Sugarcane Bagasse as an Efficient Biosorbent for Methylene Blue Removal: Kinetics, Isotherms and Thermodynamics
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Preparation of SCB
2.2. Characterization of SCB
2.3. Analytical Measurements
2.4. Effect of Particle Size and Adsorbent Concentration
2.5. Kinetics Adsorption Experiments
2.6. Isotherm Adsorption Experiments
2.7. Thermodynamic Parameters
3. Results and Discussion
3.1. Charachterization of SCB
3.2. Effect of Particle Size and Adsorbent Concentration
3.3. Kinetics Adsorption Experiments
3.4. Isotherms Adsorption Modeling
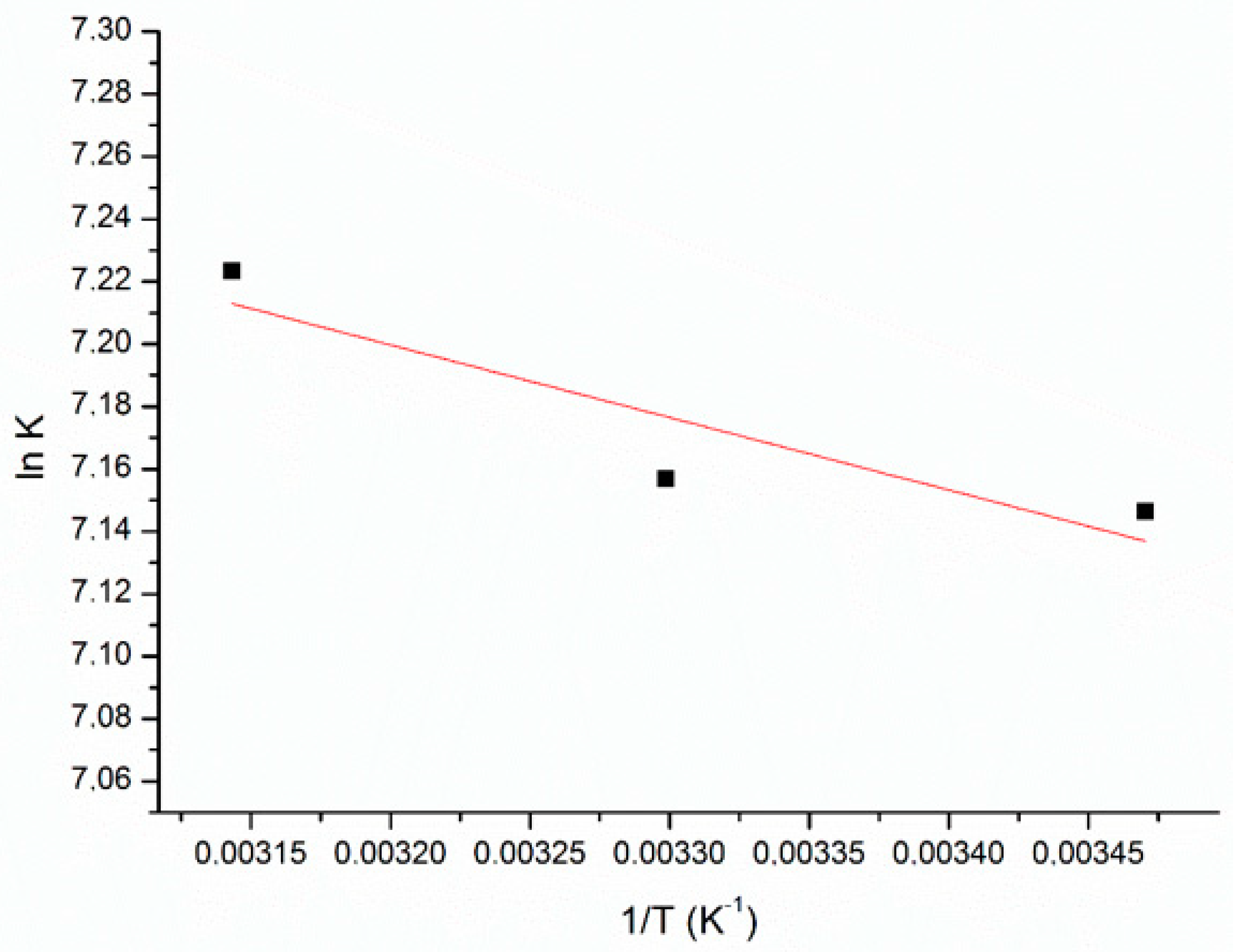
3.5. Thermodynamics Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bulgariu, L.; Escudero, L.B.; Bello, O.S.; Iqbal, M.; Nisar, J.; Adegoke, K.A.; Alakhras, F.; Kornaros, M.; Anastopoulos, I. The utilization of leaf-based adsorbents for dyes removal: A review. J. Mol. Liq. 2019, 276, 728–747. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Coll. Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Albadarin, A.B.; Mo, J.; Glocheux, Y.; Allen, S.; Walker, G.; Mangwandi, C. Preliminary investigation of mixed adsorbents for the removal of copper and methylene blue from aqueous solutions. Chem. Eng. J. 2014, 255, 525–534. [Google Scholar] [CrossRef]
- Shoukat, S.; Bhatti, H.N.; Iqbal, M.; Noreen, S. Mango stone biocomposite preparation and application for crystal violet adsorption: A mechanistic study. Microporous Mesoporous Mater. 2017, 239, 180–189. [Google Scholar] [CrossRef]
- Tahir, H.; Sultan, M.; Akhtar, N.; Hameed, U.; Abid, T. Application of natural and modified sugar cane bagasse for the removal of dye from aqueous solution. J. Saudi Chem. Soc. 2016, 20, S115–S121. [Google Scholar] [CrossRef]
- Srinivasan, A.; Viraraghavan, T. Decolorization of dye wastewaters by biosorbents: A review. J. Environ. Manag. 2010, 91, 1915–1929. [Google Scholar] [CrossRef]
- Gadd, G.M. Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. 2009, 84, 13–28. [Google Scholar] [CrossRef]
- Fomina, M.; Gadd, G.M. Biosorption: Current perspectives on concept, definition and application. Bioresour. Technol. 2014, 160, 3–14. [Google Scholar] [CrossRef]
- Soliman, E.M.; Ahmed, S.A.; Fadl, A.A. Removal of calcium ions from aqueous solutions by sugar cane bagasse modified with carboxylic acids using microwave-assisted solvent-free synthesis. Desalination 2011, 278, 18–25. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane suerfaces of glass, mica and platinium. J. Am. Chem. Soc. 1918, 40, 8. [Google Scholar] [CrossRef]
