The Effects of Different Smoking Patterns in Pregnancy on Perinatal Outcomes in the Southampton Women’s Survey
Abstract
1. Introduction
2. Materials and Methods
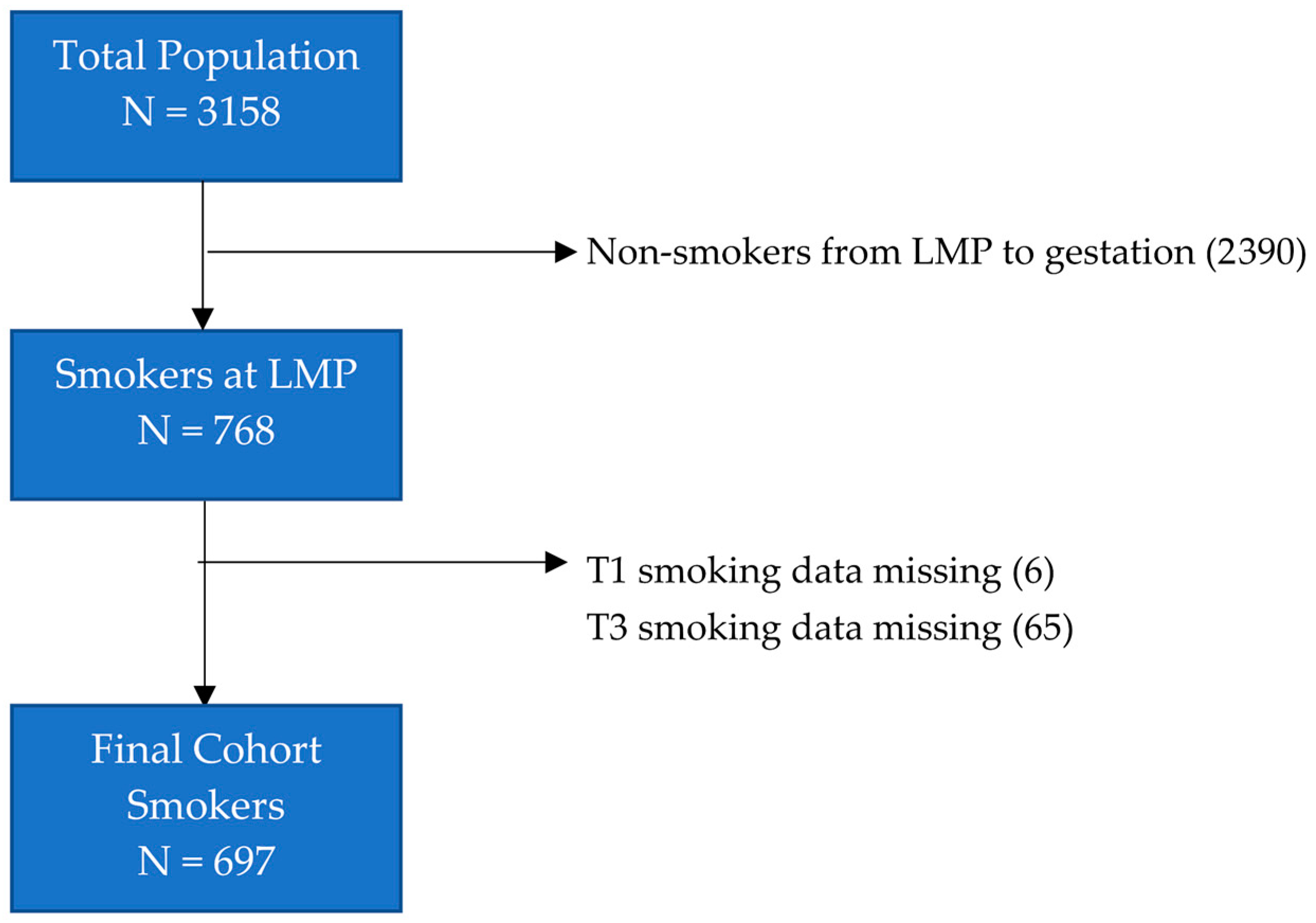
2.1. Study Cohort
2.2. Data Definitions
- Sustained quitters: those who quit smoking throughout their pregnancy (i.e., non-smoking at T1 and T3).
