] [CrossRef]
- Birgand, G.; Saliou, P.; Lucet, J.C. Influence of staff behavior on infectious risk in operating rooms: What is the evidence? Infect. Control. Hosp. Epidemiol. 2015, 36, 93–106. [Google Scholar] [CrossRef]
- Dallolio, L.; Raggi, A.; Sanna, T.; Mazzetti, M.; Orsi, A.; Zanni, A.; Farruggia, P.; Leoni, E. Surveillance of Environmental and Procedural Measures of Infection Control in the Operating Theatre Setting. Int. J. Environ. Res. Public Health 2018, 15, 46. [Google Scholar] [CrossRef]
- Kalliomaki, P.; Saarinen, P.; Tang, J.W.; Koskela, H. Airflow patterns though single hinged and sliding doors in hospital isolation rooms. Effect of ventilation, flow differential and passage. Build. Environ. 2016, 107, 154–168. [Google Scholar] [CrossRef]
- Pasquarella, C.; Vitali, P.; Saccani, E.; Manotti, P.; Boccuni, C.; Ugolotti, M.; Signorelli, C.; Mariotti, F.; Sansebastiano, G.E.; Albertini, R. Microbial air monitoring in operating rooms: Experience at the University Hospital of Parma. J. Hosp. Infect. 2012, 81, 50–57. [Google Scholar] [CrossRef]
- Pokrywka, M.; Byers, K. Traffic in the operating room: A review of factors influencing air flow and surgical wound contamination. Infect. Disord. Drug. Targets 2013, 13, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Scaltriti, S.; Cencetti, S.; Rovesti, S.; Marchesi, I.; Bargellini, A.; Borella, P. Risk factors for particulate and microbial contamination of air in operating theatres. J. Hosp. Infect. 2007, 66, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Barroso, G.; García Sanz-Calcedo, J. Evaluation of HVAC Design Parameters in High-Performance Hospital Operating Theatres. Sustainability 2019, 11, 1493. [Google Scholar] [CrossRef]
- Mears, S.C.; Blanding, R.; Belkoff, S.M. Door opening affects operating room pressure during joint arthroplasty. J. Orthoped. 2015, 38, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Villafruela, J.M.; San Josè, J.F.; Castro, F.; Zarzuelo, A. Airflow patterns through sliding door during opening and foot traffic in operating rooms. Build. Environ. 2016, 109, 190–198. [Google Scholar] [CrossRef]
- Sadrizadeh, S.; Afshari, A.; Karimipanah, T.; Håkansson, U.; Nielsen, P.V. Numerical simulation of the impact of surgeon posture on airborne particle distribution in a turbulent mixing operating theatre. Build Environ. 2016, 110, 140–147. [Google Scholar] [CrossRef]
- Pasquarella, C.; Barchitta, M.; D’Alessandro, D.; Cristina, M.L.; Mura, I.; Nobile, M.; Auxilia, F.; Agodi, A.; Collaborators. Heating, ventilation and air conditioning (HVAC) system, microbial air contamination and surgical site infection in hip and knee arthroplasties: The GISIO-SItI ISChIA study. Ann. Ig. 2018, 30 (Suppl. S2), 22–35. [Google Scholar]
- Cristina, M.L.; Sartini, M.; Schinca, E.; Ottria, G.; Spagnolo, A.M. Operating room environment and surgical site infections in arthroplasty procedures. J. Prev. Med. Hyg. 2016, 57, e142–e148. [Google Scholar]
- Cleanrooms and Associated Controlled Environments and Biocontamination Control; Part 1. General Principles and Methods; ISO 14698-1:2004; ISO: Geneva, Switzerland, 2004.
- Pasquarella, C.; Albertini, R.; Dall’Aglio, P.; Saccani, E.; Sansebastiano, G.E.; Signorelli, C. Air microbial sampling: The state of the art. Ig. San. Pubblica 2008, 64, 79–120. [Google Scholar]
- Determination of Particle Size Distribution—Single Particle Light Interaction Methods—Part 4: Light Scattering Airborne Particle Counter for Clean Spaces; ISO 21501-4:2007; ISO: Geneva, Switzerland, 2007.
- Ergonomics of the Thermal Environment Instruments for Measuring Physical Quantities; ISO 7726:1998; ISO: Geneva, Switzerland, 1998.