- Freundlich, H. Über die Adsorption in Lösungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 11. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.-J. Biosorption isotherms, kinetics and thermodynamics. Sep. Purif. Technol. 2008, 61, 229–242. [Google Scholar] [CrossRef]
- Tsuchida, J.E.; Rezende, C.A.; de Oliveira-Silva, R.; Lima, M.A.; d’Eurydice, M.N.; Polikarpov, I.; Bonagamba, T.J. Nuclear magnetic resonance investigation of water accessibility in cellulose of pretreated sugarcane bagasse. Biotechnol. Biofuels 2014, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Ben Guerrero, E.; Arneodo, J.; Bombarda Campanha, R.; Abrão de Oliveira, P.; Veneziano Labate, M.T.; Regiani Cataldi, T.; Campos, E.; Cataldi, A.; Labate, C.A.; Martins Rodrigues, C.; et al. Prospection and Evaluation of (Hemi) Cellulolytic Enzymes Using Untreated and Pretreated Biomasses in Two Argentinean Native Termites. PLoS ONE 2015, 10, e0136573. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.; Antunes, F.A.F.; Anjos, V.; Bell, M.J.V.; Rodrigues, L.N.; Polikarpov, I.; de Azevedo, E.R.; Bernardinelli, O.D.; Rosa, C.A.; Pagnocca, F.C.; et al. Multi-scale structural and chemical analysis of sugarcane bagasse in the process of sequential acid–base pretreatment and ethanol production by Scheffersomyces shehatae and Saccharomyces cerevisiae. Biotechnol. Biofuels 2014, 7, 63. [Google Scholar] [CrossRef]
- Moubarik, A.; Grimi, N. Valorization of olive stone and sugar cane bagasse by-products as biosorbents for the removal of cadmium from aqueous solution. Food Res. Int. 2015, 73, 169–175. [Google Scholar] [CrossRef]
- Antonio Bizzo, W.; Lenço, P.C.; Carvalho, D.J.; Veiga, J.P.S. The generation of residual biomass during the production of bio-ethanol from sugarcane, its characterization and its use in energy production. Renew. Sustain. Energy Rev. 2014, 29, 589–603. [Google Scholar] [CrossRef]
- Meili, L.; Lins, P.V.S.; Costa, M.T.; Almeida, R.L.; Abud, A.K.S.; Soletti, J.I.; Dotto, G.L.; Tanabe, E.H.; Sellaoui, L.; Carvalho, S.H.V.; et al. Adsorption of methylene blue on agroindustrial wastes: Experimental investigation and phenomenological modelling. Prog. Biophys. Mol. Biol. 2019, 141, 60–71. [Google Scholar] [CrossRef]