- Partial quitters: those who stated they were not smoking at T1 but were smoking at T3 (first trimester quitters) or who stated they were smoking at T1 and not at T3 (third trimester quitters).
- Sustained smokers: those who continued to smoke throughout their pregnancy (i.e., smoking at T1 and T3).
2.3. Analysis
3. Results
3.1. Study Population Characteristics
3.2. Perinatal Outcomes
4. Discussion
4.1. Study Findings
4.2. Partial Quitting and other Harm Reduction Strategies in Practice
4.3. Study Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- Centers for Disease Control and Prevention (US); National Center for Chronic Disease Prevention and Health Promotion (US); Office on Smoking and Health (US). How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General; Publications and Reports of the Surgeon General; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2010; ISBN 978-0-16-084078-4.
- Rogers, J.M. Tobacco and pregnancy. Reprod. Toxicol. 2009, 28, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Salmasi, G.; Grady, R.; Jones, J.; McDonald, S.D. Environmental tobacco smoke exposure and perinatal outcomes: A systematic review and meta-analyses. Acta Obstet. Gynecol. Scand. 2010, 89, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Zdravkovic, T.; Genbačev, O.; McMaster, M.; Fisher, S. The adverse effects of maternal smoking on the human placenta: A review. Placenta 2005, 26, S81–S86. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.; Probst, C.; Rehm, J.; Popova, S. National, regional, and global prevalence of smoking during pregnancy in the general population: A systematic review and meta-analysis. Lancet Glob. Health 2018, 6, e769–e776. [Google Scholar] [CrossRef]
- Hiscock, R.; Bauld, L.; Amos, A.; Fidler, J.A.; Munafo, M.R. Socioeconomic status and smoking: A review. Ann. N. Y. Acad. Sci. 2012, 1248, 107–123. [Google Scholar] [CrossRef]
- Quinn, V.P.; Mullen, P.D.; Ershoff, D.H. Women who stop smoking spontaneously prior to prenatal care and predictors of relapse before delivery. Addict. Behav. 1991, 16, 29–40. [Google Scholar] [CrossRef]
- Woodby, L.L.; Windsor, R.A.; Snyder, S.W.; Kohler, C.L.; DiClemente, C.C. Predictors of smoking cessation during pregnancy. Addiction 1999, 94, 283–292. [Google Scholar] [CrossRef]
- Kong, G.W.S.; Tam, W.H.; Sahota, D.S.; Nelson, E.A.S. Smoking pattern during pregnancy in Hong Kong Chinese. Aust. N. Z. J. Obstet. Gynaecol. 2008, 48, 280–285. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Smoking: Stopping in Pregnancy and after Childbirth (PH26); National Institute for Health and Care Excellence: London, UK, 2018. [Google Scholar]
- Coleman, T.; Chamberlain, C.; Davey, M.-A.; E Cooper, S.; Leonardi-Bee, J. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Chamberlain, C.; O’Mara-Eves, A.; Porter, J.; Coleman, T.; Perlen, S.M.; Thomas, J.; McKenzie, J.E. Psychosocial interventions for supporting women to stop smoking in pregnancy. Cochrane Database Syst. Rev. 2017, 2, CD001055. [Google Scholar] [CrossRef]
