Emerging Natural Focal Infectious Diseases in Russia: A Medical–Geographical Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Newly Emerging or Newly Identified Diseases
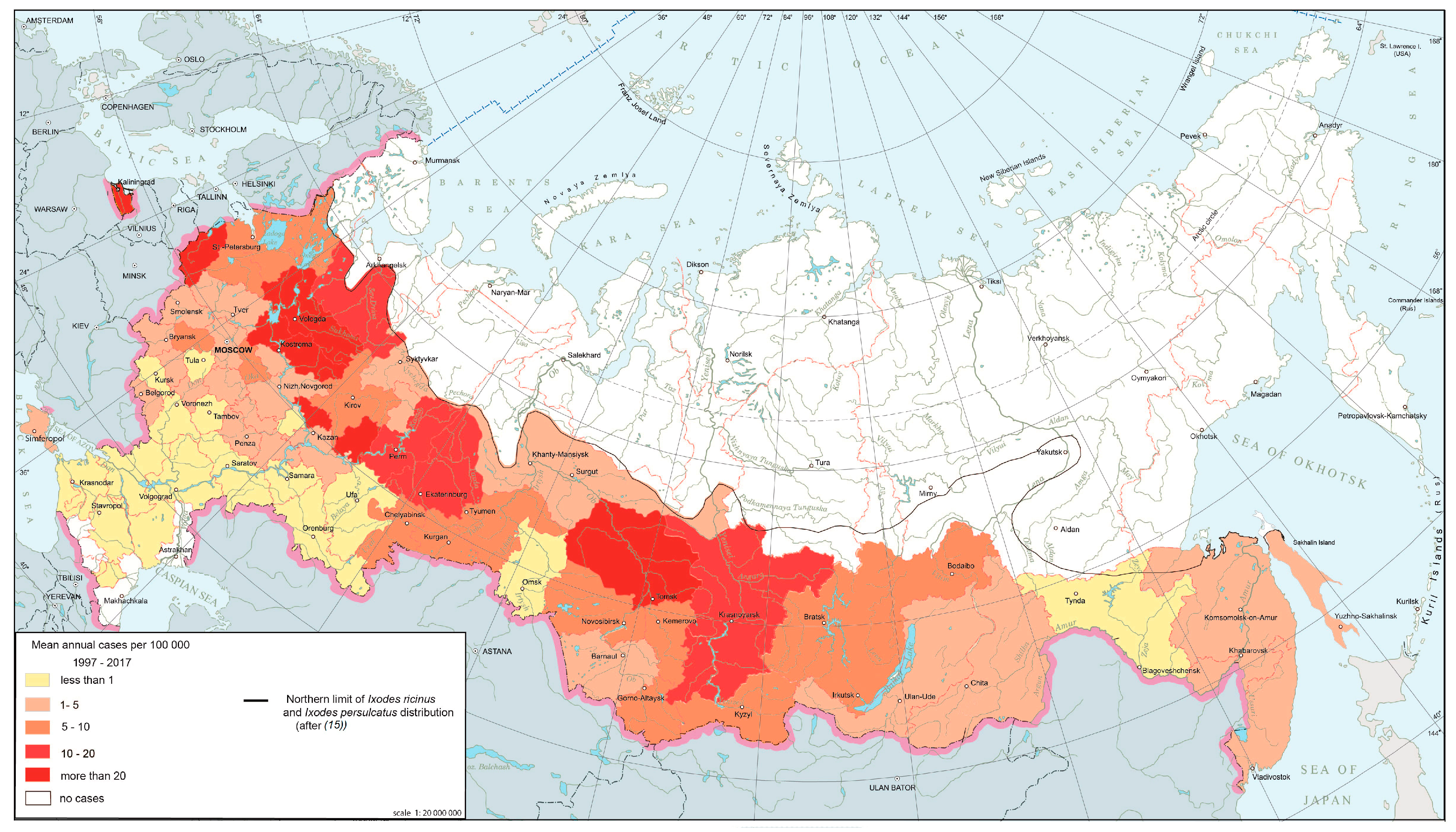
3.2. Diseases with Their Areal Limits Static while Changes in Internal Structure and Dynamics Occur
3.3. Diseases Spreading through Expansion of Their Nosoareals to New Territories
3.4. Diseases That Periodically Give Epidemic Outbreaks against the Background of Relatively Stable Mean Morbidity Rates
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lvov, D.K. Influenza and other emerging infectious diseases of Northern Eurasia. Global consequences. Fed. Man. Public Health Serv. Russia 2010, 11, 209–220. [Google Scholar]
- Morens, D.M.; Fauci, A.S. Emerging Infectious Diseases: Threats to Human Health and Global Stability. PLoS Pathog. 2013, 9, e1003467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semenza, J.C.; Lindgren, E.; Balkanyi, L.; Espinosa, L.; Almqvist, M.S.; Penttinen, P.; Rocklöv, J. Determinants and Drivers of Infectious Disease Threat Events in Europe. Emerg. Infect. Dis. 2016, 22, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Lvov, D.K.; Butenko, A.M.; Gromashevsky, V.L.; Kovtunov, A.I.; Prilipov, A.G.; Kinney, R.; Aristova, V.A.; Dzharkenov, A.F.; Samokhvalov, E.I.; Savage, H.M.; et al. West Nile virus and other zoonotic viruses in Russia: Examples of emerging-reemerging situations. In Emergence and Control of Zoonotic Viral Encephalitides; Calisher, C.H., Griffin, D.E., Eds.; Springer: Vienna, Austria, 2004; pp. 85–96. ISBN 978-3-211-20454-2. [Google Scholar]
- Butenko, A.M.; Karganova, G.G. Crimean-Congo Hemorrhagic Fever in Russia and Other Countries of the Former Soviet Union. In Crimean-Congo Hemorrhagic Fever; Ergonul, O., Whitehouse, C.A., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 99–114. ISBN 978-1-4020-6105-9. [Google Scholar]
- Maltezou, H.C.; Papa, A. Crimean–Congo hemorrhagic fever: Risk for emergence of new endemic foci in Europe? Travel Med. Infect. Dis. 2010, 8, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Kulichenko, A.N.; Maletskaya, O.V.; Vasilenko, N.F.; Beyer, A.P.; Sannikova, I.V.; Pasechnikov, V.D. Crimean-Congo Hemorrhagic Fever in Eurasia in 21st Century: Epidemiological Aspects. Curr. Items Epidemiol. Infect. Dis. 2012, 3, 42–53. [Google Scholar]