- Marino, M.J. The use and misuse of statistical methodologies in pharmacology research. Biochem. Pharmacol. 2014, 87, 78–92. [Google Scholar] [CrossRef]
- Montagna, M.T.; Rutigliano, S.; Trerotoli, P.; Napoli, C.; Apollonio, F.; D’Amico, A.; De Giglio, O.; Diella, G.; Lopuzzo, M.; Marzella, A.; et al. Evaluation of Air Contamination in Orthopaedic Operating Theatres in Hospitals in Southern Italy: The IMPACT Project. Int. J. Environ. Res Public. Health 2019, 16, 3581. [Google Scholar] [CrossRef] [PubMed]
- Vonci, N.; De Marco, M.F.; Grasso, A.; Spataro, G.; Cevenini, G.; Messina, G. Association between air changes and airborne microbial contamination in operating rooms. J. Infect. Public Health 2019. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, R.E.; Ballard, E.L.; O’Rourke, P.; Knibbs, L.D.; Morawska, L.S.; Bell, C. Indoor hospital air and the impact of ventilation on bioaerosols: A systematic review. J. Hosp. Infect. 2019, 103, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Albertini, R.; Colucci, M.E.; Turchi, S.; Vitali, P. The management of air contamination control in operating theaters: The experience of the Parma University Hospital (IT). Aerobiologia 2019. [Google Scholar] [CrossRef]
- Talon, D.; Schoenleber, T.; Bertrand, X.; Vichard, P. What type of airflow system should be used in orthopaedic operating theatres? Hosp. Infect. Soc. 2006. [Google Scholar] [CrossRef]
- Din, A.; Foden, P.; Mathew, M.; Periasamy, K. Does laminar flow reduce the risk of early surgical site infection in hip fracture patients? J. Orthop. 2019. [Google Scholar] [CrossRef]
- ANSI/ASHRAE-170. Ventilation of Health Care Facilities; ASHRAE Standards Committee, The ASHRAE Board of Directors: Atlanta, GA, USA; The American National Standards Institute: New York, NY, USA, 2008. [Google Scholar]
- ANSI/ASHRAE-62-1. Ventilation for Acceptable Indoor Air Quality; ASHRAE: Atlanta, GA, USA, 2007. [Google Scholar]




| Operating Table | Exhaust Grille | Entrance Door | |||||||
|---|---|---|---|---|---|---|---|---|---|
| R | C | NC | R | C | NC | R | C | NC | |
| CFU/m3 | 2 | 13 | 74 | 3 | 23 | 44 | 4 | 29 | 93 |
| IMA | 0 | 2 | 8 | 0 | 6 | 8 | 2 | 4 | 16 |
| Particles ≥ 0.5 µm | 1848 | 64,783 | 82,696 | - | - | - | - | - | - |
| Air velocity [m/s] | Temperature [°C] | Relative Humidity [%] | CO2 [ppm] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OPERATING TABLE | Entrance Door | Operating Table Radiant | Operating Table | Exhaust Grille | Entrance Door | Operating Table | Exhaust Grille | Entrance Door | Operating Table | ||
| Median | 0.023 | 0.135 | 20.69 | 20.26 | 20.50 | 19.98 | 65.60 | 67.10 | 66.00 | 410.39 | At rest |
| Average | 0.031 | 0.134 | 20.68 | 20.27 | 20.49 | 19.98 | 65.57 | 67.05 | 66.00 | 411.99 | |
| Standard deviation | 0.04 | 0.02 | 0.02 | 0.03 | 0.03 | 0.01 | 0.12 | 0.16 | 0.10 | 0.18 | |
| Median | 0.051 | 0.093 | 21.28 | 20.94 | 20.98 | 20.50 | 64.30 | 66.20 | 65.20 | 505.91 | Correct condition |
| Average | 0.069 | 0.095 | 21.27 | 20.93 | 20.97 | 20.51 | 64.21 | 65.98 | 65.16 | 506.31 | |
| Standard deviation | 0.06 | 0.05 | 0.03 | 0.06 | 0.03 | 0.05 | 0.26 | 0.25 | 0.13 | 0.52 | |
| Median | 0.19 | 0.092 | 21.53 | 21.22 | 21.21 | 20.78 | 63.80 | 65.80 | 64.71 | 522.50 | Not correct condition |
| Average | 0.15 | 0.11 | 21.50 | 21.20 | 21.20 | 20.80 | 63.60 | 65.70 | 64.70 | 520.52 | |
| Standard deviation | 0.07 | 0.07 | 0.05 | 0.05 | 0.05 | 0.05 | 0.21 | 0.22 | 0.12 | 0.86 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasquarella, C.; Balocco, C.; Colucci, M.E.; Saccani, E.; Paroni, S.; Albertini, L.; Vitali, P.; Albertini, R. The Influence of Surgical Staff Behavior on Air Quality in a Conventionally Ventilated Operating Theatre during a Simulated Arthroplasty: A Case Study at the University Hospital of Parma. Int. J. Environ. Res. Public Health 2020, 17, 452. https://doi.org/10.3390/ijerph17020452
Pasquarella C, Balocco C, Colucci ME, Saccani E, Paroni S, Albertini L, Vitali P, Albertini R. The Influence of Surgical Staff Behavior on Air Quality in a Conventionally Ventilated Operating Theatre during a Simulated Arthroplasty: A Case Study at the University Hospital of Parma. International Journal of Environmental Research and Public Health. 2020; 17(2):452. https://doi.org/10.3390/ijerph17020452
Chicago/Turabian StylePasquarella, Cesira, Carla Balocco, Maria Eugenia Colucci, Elisa Saccani, Samuel Paroni, Lara Albertini, Pietro Vitali, and Roberto Albertini. 2020. "The Influence of Surgical Staff Behavior on Air Quality in a Conventionally Ventilated Operating Theatre during a Simulated Arthroplasty: A Case Study at the University Hospital of Parma" International Journal of Environmental Research and Public Health 17, no. 2: 452. https://doi.org/10.3390/ijerph17020452
APA StylePasquarella, C., Balocco, C., Colucci, M. E., Saccani, E., Paroni, S., Albertini, L., Vitali, P., & Albertini, R. (2020). The Influence of Surgical Staff Behavior on Air Quality in a Conventionally Ventilated Operating Theatre during a Simulated Arthroplasty: A Case Study at the University Hospital of Parma. International Journal of Environmental Research and Public Health, 17(2), 452. https://doi.org/10.3390/ijerph17020452