- Lumley, J.; Chamberlain, C.; Dowswell, T.; Oliver, S.; Oakley, L.; Watson, L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database of Syst. Rev. 2009, CD001055. [Google Scholar] [CrossRef]
- Hayes, C.B.; Kearney, M.; O’Carroll, H.; Zgaga, L.; Geary, M.; Kelleher, C.C. Patterns of Smoking Behaviour in Low-Income Pregnant Women: A Cohort Study of Differential Effects on Infant Birth Weight. Int. J. Environ. Res. Public Health 2016, 13, 1060. [Google Scholar] [CrossRef] [PubMed]
- Datar, A.; Jacknowitz, A. Birth Weight Effects on Children’s Mental, Motor, and Physical Development: Evidence from Twins Data. Matern. Child Health J. 2009, 13, 780–794. [Google Scholar] [CrossRef] [PubMed]
- Risnes, K.R.; Vatten, L.J.; Baker, J.L.; Jameson, K.; Sovio, U.; Kajantie, E.; Osler, M.; Morley, R.; Jokela, M.; Painter, R.C.; et al. Birthweight and mortality in adulthood: A systematic review and meta-analysis. Int. J. Epidemiol. 2011, 40, 647–661. [Google Scholar] [CrossRef]
- Belbasis, L.; Savvidou, M.D.; Kanu, C.; Evangelou, E.; Tzoulaki, I. Birth weight in relation to health and disease in later life: An umbrella review of systematic reviews and meta-analyses. BMC Med. 2016, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Butler, N.R.; Goldstein, H.; Ross, E.M. Cigarette Smoking in Pregnancy: Its Influence on Birth Weight and Perinatal Mortality. BMJ 1972, 2, 127–130. [Google Scholar] [CrossRef] [PubMed]
- MacArthur, C.; Knox, E.G. Smoking in pregnancy: Effects of stopping at different stages. Int. J. Obstet. Gynaecol. 1988, 95, 551–555. [Google Scholar] [CrossRef]
- England, L.J.; Kendrick, J.S.; Gargiullo, P.M.; Zahniser, S.C.; Hannon, W.H. Measures of maternal tobacco exposure and infant birth weight at term. Am. J. Epidemiol. 2001, 153, 954–960. [Google Scholar] [CrossRef]
- Jaddoe, V.W.V.; Troe, E.-J.W.M.; Hofman, A.; MacKenbach, J.P.; Moll, H.A.; Steegers, E.A.P.; Witteman, J.C.M. Active and passive maternal smoking during pregnancy and the risks of low birthweight and preterm birth: The Generation R Study. Paediatr. Périnat. Epidemiol. 2008, 22, 162–171. [Google Scholar] [CrossRef]
- Windsor, R.A.; Perkins, L.; Goldenberg, R.L.; Lowe, J.B. The Impact on Infant Birth Weight and Gestational Age of Cotinine-Validated Smoking Reduction During Pregnancy. JAMA 1993, 269, 1519. [Google Scholar] [CrossRef]
- A Lindley, A.; Becker, S.; Gray, R.H.; Herman, A.A. Effect of continuing or stopping smoking during pregnancy on infant birth weight, crown-heel length, head circumference, ponderal index, and brain:body weight ratio. Am. J. Epidemiol. 2000, 152, 219–225. [Google Scholar] [CrossRef]
- Prabhu, N.; Smith, N.; Campbell, D.; Craig, L.C.; Seaton, A.; Helms, P.J.; Devereux, G.; Turner, S.W. First trimester maternal tobacco smoking habits and fetal growth. Thorax 2010, 65, 235–240. [Google Scholar] [CrossRef]
- Cliver, S.P.; Goldenberg, R.L.; Cutter, G.R.; Hoffman, H.J.; Davis, R.O.; Nelson, K.G. The Effect of Cigarette Smoking on Neonatal Anthropometric Measurements. Obstet. Gynecol. 1995, 85, 625–630. [Google Scholar] [CrossRef]