- Lvov, D.K. Isolation of Two Strains of West Nile Virus during an Outbreak in Southern Russia, 1999. Emerg. Infect. Dis. 2000, 6, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Morse, S.S. Factors in the Emergence of Infectious Diseases. Emerg. Infect. Dis. 1995, 1, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.E.; Patel, N.G.; Levy, M.A.; Storeygard, A.; Balk, D.; Gittleman, J.L.; Daszak, P. Global trends in emerging infectious diseases. Nature 2008, 451, 990–993. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.H.; Latham, S.M.; Woolhouse, M.E. Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Pavlovskij, E.N. Natural Focality of Vector-Borne Diseases; Nauka: Moscow, Russia, 1964. [Google Scholar]
- Malkhazova, S.M.; Kotova, T.V.; Mironova, V.; Shartova, N.; Ryabova, N.V. Medico-Geographical Atlas of Russia “Natural Focal Diseases”, 2nd ed.; Faculty of Geography, Lomonosov Moscow State University: Moscow, Russia, 2017. [Google Scholar]
- Malkhazova, S.M.; Mironova, V.A.; Kotova, T.V.; Shartova, N.V.; Orlov, D.S. Natural-focal diseases: Mapping experience in Russia. Int. J. Health Geogr. 2014, 13, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korenberg, E.I.; Pomelova, V.G.; Osin, N.S. Ixodid Tick-Borne Natural Focal Diseases; OOO Kommentary: Moscow, Russia, 2013. [Google Scholar]
- Ogureeva, G.N.; Kotova, T.V. MAP “BIOMES OF RUSSIA” AND ITS ROLE IN THE IMPROVEMENT OF ECOLOGICAL EDUCATION AND NATURE PROTECTION ACTIVITY. Proc. Int. Conf. InterCartoInterGIS 2014, 1, 632–641. [Google Scholar] [CrossRef] [Green Version]
- Lurie, I.K. Geoinformatic Mapping. Methods of Geoinformatics and Image Visual Prosessing; Knizhnyj dom Universitet: Moscow, Russia, 2016. [Google Scholar]
- Ogureeva, G.N.; Leonova, N.B.; Buldakova, E.V.; Kadetov, N.G.; Arkhipova, M.V.; Miklyaeva, I.M.; Bocharnikov, M.V.; Dudov, S.V.; Ignatova, E.A.; Ignatov, M.S.; et al. Biomes of Russia, Scale 1:7 500 000. Wall Map for Institutions of Higher Education; OOO Finansovyj i Organizacionnyj Konsalting: Moscow, Russia, 2016. [Google Scholar]
- Malkhazova, S.M. Medical and Geographical Analysis of Territories: Mapping, Assessment, Forecast; Scientific World: Moscow, Russia, 2001; 239p. [Google Scholar]
- Homyakov, Y.N.; Homyakova, T.I.; Severin, S.E. New infectious diseases hazard. Biosafety and molecular mechanisms of the pathogenesis. Vestn. NII Mol. Med. 2004, 4, 6–24. [Google Scholar]
- Shkarin, V.V.; Kovalishena, O.V. Contemporary Classification of New Infections: A New Look on the Old Problem. Epidemiol. Infekc. Bolezn. Aktual’nye Vopr. 2012, 4, 53–57. [Google Scholar]
- Morens, D.M.; Folkers, G.K.; Fauci, A.S. The challenge of emerging and re-emerging infectious diseases. Nature 2004, 430, 242–249. [Google Scholar] [CrossRef] [PubMed]
- The State Report. On the Status of Population Sanitary and Epidemiological Well-Being in Russian Federation in 2014; Federal Service on Customers’ Rights Protection and Human Well-Being Surveillance: Moscow, Russia, 2015; 206p. [Google Scholar]
- Tarasevich, I.V. Astrakhan Spotted Fever; Meditsina: Moscow, Russia, 2002. [Google Scholar]