- Dadrasnia, A.; Chuan Wei, K.S.; Shahsavari, N.; Azirun, M.S.; Ismail, S. Biosorption Potential of Bacillus salmalaya Strain 139SI for Removal of Cr (VI) from Aqueous Solution. Int. J. Environ. Res. Public Health 2015, 12, 15321–15338. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Feng, C.; Wang, C.; Wang, Z. A facile one-pot solvothermal method to produce superparamagnetic graphene–Fe3O4 nanocomposite and its application in the removal of dye from aqueous solution. Coll. Surf. B Biointerfaces 2013, 101, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Dey, M.D.; Ahmed, M.; Singh, R.; Boruah, R.; Mukhopadhyay, R. Utilization of two agrowastes for adsorption and removal of methylene blue: Kinetics and isotherm studies. Water Sci. Technol. 2016, 75, 1138–1147. [Google Scholar] [CrossRef] [PubMed]
- Pavan, F.A.; Mazzocato, A.C.; Gushikem, Y. Removal of methylene blue dye from aqueous solutions by adsorption using yellow passion fruit peel as adsorbent. Bioresour. Technol. 2008, 99, 3162–3165. [Google Scholar] [CrossRef]
- Hameed, B.H.; Ahmad, A.A. Batch adsorption of methylene blue from aqueous solution by garlic peel, an agricultural waste biomass. J. Hazard. Mater. 2009, 164, 870–875. [Google Scholar] [CrossRef]
- Guechi, E.-K.; Hamdaoui, O. Biosorption of methylene blue from aqueous solution by potato (Solanum tuberosum) peel: Equilibrium modelling, kinetic, and thermodynamic studies. Desalin. Water Treatment 2016, 57, 10270–10285. [Google Scholar] [CrossRef]
- Contreras, E.; Sepúlveda, L.; Palma, C. Valorization of Agroindustrial Wastes as Biosorbent for the Removal of Textile Dyes from Aqueous Solutions. Int. J. Chem. Eng. 2012, 2012, 9. [Google Scholar] [CrossRef]
- Jawad, A.H.; Ngoh, Y.S.; Radzun, K.A. Utilization of watermelon (Citrullus lanatus) rinds as a natural low-cost biosorbent for adsorption of methylene blue: Kinetic, equilibrium and thermodynamic studies. J. Taibah Univ. Sci. 2018, 12, 371–381. [Google Scholar] [CrossRef]
- Zaki, A.B.; El-Sheikh, M.Y.; Evans, J.; El-Safty, S.A. Kinetics and Mechanism of the Sorption of Some Aromatic Amines onto Amberlite IRA-904 Anion-Exchange Resin. J. Coll. Interface Sci. 2000, 221, 58–63. [Google Scholar] [CrossRef]
- Shah, I.; Adnan, R.; Wan Ngah, W.S.; Mohamed, N. Iron Impregnated Activated Carbon as an Efficient Adsorbent for the Removal of Methylene Blue: Regeneration and Kinetics Studies. PLoS ONE 2015, 10, e0122603. [Google Scholar] [CrossRef]






| Experimental | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| qe (mg g−1) | K1 (min−1) | qe,cal (mg g−1) | R2 | K2 (g mg−1 min−1) | qe,cal (mg g−1) | R2 |
| 4.41 | 0.0080 | 0.7365 | 0.7315 | 0.0518 | 4.4228 | 0.9999 |
| Model | Parameters | Temperature | ||
|---|---|---|---|---|
| 15 °C | 30 °C | 45 °C | ||
| Experimental | qm (mg g−1) | 9.4094 | 9.4575 | 9.4109 |
| Langmuir | qm (mg g−1) | 21.7799 | 27.4314 | 24.3229 |
| KL (L mg−1) | 1.0373 | 0.8044 | 0.8626 | |