- Dürmuş, B.; Kruithof, C.J.; Gillman, M.H.; Willemsen, S.P.; Hofman, A.; Raat, H.; Eilers, P.H.C.; Steegers, E.A.P.; Jaddoe, V.W.V. Parental smoking during pregnancy, early growth, and risk of obesity in preschool children: The Generation R Study. Am. J. Clin. Nutr. 2011, 94, 164–171. [Google Scholar] [CrossRef]
- Vardavas, C.I.; Chatzi, L.; Patelarou, E.; Plana, E.; Sarri, K.; Kafatos, A.; Koutis, A.D.; Kogevinas, M. Smoking and smoking cessation during early pregnancy and its effect on adverse pregnancy outcomes and fetal growth. Eur. J. Pediatr. 2010, 169, 741–748. [Google Scholar] [CrossRef]
- Blatt, K.; Moore, E.; Chen, A.; Van Hook, J.; DeFranco, E.A. Association of Reported Trimester-Specific Smoking Cessation and Fetal Growth Restriction. Obstet. Gynecol. 2015, 125, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- McCowan, L.M.E.; Dekker, G.A.; Chan, E.; Stewart, A.; Chappell, L.C.; Hunter, M.; Moss-Morris, R.; A North, R.; on behalf of the SCOPE Consortium. Spontaneous preterm birth and small for gestational age infants in women who stop smoking early in pregnancy: Prospective cohort study. BMJ 2009, 338, b1081. [Google Scholar] [CrossRef] [PubMed]
- Polakowski, L.L.; Akinbami, L.J.; Mendola, P. Prenatal Smoking Cessation and the Risk of Delivering Preterm and Small-for-Gestational-Age Newborns. Obstet. Gynecol. 2009, 114, 318–325. [Google Scholar] [CrossRef]
- Philips, E.M.; Santos, S.; Trasande, L.; Aurrekoetxea, J.J.; Barros, H.; Von Berg, A.; Bergström, A.; Bird, P.K.; Brescianini, S.; Chaoimh, C.N.; et al. Changes in parental smoking during pregnancy and risks of adverse birth outcomes and childhood overweight in Europe and North America: An individual participant data meta-analysis of 229,000 singleton births. PLoS Med. 2020, 17, e1003182. [Google Scholar] [CrossRef]
- Räisänen, S.; Sankilampi, U.; Gissler, M.; Kramer, M.R.; Hakulinen-Viitanen, T.; Saari, J.; Heinonen, S. Smoking cessation in the first trimester reduces most obstetric risks, but not the risks of major congenital anomalies and admission to neonatal care: A population-based cohort study of 1 164 953 singleton pregnancies in Finland. J. Epidemiol. Community Health. 2014, 68, 159–164. [Google Scholar] [CrossRef]
- Xaverius, P.K.; O’Reilly, Z.; Li, A.; Flick, L.H.; Arnold, L.D. Smoking Cessation and Pregnancy: Timing of Cessation Reduces or Eliminates the Effect on Low Birth Weight. Matern. Child Health J. 2019, 23, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Saigal, S.; Doyle, L.W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008, 371, 261–269. [Google Scholar] [CrossRef]
- Soneji, S.; Beltrán-Sánchez, H. Association of Maternal Cigarette Smoking and Smoking Cessation with Preterm Birth. JAMA Netw. Open 2019, 2, e192514. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.; Blatt, K.; Chen, A.; Van Hook, J.; DeFranco, E.A. Relationship of trimester-specific smoking patterns and risk of preterm birth. Am. J. Obstet. Gynecol. 2016, 215, 109.e1–109.e6. [Google Scholar] [CrossRef]
- Physical Status: The Use and Interpretation of Anthropometry; Technical Report Series; World Health Organisation: Geneva, Switzerland, 1998; p. 452.