- Ecker, M.; Allison, S.L.; Meixner, T.; Heinz, F.X. Sequence analysis and genetic classification of tick-borne encephalitis viruses from Europe and Asia. J. Gen. Virol. 1999, 80, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Korenberg, E.I.; Kovalevskii, Y.V. A Model for Relationships among the Tick-Borne Encephalitis Virus, Its Main Vectors, and Hosts. In Advances in Disease Vector Research; Harris, K.F., Ed.; Advances in Disease Vector Research; Springer: New York, NY, USA, 1994; Volume 10, pp. 65–92. ISBN 978-1-4612-7596-1. [Google Scholar]
- Rudakova, S.A. Ixodid tick-borne borrelioses in multi-infection foci of Western Siberia. Bull. VSNC SO RAMN 2007, 7, 151–155. [Google Scholar]
- Maleev, V.V.; Galimzyanov, K.M.; Butenko, A.M.; Cherenov, I.V. Crimean-Congo Hemorrhagic Fever; Izdatel’stvo Astrakhanskoj Meditsinskoj Akademii: Moscow-Astrakhan, Russia, 2003. [Google Scholar]
- Platonov, A.; Tolpin, V.; Gridneva, K.; Titkov, A.; Platonova, O.; Kolyasnikova, N.; Busani, L.; Rezza, G. The Incidence of West Nile Disease in Russia in Relation to Climatic and Environmental Factors. Int. J. Environ. Res. Public. Health 2014, 11, 1211–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adishcheva, O.S.; Malhazova, S.M.; Orlov, D.S. The spread of West Nile fever in Russia. Vestn. MSU Seriya 5 Geogr. 2016, 4, 48–55. [Google Scholar]
- Meshcheryakova, I.S.; Dobrovol’skij, A.A.; Demidova, T.N.; Kormilitsyna, M.I.; Mihajlova, T.V. Vector-Borne Tularemia Epidemic Outbreak in Khanty-Mansijsk in 2013. Epidemiologiya i Vakcinoprofilaktika 2014, 5, 14–20. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malkhazova, S.; Pestina, P.; Prasolova, A.; Orlov, D. Emerging Natural Focal Infectious Diseases in Russia: A Medical–Geographical Study. Int. J. Environ. Res. Public Health 2020, 17, 8005. https://doi.org/10.3390/ijerph17218005
Malkhazova S, Pestina P, Prasolova A, Orlov D. Emerging Natural Focal Infectious Diseases in Russia: A Medical–Geographical Study. International Journal of Environmental Research and Public Health. 2020; 17(21):8005. https://doi.org/10.3390/ijerph17218005
Chicago/Turabian StyleMalkhazova, Svetlana, Polina Pestina, Anna Prasolova, and Dmitry Orlov. 2020. "Emerging Natural Focal Infectious Diseases in Russia: A Medical–Geographical Study" International Journal of Environmental Research and Public Health 17, no. 21: 8005. https://doi.org/10.3390/ijerph17218005
APA StyleMalkhazova, S., Pestina, P., Prasolova, A., & Orlov, D. (2020). Emerging Natural Focal Infectious Diseases in Russia: A Medical–Geographical Study. International Journal of Environmental Research and Public Health, 17(21), 8005. https://doi.org/10.3390/ijerph17218005