| R2 | 0.9413 | 0.9329 | 0.9163 | |
| Freundlich | KF (L mg−1) | 12.1294 | 13.6724 | 12.2655 |
| 1/n | 0.7653 | 0.8268 | 0.7979 | |
| R2 | 0.9103 | 0.9128 | 0.8890 | |
| Sips | qm (mg g−1) | 10.2134 | 10.4478 | 9.5644 |
| KS | 3.9698 | 4.0111 | 4.2878 | |
| βs | 2.0008 | 2.0339 | 2.4233 | |
| R2 | 0.9856 | 0.9703 | 0.9798 | |
| Toth | qm (mg g−1) | 9.7364 | 9.9577 | 9.7307 |
| K | 16.4588 | 19.6479 | 17.2374 | |
| t | 4.2907 | 4.5530 | 4.6464 | |
| R2 | 0.9547 | 0.9370 | 0.9254 | |
| Biosorbent | qm (mg g−1) | Reference |
|---|---|---|
| Betel nut husk | 0.32 | [23] |
| Passion fruit peel | 2.17 | [24] |
| Banana peel | 4.91 | [23] |
| Garlic peel | 82.74 | [25] |
| Potato peel | 105.26 | [26] |
| Orange peel | 157.20 | [27] |
| Watermelon winds | 188.68 | [28] |
| Sugarcane bagasse | 9.41 | This work |
| Temperature (°C) | ΔG° (kJ mol−1) | ΔH° (kJ mol−1) | ΔS° (J mol−1 K−1) |
|---|---|---|---|
| 15 | −17.12 | 1.93 | 66.04 |
| 30 | −18.04 | ||
| 45 | −19.11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade Siqueira, T.C.; Zanette da Silva, I.; Rubio, A.J.; Bergamasco, R.; Gasparotto, F.; Aparecida de Souza Paccola, E.; Ueda Yamaguchi, N. Sugarcane Bagasse as an Efficient Biosorbent for Methylene Blue Removal: Kinetics, Isotherms and Thermodynamics. Int. J. Environ. Res. Public Health 2020, 17, 526. https://doi.org/10.3390/ijerph17020526
Andrade Siqueira TC, Zanette da Silva I, Rubio AJ, Bergamasco R, Gasparotto F, Aparecida de Souza Paccola E, Ueda Yamaguchi N. Sugarcane Bagasse as an Efficient Biosorbent for Methylene Blue Removal: Kinetics, Isotherms and Thermodynamics. International Journal of Environmental Research and Public Health. 2020; 17(2):526. https://doi.org/10.3390/ijerph17020526
Chicago/Turabian StyleAndrade Siqueira, Thaisa Caroline, Isabella Zanette da Silva, Andressa Jenifer Rubio, Rosângela Bergamasco, Francielli Gasparotto, Edneia Aparecida de Souza Paccola, and Natália Ueda Yamaguchi. 2020. "Sugarcane Bagasse as an Efficient Biosorbent for Methylene Blue Removal: Kinetics, Isotherms and Thermodynamics" International Journal of Environmental Research and Public Health 17, no. 2: 526. https://doi.org/10.3390/ijerph17020526
APA StyleAndrade Siqueira, T. C., Zanette da Silva, I., Rubio, A. J., Bergamasco, R., Gasparotto, F., Aparecida de Souza Paccola, E., & Ueda Yamaguchi, N. (2020). Sugarcane Bagasse as an Efficient Biosorbent for Methylene Blue Removal: Kinetics, Isotherms and Thermodynamics. International Journal of Environmental Research and Public Health, 17(2), 526. https://doi.org/10.3390/ijerph17020526