- Borlee, I.; Bouckaert, A.; Lechat, M.; Misson, C. Smoking patterns during and before pregnancy: Weight, length and head circumference of progeny. Eur. J. Obstet. Gynecol. Reprod. Biol. 1978, 8, 171–177. [Google Scholar] [CrossRef]
- Källén, K. Maternal smoking during pregnancy and infant head circumference at birth. Early Hum. Dev. 2000, 58, 197–204. [Google Scholar] [CrossRef]
- Inoue, S.; Naruse, H.; Yorifuji, T.; Kato, T.; Murakoshi, T.; Doi, H.; Subramanian, S. Impact of maternal and paternal smoking on birth outcomes. J. Public Health Oxf. Engl. 2017, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kharkova, O.A.; Grjibovski, A.M.; Krettek, A.; Nieboer, E.; Odland, J.Ø. Effect of Smoking Behavior before and during Pregnancy on Selected Birth Outcomes among Singleton Full-Term Pregnancy: A Murmansk County Birth Registry Study. Int. J. Environ. Res. Public Health 2017, 14, 867. [Google Scholar] [CrossRef]
- Abraham, M.; Alramadhan, S.; Iniguez, C.; Duijts, L.; Jaddoe, V.W.V.; Dekker, H.T.D.; Crozier, S.; Godfrey, K.M.; Hindmarsh, P.; Vik, T.; et al. A systematic review of maternal smoking during pregnancy and fetal measurements with meta-analysis. PLoS ONE 2017, 12, e0170946. [Google Scholar] [CrossRef]
- Inskip, H.M.; Godfrey, K.M.; Robinson, S.M.; Law, C.; Barker, D.J.P.; Cooper, C.; SWS Study Group. Cohort profile: The Southampton Women’s Survey. Int. J. Epidemiol. 2014, 35, 42–48. [Google Scholar] [CrossRef]
- Robinson, S.; Godfrey, K.; Osmond, C.; Cox, V.; Barker, D. Evaluation of a food frequency questionnaire used to assess nutrient intakes in pregnant women. Eur. J. Clin. Nutr. 1996, 50, 302–308. [Google Scholar]
- Crozier, S.; SWS Study Group; Robinson, S.M.; Borland, S.E.; Inskip, H.M. Dietary patterns in the Southampton Women’s Survey. Eur. J. Clin. Nutr. 2006, 60, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- West, R.; Hajek, P.; Stead, L.; Stapleton, J. Outcome criteria in smoking cessation trials: Proposal for a common standard. Addiction 2005, 100, 299–303. [Google Scholar] [CrossRef]
- Abrahamsson, A.; Ejlertsson, G. Smoking patterns during pregnancy: Differences in socioeconomic and health-related variables. Eur. J. Public Health 2000, 10, 208–213. [Google Scholar] [CrossRef]
- Greenland, S.; Pearl, J.; Robins, J.M. Causal Diagrams for Epidemiologic Research. Epidemiology 1999, 10, 37–48. [Google Scholar] [CrossRef]
- Textor, J.; Van Der Zander, B.; Gilthorpe, M.S.; Liśkiewicz, M.; Ellison, G.T. Robust causal inference using directed acyclic graphs: The R package ‘dagitty’. Int. J. Epidemiol 2016, 45, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Staplin, N.; Herrington, W.G.; Judge, P.K.; Reith, C.A.; Haynes, R.; Landray, M.J.; Baigent, C.; Emberson, J. Use of Causal Diagrams to Inform the Design and Interpretation of Observational Studies: An Example from the Study of Heart and Renal Protection (SHARP). Clin. J. Am. Soc. Nephrol. 2016, 12, 546–552. [Google Scholar] [CrossRef]
- McBride, C.M.; Emmons, K.M.; Lipkus, I.M. Understanding the potential of teachable moments: The case of smoking cessation. Health Educ. Res. 2003, 18, 156–170. [Google Scholar] [CrossRef]
- A Williams, L.; Evans, S.F.; Newnham, J.P. Prospective cohort study of factors influencing the relative weights of the placenta and the newborn infant. BMJ 1997, 314, 1864. [Google Scholar] [CrossRef]
- Andriani, H.; Kuo, H.-W. Adverse effects of parental smoking during pregnancy in urban and rural areas. BMC Pregnancy Childbirth 2014, 14, 414. [Google Scholar] [CrossRef]
- Larsen, S.; Haavaldsen, C.; Bjelland, E.K.; Dypvik, J.; Jukic, A.M.; Eskild, A. Placental weight and birthweight: The relations with number of daily cigarettes and smoking cessation in pregnancy. A population study. Int. J. Epidemiol. 2018, 47, 1141–1150. [Google Scholar] [CrossRef]
- McDonnell, B.P.; Dicker, P.; Regan, C.L. Electronic cigarettes and obstetric outcomes: A prospective observational study. Int. J. Obstet. Gynaecol. 2020, 127, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Dinakar, C.; O’Connor, G. The Health Effects of Electronic Cigarettes. N. Engl. J. Med. 2016, 375, 1372–1381. [Google Scholar] [CrossRef]
- Stephenson, J.; Vogel, C.; Hall, J.; Hutchinson, J.; Mann, S.; Duncan, H.; Woods-Townsend, K.; De Lusignan, S.; Poston, L.; Cade, J.; et al. Preconception health in England: A proposal for annual reporting with core metrics. Lancet 2019, 393, 2262–2271. [Google Scholar] [CrossRef]
- Ng, S.; Aris, I.M.; Tint, M.T.; Gluckman, P.D.; Godfrey, K.M.; Shek, L.P.-C.; Yap, F.; Ciptaningtyas, R.; Lek, N.; Teoh, O.H.; et al. High Maternal Circulating Cotinine During Pregnancy is Associated With Persistently Shorter Stature From Birth to Five Years in an Asian Cohort. Nicotine Tob. Res. 2018, 21, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Rauschert, S.; Melton, P.E.; Burdge, G.C.; Craig, J.M.; Godfrey, K.M.; Holbrook, J.D.; Lillycrop, K.A.; Mori, T.A.; Beilin, L.J.; Oddy, W.H.; et al. Maternal Smoking During Pregnancy Induces Persistent Epigenetic Changes Into Adolescence, Independent of Postnatal Smoke Exposure and Is Associated with Cardiometabolic Risk. Front. Genet. 2019, 10, 770. [Google Scholar] [CrossRef] [PubMed]
| Non-Smokers (N = 2461) | Smokers (N = 697) | Sustained Smokers (N = 355) | Partial Quitters (N = 81) | Sustained Quitters (N = 261) | |
|---|---|---|---|---|---|
| Age (years) (mean, SD) | 31.0 (3.7) | 29.4 (4.2) | 29.5 (4.1) | 29.4 (4.7) | 29.2 (4.1) |
| Ethnicity (N, %) | |||||
| White | 2332 (94.8) | 684 (98.1) | 349 (98.3) | 78 (96.3) | 257 (98.5) |
| Non-White | 128 (5.2) | 13 (1.9) | 6 (1.7) | 3 (3.7) | 4 (1.5) |
| Gestational Diabetes Mellitus (N, %) | 36 (1.5) | 2 (0.3) | 1 (0.3) | 1 (1.2) | 0 (0.0) |
| Maternal Pre-pregnancy Body Mass Index (kilograms/metres2) (median, IQR) | 24.1 (21.9, 27.3) | 24.3 (21.9, 27.7) | 24.5 (22.0, 28.0) | 25.3 (22.5, 28.9) | 23.8 (21.5, 26.7) |
| Primiparous (N, %) | 1093 (51.5) | 295 (42.3) | 114 (32.1) | 36 (44.4) | 145 (55.6) |
| Townsend Deprivation Index Score (mean, SD) | −0.2 (3.1) | 1.0 (3.3) | 1.4 (3.2) | 1.1 (3.5) | 0.5 (3.2) |
| Receiving Benefits (N, %) | 263 (10.7) | 215 (30.8) | 153 (43.1) | 15 (18.5) | 47 (18.0) |
| Educational Attainment (N, %) | |||||
| None | 49 (2.0) | 50 (7.2) | 32 (9.0) | 5 (6.3) | 13 (5.0) |
| Certificate of Secondary Education | 182 (7.4) | 113 (16.2) | 75 (21.1) | 13 (16.3) | 25 (9.6) |
| O-levels | 651 (26.5) | 260 (37.4) | 146 (41.1) | 22 (27.5) | 92 (35.2) |
| A-levels | 762 (31.1) | 191 (27.4) | 86 (24.2) | 30 (37.5) | 75 (28.7) |
| Higher National Diploma | 172 (7.0) | 26 (3.7) | 6 (1.7) | 4 (5.0) | 16 (6.1) |
| Degree or above | 637 (26.0) | 56 (8.0) | 10 (2.8) | 6 (7.5) | 40 (15.3) |
| Early-pregnancy Prudent Diet Score (z-score) (mean, SD) | 0.2 (0.9) | −0.6 (1.0) | −0.9 (0.9) | −0.4 (1.1) | −0.2 (1.0) |
| All Smokers (N = 697) | Sustained Smokers (N = 355) | Partial Quitters (N = 81) | Sustained Quitters (N = 261) | ||
|---|---|---|---|---|---|
| First Trimester Quitters (N = 32) | Third Trimester Quitters (N = 49) | ||||
| Age commenced smoking (years) (median, IQR) | 16 (14–17) | 15 (14–17) | 16 (15–17) | 16 (14–18) | 16 (15–18) |
| Cigarettes/day at the last menstrual period (median, IQR) | 15 (7–20) | 18 (10–20) | 11 (8–18) | 10 (6–20) | 7 (4–15) |
| Cigarettes/day in the first trimester (median, IQR) | 10 (5–10) | 10 (5–12) | - | 5 (2–6) | - |
| Cigarettes/day in the third trimester (median, IQR) | 10 (5–15) | 10 (5–15) | 3 (2–10) | - | - |
| Non-Smokers (N = 2461) | Smokers (N = 697) | Sustained Smokers (N = 355) | Partial Quitters (N = 81) | Sustained Quitters (N = 261) | |
|---|---|---|---|---|---|
| Birthweight (WHO z-score) (mean, SD) | 0.1 (1.0) | −0.1 (1.0) | −0.4 (1.0) | 0.1 (1.0) | 0.2 (0.9) |
| Head circumference (WHO z-score) (mean, SD) | 0.4 (1.0) | 0.3 (1.0) | 0.1 (1.0) | 0.5 (1.1) | 0.5 (1.1) |
| Crown–heel length (WHO z-score) (mean, SD) | −0.3 (0.8) | −0.5 (0.9) | −0.7 (0.9) | −0.5 (1.0) | −0.2 (0.8) |
| Gestation (weeks) (median, IQR) | 40.0 (39.0, 41.0) | 40.1 (39.1, 41.0) | 39.8 (38.9, 40.7) | 40.0 (38.9, 41.0) | 40.3 (39.6, 41.1) |
| Partial Quitters | Sustained Quitters | ||||
|---|---|---|---|---|---|
| N | β | 95% CI | β | 95% CI | |
| Birthweight (WHO z-score) | 686 | 0.48 | (0.24–0.72) | 0.64 | (0.47–0.80) |
| Head circumference (WHO z-score) | 679 | 0.38 | (0.12–0.64) | 0.41 | (0.23–0.59) |
| Crown–heel length (WHO z-score) | 671 | 0.23 | (0.02–0.45) | 0.54 | (0.40–0.45) |
| Gestation (weeks) | 691 | 0.10 | (−0.25–0.45) | 0.50 | (0.26–0.74) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Donnell, M.M.; Baird, J.; Cooper, C.; Crozier, S.R.; Godfrey, K.M.; Geary, M.; Inskip, H.M.; Hayes, C.B. The Effects of Different Smoking Patterns in Pregnancy on Perinatal Outcomes in the Southampton Women’s Survey. Int. J. Environ. Res. Public Health 2020, 17, 7991. https://doi.org/10.3390/ijerph17217991
O’Donnell MM, Baird J, Cooper C, Crozier SR, Godfrey KM, Geary M, Inskip HM, Hayes CB. The Effects of Different Smoking Patterns in Pregnancy on Perinatal Outcomes in the Southampton Women’s Survey. International Journal of Environmental Research and Public Health. 2020; 17(21):7991. https://doi.org/10.3390/ijerph17217991
Chicago/Turabian StyleO’Donnell, Martin M., Janis Baird, Cyrus Cooper, Sarah R. Crozier, Keith M. Godfrey, Michael Geary, Hazel M. Inskip, and Catherine B. Hayes. 2020. "The Effects of Different Smoking Patterns in Pregnancy on Perinatal Outcomes in the Southampton Women’s Survey" International Journal of Environmental Research and Public Health 17, no. 21: 7991. https://doi.org/10.3390/ijerph17217991
APA StyleO’Donnell, M. M., Baird, J., Cooper, C., Crozier, S. R., Godfrey, K. M., Geary, M., Inskip, H. M., & Hayes, C. B. (2020). The Effects of Different Smoking Patterns in Pregnancy on Perinatal Outcomes in the Southampton Women’s Survey. International Journal of Environmental Research and Public Health, 17(21), 7991. https://doi.org/10.3390/ijerph17217991






